SUMMARY
Many patients with type 1 diabetes (T1D) have residual β cells producing small amounts of C-peptide long after disease onset but develop an inadequate glucagon response to hypoglycemia following T1D diagnosis. The features of these residual β cells and α cells in the islet endocrine compartment are largely unknown, due to the difficulty of comprehensive investigation. By studying the T1D pancreas and isolated islets, we show that remnant β cells appeared to maintain several aspects of regulated insulin secretion. However, the function of T1D α cells was markedly reduced, and these cells had alterations in transcription factors constituting α and β cell identity. In the native pancreas and after placing the T1D islets into a non-autoimmune, normoglycemic in vivo environment, there was no evidence of α-to-β cell conversion. These results suggest an explanation for the disordered T1D counterregulatory glucagon response to hypoglycemia.
In Brief
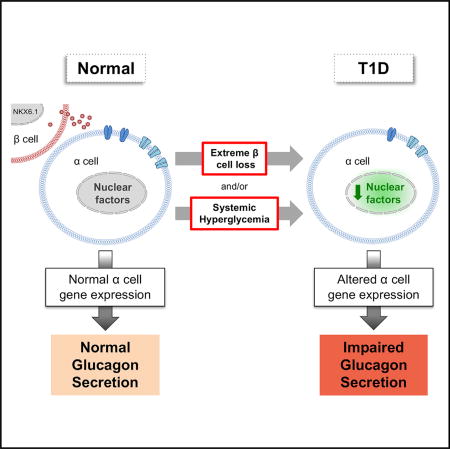
Brissova et al. find that β cells in the type 1 diabetic (T1D) pancreas maintain several functional and molecular features, but α cells have impaired glucagon secretion and an altered gene expression profile. These findings provide insight into the mechanism of α cell dysfunction in T1D.
INTRODUCTION
The events related to type 1 diabetes (T1D) pathophysiology in humans are poorly defined. For example, we do not understand the initiating trigger for T1D, how β cell loss proceeds, whether the loss is inevitable or can be abrogated, or the potential for residual β cell recovery. The long-standing view of T1D pathogenesis was that autoimmune β cell destruction resulted in complete loss of pancreatic insulin secretion. The improved sensitivity of C-peptide detection as well as studies using pancreatic specimens have recently led to the realization that many individuals with T1D have insulin-secreting cells, even 50 years after diagnosis (Keenan et al., 2010; Oram et al., 2014). Additionally, little is known about the properties of the glucagon-producing α cells in the T1D pancreas and whether they share the plasticity recently described in mouse models of profound β cell loss (Chera et al., 2014; Thorel et al., 2010). Moreover, it is unclear why T1D α cells have impaired glucagon secretion (Bolli et al., 1983; Gerich et al., 1973; Sherr et al., 2014), which contributes to hypoglycemia susceptibility.
To comprehensively define the functional and molecular properties of T1D islets, we used an approach that allows study of the pancreas and isolated islets from the same organ donor. Our findings show that remnant β cells appeared to maintain several features of regulated insulin secretion. In contrast, glucagon secretion was significantly compromised, and the levels of essential α cell transcription factors and their downstream targets involved in α cell electrical activity were reduced. Moreover, an important β-cell-enriched transcription factor was misexpressed in T1D α cells. These results provide insight into the functional and molecular profile of α cells in T1D.
RESULTS
Procurement of Pancreatic Islets and Tissue from the Same Organ Donor Allows for Multifaceted Phenotypic Analysis of T1D Islets
Our methodology for islet isolation and tissue procurement from the same pancreas allowed coupling of islet functional and molecular analysis with histological assessment of islets in the native organ (Figure S1A). In this way, we were able to study 5 donors with recent-onset T1D (<10 years of T1D duration) and 3 donors with long-standing T1D (>10 years of T1D duration) receiving continuous insulin therapy compared to the appropriate non-diabetic controls (Tables 1 and S1). Experimental approaches used for analysis of each T1D donor are indicated in Table 1 and labeled accordingly in figure legends. Due to clinical heterogeneity of T1D, we confirmed disease status by DNA sequencing (Sanyoura et al., 2018) as described in the Supplemental Experimental Procedures. DNA sequencing covering coding regions and splice junctions of 148 genes associated with monogenic diabetes did not detect variants associated with monogenic diabetes (Alkorta-Aranburu et al., 2016; Table S2). By flow cytometry analysis, recent-onset T1D islets contained 7-fold more α cells than β cells, and the β cell fraction was reduced approximately 6-fold compared to normal islets (Blodgett et al., 2015; Figures S1B–S1D).
Table 1.
Demographic Information and Phenotype of T1D Donors
| Donors | Age (Years) |
T1D Duration (Years) |
Ethnicity/Race | Gender | BMI | Cause of Death |
High-risk HLAa | AutoAb | C-Peptide (ng/mL) |
HbA1Cb |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 3 | Caucasian | F | 26.6 | anoxia | DR3, DQ2 | mIAA | 0.05 | 9.8 |
| 2 | 13 | 5 | Caucasian | M | 19.1 | anoxia | DR4, DQ2, DQ8 | IA2A, mIAA | <0.02 | NA |
| 3 nPOD case no. 6342 | 14 | 2 | Caucasian | F | 24.3 | anoxia | DR4 | IA2A, mIAA | 0.26 | 9.2 |
| 4 | 20 | 7 | Caucasian | M | 25.5 | anoxia | DR4, DQ2, DQ8 | IA2A | 0.43 | NA |
| 5 nPOD case no. 6323 | 22 | 6 | Caucasian | F | 24.7 | anoxia | DR3, DR4, DQ2 | GADA, IA2A | <0.02 | 6.6 |
| 6 | 27 | 17 | Caucasian | M | 18.5 | anoxia | DR4, DQ2, DQ8 | ND | <0.02 | NA |
| 7 | 30 | 20 | Caucasian | M | 29.8 | anoxia | DR4, DQ8 | ND | <0.02 | NA |
| 8 | 58 | 31 | Caucasian | M | 21.7 | anoxia | DR4 | NA | NA | 8.8 |
The nature of T1D pancreas, organ scarcity, and logistics of organ procurement and processing precluded us from collecting the entire dataset on each T1D donor. Perifusion, donors nos. 1, 3, 4, and 5; qRT-PCR, donors nos. 1, 4, and 5; islet endocrine cell composition by FACS, donors nos. 1, 4, 5, and 8; histology, donors nos. 1, 2, 5, and 8; islet transplantation, donors nos. 1, 5, and 8; α cell purification and RNA sequencing, donors nos. 3, 6, and 7.
AutoAb, autoantibodies; GADA, glutamic acid decarboxylase autoantibody; HbA1C, hemoglobin A1C; IA2A, autoantibody to transmembrane protein of the protein tyrosine phosphatase family; mIAA, insulin autoantibody; NA, not available; ND, non-detectable.
HLA typing provided by Organ Procurement Organization.
HbA1C collected from donor’s redacted medical chart.
T1D β Cells Have Regulated Insulin Secretion and Express Key Transcriptional Regulators
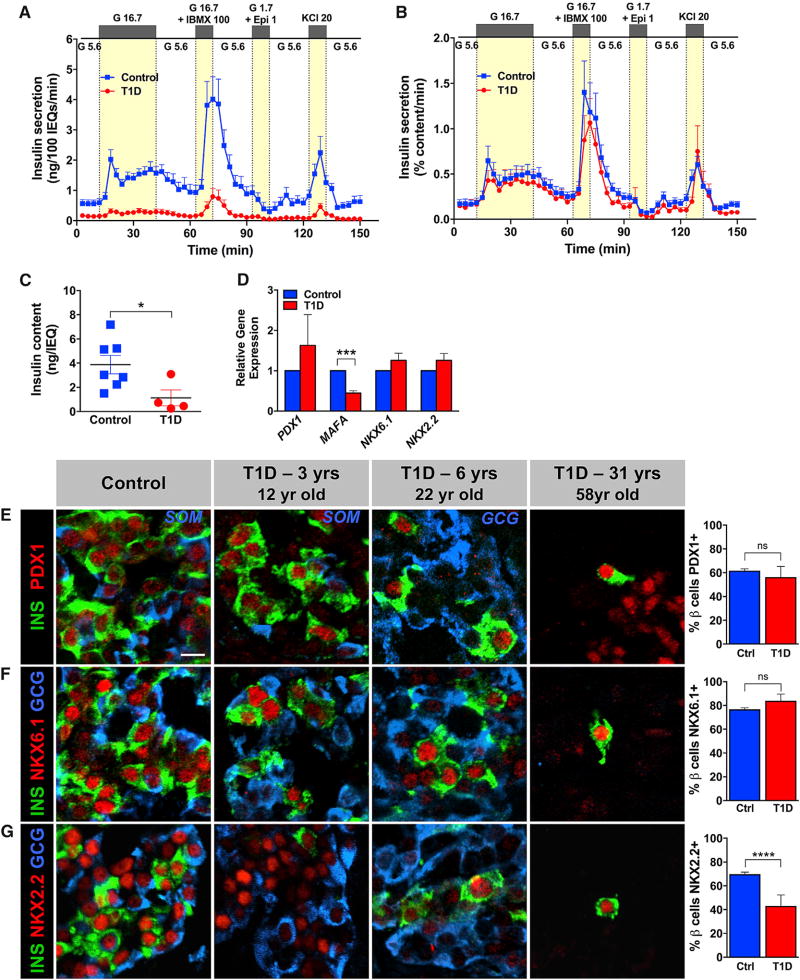
Next, we analyzed the secretory function of the T1D islets in a dynamic cell perifusion system and compared it with islets from normal donors (Kayton et al., 2015). We found that the few remaining T1D β cells responded to glucose, cyclic AMP (cAMP)-evoked stimulation, and KCl-mediated depolarization with a similar pattern as controls (Figures 1A and 1B). The biphasic glucose-stimulated insulin secretion in islets at T1D onset was also shown recently by Krogvold and colleagues (Krogvold et al., 2015). As expected, insulin secretion by T1D islets was diminished when normalized to overall islet cell volume (expressed in islet equivalents [IEQs]; Figure 1A) due to the greatly reduced β cell number (Figure S1C). However, insulin secretion normalized to islet insulin content (reflecting β cell number) by T1D islets nearly overlapped in terms of magnitude with the secretory response of controls (Figure 1B). Consistent with flow cytometry data in Figure S1C, the T1D β cell population was 4- to 6-fold less than in control islets when adjusted to islet insulin content (Figure 1C). Furthermore, the expression of transcription factors critical for β cell identity PDX1 (Gao et al., 2014) and NKX6.1 (Taylor et al., 2013) was not changed in either isolated T1D islets (Figure 1D) or by protein analysis of the native pancreatic tissue (Figures 1E, 1F, and S2). Even in the 58-year-old T1D donor with long-standing T1D, these transcription factors were expressed in rare insulin+ cells found scattered in the exocrine parenchyma (Figures 1E, 1F, and S2). However, MAFA (Guo et al., 2013), a transcription factor known to be required for murine β cell maturation, was reduced in the T1D islet (Figure 1D), and there were fewer NKX2.2-expressing T1D β cells compared to controls (Figures 1G and S2), even though islet NKX2-2 mRNA was unchanged (Figure 1D). These studies allowed us to directly access multiple pathways of insulin secretion and suggest that the T1D β cells appear to maintain several functional features of normal β cells, supporting the notion that T1D is a disease primarily of β cell loss. Due to very few T1D β cells available for deeper analyses, we focused our efforts on comprehensive characterization of the most abundant endocrine cell type in T1D islets, the α cell (Figure S1C).
Figure 1. T1D β Cells in Recent-Onset T1D Retain Secretory Properties and Gene Expression Pattern Similar to Normal β Cells.
(A and B) Insulin secretion was assessed in islets isolated from donors with recent-onset T1D (n = 4; ages 12–22 years; donors nos. 1, 3, 4, and 5) and compared to normal controls (n = 7; ages 7–21 years); G 5.6–5.6 mM glucose, G 16.7–16.7 mM glucose, G 16.7 + IBMX 100–16.7 mM glucose + 100 µM isobutylmethylxanthine (IBMX), G 1.7 + Epi 1–1.7 mM glucose + 1 µM epinephrine, KCl 20–20 mM potassium chloride.
(A) Insulin secretion normalized to overall islet cell volume (expressed as islet equivalents [IEQs]); ****p < 0.0001.
(B) Insulin secretion normalized to islet insulin content; ***p = 0.0005. Data in (A) and (B) were compared by two-way ANOVA.
(C) Insulin content of control (3.873 ± 0.763 ng/IEQ) and T1D islets (1.131 ± 0.660 ng/IEQ); p = 0.0394; data are represented as mean ± SEM.
(D) Expression of β-cell-enriched transcription factors by qRT-PCR in whole T1D islets (n = 3 donors; ages 12–22 years; donors nos. 1, 4, and 5) and controls (n = 3 donors; ages 11–29 years) was normalized to endogenous control and INS expression; ***p < 0.0007.
(E–G) Expression of β-cell-enriched transcription factors in the native pancreatic tissue from donors with recent-onset T1D (n = 2; ages 12–22 years; donors nos. 1 and 5) was compared to 58-year-old donor with 31 years of T1D duration (donor no. 8) and controls (n = 7; ages 8–55 years). The pancreas of 58-year-old T1D donor did not have any insulin+ islets; only rare β cells were found in exocrine parenchyma. T1D β cells (n = 3 donors; ages 12–58 years; donors nos. 1, 5, and 8) had normal expression of β-cell-enriched transcription factors PDX1 (E) and NKX6.1 (F) but decreased expression of NKX2.2 (G) compared to controls (n = 7 donors; ages 8–55 years); ****p < 0.0001; ns, not significant.
Data in (C)–(G) were compared by two-tailed Student’s t test. Data in (A)–(G) are shown as mean ± SEM. The scale bar in (E) represents 10 µm and also corresponds to (F) and (G). See also Figures S1 and S2.
T1D α Cells Are Functionally Impaired and Have Altered Expression of Transcription Factors Constituting α and β Cell Identity
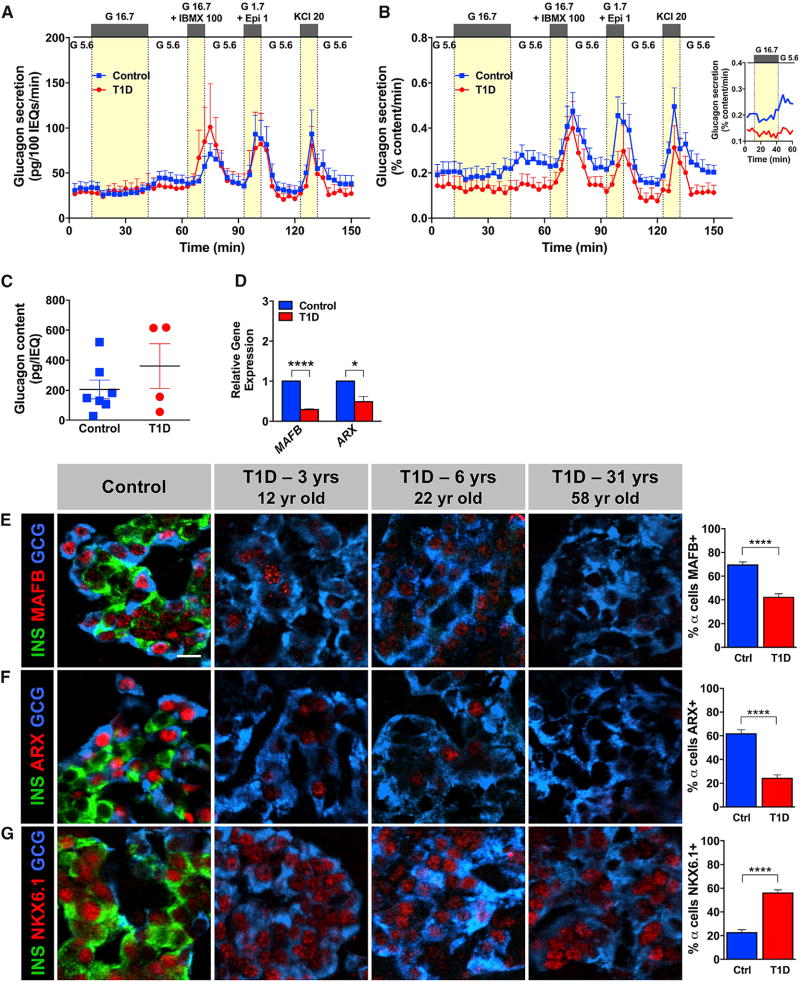
Surprisingly, in spite of T1D islets containing 2-fold more α cells than normal islets (Figure S1C), their glucagon secretion was not significantly increased compared to controls when normalized to overall islet cell volume (expressed as IEQs; Figure 2A). The response was reduced when normalized to islet glucagon content (Figures 2B and 2C) and lacked the appropriate increase at low glucose following 30-min high glucose inhibition (Figure 2B, inset). Marchetti and colleagues (Marchetti et al., 2000) observed a similar defect in glucagon secretion in islets isolated from a single T1D donor 8 months after the disease onset. These functional changes in T1D α cells were accompanied by reduced mRNA expression of two bona fide α cell regulators ARX (Courtney et al., 2013) and MAFB (Guo et al., 2013) in isolated islets (Figure 2D). Notably, histological analysis of native tissues further revealed that most α cells from T1D donors did not express MAFB and ARX (Figures 2E, 2F, and S3) but did express low levels of NKX6.1 (Figures 2G and S3), which is normally only found in β cells (Figures 1G and S2). A similar pattern has been seen in a mouse model with extreme β cell loss (Chera et al., 2014; Thorel et al., 2010). To test whether there was evidence of α-to-β cell conversion in the T1D donor pancreas, we searched for, but did not find, islet cells co-expressing insulin and glucagon (data not shown). This observation differs from the recently described α-to-β cell conversion in a mouse model of 99% β cell loss (Chera et al., 2014; Thorel et al., 2010).
Figure 2. T1D α Cells in Recent-Onset T1D Have Reduced Glucagon Secretion and Dysregulated Gene Expression.
The same sets of islets shown in Figures 1A and 1B were simultaneously analyzed for glucagon secretion. The same non-diabetic controls were used as Figure 1. The labeling of islet stimuli is identical to that in Figure 1.
(A) Glucagon secretion normalized to overall islet cell volume (expressed as IEQs); p = 0.2470.
(B) Glucagon secretion normalized to islet glucagon content; ****p < 0.0001. Data in (A) and (B) were compared by two-way ANOVA. Inset shows mean glucagon response to low glucose following the 30-min inhibition with high glucose.
(C) Glucagon content in control (206 ± 62 pg/IEQ) and T1D islets (362 ± 149 pg/IEQ); p = 0.2831; data are represented as mean ± SEM.
(D) Expression of α-cell-enriched factors by qRT-PCR in whole T1D islets (n = 3 donors; ages 12–22 years; donors nos. 1, 4, and 5) and controls (n = 3 donors; ages 11–29 years) was normalized to endogenous control and GCG expression; ****p < 0.0001; *p = 0.0184.
(E–G) Analysis of native pancreatic tissue for expression of islet-enriched transcription factors. T1D α cells (n = 4 donors; ages 12–58 years; donors nos. 1, 2, 5, and 8) expressed β cell marker NKX6.1 (G) and lost bona fide α cell markers MAFB (E) and ARX (F) in most T1D α cells compared to controls (n = 7 donors; ages 8–55 years); ****p < 0.0001.
Data in (C)–(G) were compared by two-tailed Student’s t test. Data in (A)–(G) are shown as mean ± SEM. The scale bar in (E) represents 10 µm and also corresponds to (F) and (G). See also Figures S1 and S3.
Non-autoimmune, Normoglycemic Environment Does Not Promote Conversion of T1D α Cells into β Cells
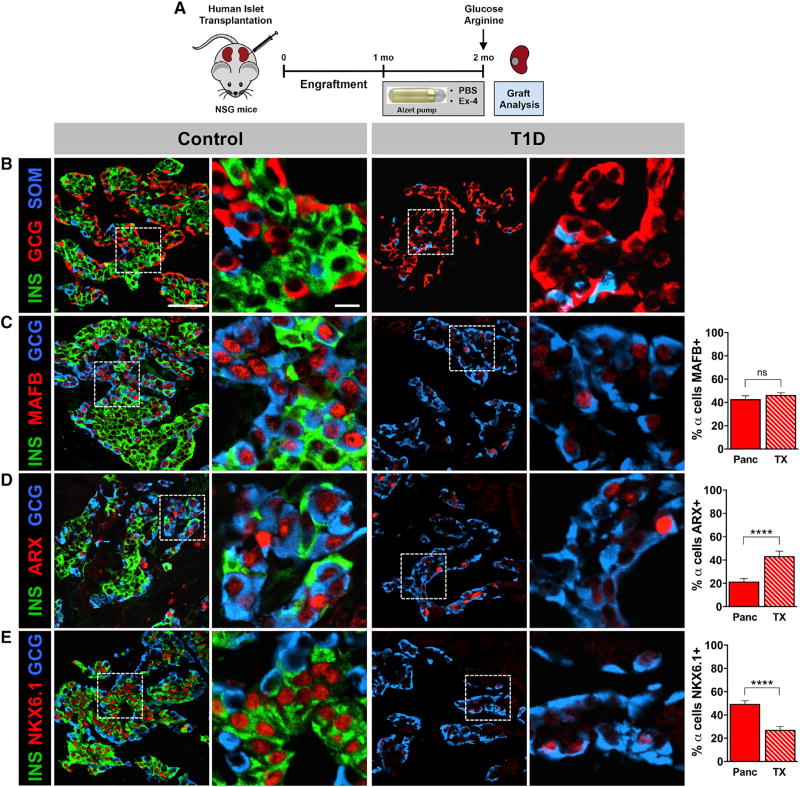
To determine whether human T1D α cells following extreme β cell loss can give rise to β cells when placed in a normoglycemic, non-autoimmune environment, we transplanted islets from the same T1D donors into immunodeficient Nod-SCID-IL2Rγnull (NSG) mice (Brissova et al., 2014; Figure 3A). After one-month engraftment, mice were treated with either PBS or exendin-4 (Dai et al., 2017), a GLP-1 analog reported to promote β cell maturation or proliferation for an additional 1 month. At the end of the treatment, in vivo insulin secretion was stimulated by a bolus of high glucose and arginine. Although a species-specific assay readily detected a rise in mouse plasma insulin levels, human insulin was undetectable (data not shown), indicating the absence of functional human β cells in T1D islet grafts. Similar to native tissue, graft immunocytochemistry showed that β cells were very rare and did not detect insulin/glucagon co-expression (Figure 3B; data not shown). Because there were no significant phenotypic differences between PBS- and exendin-4-treated groups, these treatment groups were combined to assess α cell transcription factor expression. After transplantation, the number of α cells expressing ARX in T1D islet grafts was greater (Figure 3D) with a decrease in the number of NKX6.1+ α cells (Figure 3E) compared to α cells in the native T1D pancreas, suggesting that the normoglycemic, non-autoimmune environment allowed for partial recovery of α cell identity marker expression.
Figure 3. T1D α Cells Do Not Show Evidence of α-to-β Cell Reprogramming in Normoglycemic, Non-autoimmune Environment.
(A) Islets from donors with recent-onset and long-standing T1D (n = 3 donors; 12–58 years; donors nos. 1, 5, and 8) depicted in Figures 1 and 2 were transplanted into NSG mice. After 1-month engraftment, mice were treated with either PBS or Ex-4 for an additional 1 month. Representative images of islet grafts are from the 12-year-old individual with 3-year T1D duration (donor no. 1). In control and T1D columns, regions denoted by the dashed line in images on the left (B)–(E) (scale bar in B is 50 µm) are displayed on the right (scale bar is 10 µm).
(B) Insulin (INS) and glucagon (GCG) double-positive cells were not detected in either type of T1D islet grafts (PBS or Ex-4).
(C–E) As there were no phenotypic differences between PBS and Ex-4 treatment groups, representative images were taken from both cohorts and analyzed for α cell transcription factor expression. Change in number of GCG+ cells expressing MAFB (C), ARX (D), and NKX6.1 (E) in transplanted T1D islets (TX) relative to donor’s native pancreas (Panc) is shown. ****p < 0.0001; ns, not significant.
Data in (C)–(E) are shown as mean ± SEM and were compared by two-tailed Student’s t test.
Genes Critical to α Cell Identity and Function Are Differentially Expressed between T1D and Control α Cells
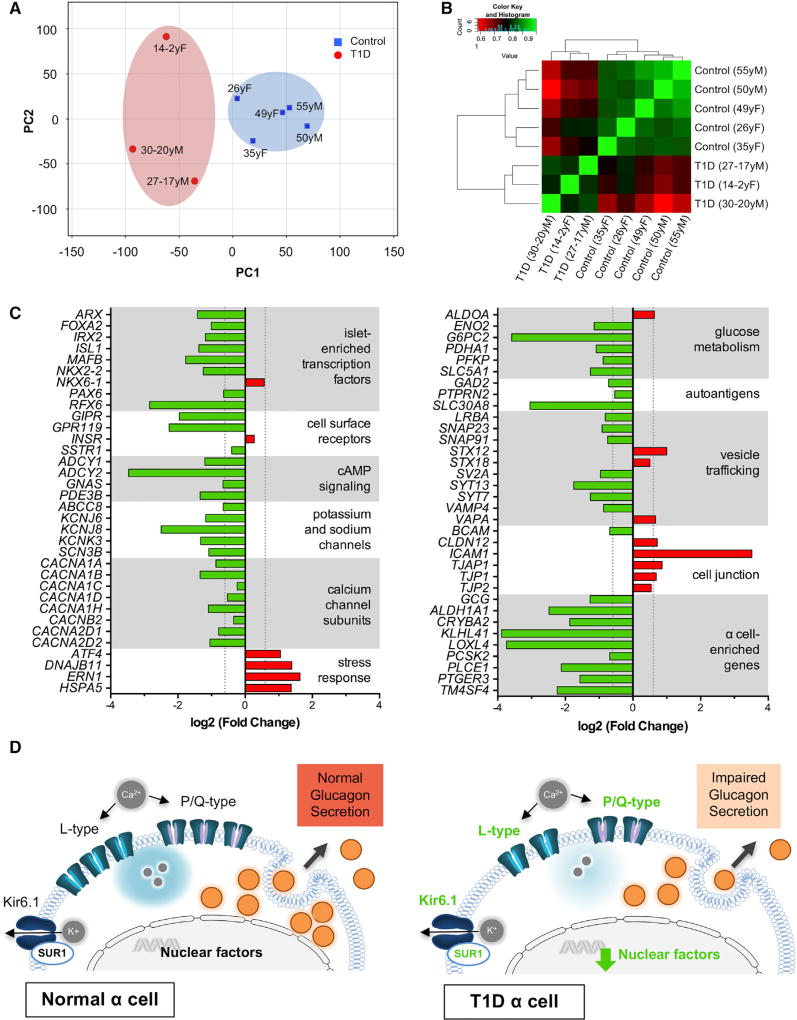
T1D and control islet α cells were purified by fluorescence-activated cell sorting (FACS) (Figure S4A). RNA-sequencing analysis (RNA-seq) performed on these cells indicated significant differences in the gene expression profiles (Figures 4A, 4B, and S4B). Ingenuity pathway analysis (IPA) and Gene Ontology (GO) term analysis (Tables S3 and S4; Figures S4C and S4D) identified differences in processes associated with protein synthesis and handling, immune-activated signaling, and cell stress response pathways. Specifically, T1D α cells had increased expression of genes important in the unfolded protein response and formation of tight and adhesive junctions. Conversely, T1D α cells had significantly reduced expression of genes recently identified by single-cell RNA-seq as α cell enriched, such as KLHL41, LOXL4, and PTGER3 (Muraro et al., 2016; Segerstolpe et al., 2016; Figure 4C). Our RNA-seq analysis further confirmed dysregulated expression of several islet-enriched transcription factors in T1D α cells, which we initially detected by RT-PCR in whole islets and at a protein level in pancreatic tissues (MAFB, ARX, and NKX6-1; Figures 2D–2G and 4C). Among islet-enriched transcription factors, RFX6, which lies upstream of MAFB, ARX, and NKX6-1 in endocrine cell differentiation (Piccand et al., 2014; Smith et al., 2010), had the most reduced expression (7.2-fold). In mature mouse and human β cells, RFX6 directly controls expression of P/Q and L-type voltage-gated calcium channels (CACNA1A, CACNA1C, and CACNA1D) and the KATP channel subunit sulfonylurea receptor 1 (ABCC8) (Chandra et al., 2014; Piccand et al., 2014; Figure 4C) that associates with Kir6.x pore-forming subunits (Winkler et al., 2009). T1D α cells also had altered expression of potassium and sodium ion channels, vesicle trafficking proteins, and cAMP signaling molecules, which collectively point to altered T1D α cell electrical activity and impaired glucagon exocytosis (Figure 4D).
Figure 4. Genes Critical to α Cell Function Are Differentially Expressed in T1D α Cells.
Transcriptome by RNA-sequencing analysis of purified human α cells from T1D donors (n = 3; ages 14–30 years; donors nos. 3, 6, and 7) and controls (n = 5; ages 26–55 years).
(A) Principal-component analysis (PCA) plot shows clustering of α cell samples from control and T1D donors.
(B) Heatmap of the pairwise correlation between all samples based on the Spearman correlation coefficient. Perfect correlation is indicated by 1.
(C) Genes associated with α cell identity and function are significantly downregulated in the T1D α cells with increased expression of stress response factors and cell-cell contact proteins. Vertical dotted lines represent point of significance for fold change (FC) = 1.53 threshold analysis; p < 0.05 for all values shown.
(D) Proposed model for disrupted glucagon secretion in T1D α cells. Normal α cell function is maintained by islet-enriched transcription factors, which regulate α cell machinery necessary for glucagon synthesis and secretion (left panel). Altered expression of transcription factors likely leads to reduced α cell glucagon production, disrupted calcium signaling, and electrical activity that results in impaired glucagon secretion (right panel; green font indicates downregulation). See also data in Figure S4 and Tables S3 and S4.
DISCUSSION
These results show the utility and advantages of an experimental approach that studies the pancreatic tissue and isolated islets from the same T1D individual and incorporates the in vitro and in vivo analysis of islets removed from the autoimmune, hyperglycemic environment. This approach also allowed us to directly test multiple pathways of hormone secretion and uncouple effects of decreased β cell mass and β cell dysfunction not possible in clinical studies in vivo. We found that the rare β cells in the pancreas present not only in recent-onset T1D but also many years after T1D diagnosis maintained features of regulated insulin secretion and/or produced key transcriptional regulators known to play a critical role in the maintenance of β cell fate and function. In contrast, T1D α cells, while highly abundant, were functionally impaired. Impaired glucagon secretion by T1D islets was associated with altered expression of multiple nuclear regulators (e.g., ARX, MAFB, and RFX6) and their downstream targets, suggesting that these changes directly and indirectly impact glucagon secretory pathways by altering expression of potassium and sodium ion channels, vesicle trafficking proteins, and cAMP signaling molecules. Abnormal glucagon secretion is a common complication of T1D, including impaired counterregulatory response of glucagon to hypoglycemia (Gerich et al., 1973) and an inappropriate rise in circulating glucagon in response to a mixed meal challenge (Sherr et al., 2014). Defects in neural glucose sensing, impaired islet innervation, or intra-islet insulin deficiency have been proposed to explain these abnormalities in glucagon secretion (Mundinger et al., 2016). The current analysis provides a new explanation and molecular mechanism for the dysregulated glucagon secretion in T1D, namely an intrinsic α cell defect (Figure 4D). Our observation that the changes in α cell gene expression partially resolved when T1D islets were transplanted into a normoglycemic, non-autoimmune environment suggests that interventions might be developed to improve α cell gene expression and glucagon secretion in T1D.
These data provide insight and raise important questions about the molecular and functional changes in human T1D α cells. After massive β cell loss in mice, β cells can be gradually and partially replenished by a sustained α-to-β cell reprogramming (Chera et al., 2014; Thorel et al., 2010). Unlike in mice, the current analysis did not identify cells co-expressing insulin and glucagon in the native pancreas or after transplantation into a normoglycemic, non-autoimmune environment, further supporting the notion that α-to-β cell conversion in humans is a very rare event (Chakravarthy et al., 2017). Our findings do suggest that T1D α cells have reduced key molecular regulators (ARX and MAFB) and express a transcription factor, NKX6.1, that is usually β cell specific, raising the possibility of partial change toward a β cell phenotype. Perhaps an additional stimulus or multiple stimuli may be required for human α cell reprogramming. Lineage-tracing studies of human α cells are needed to investigate the plasticity of human α cells.
These results stimulate a number of questions about the molecular and cellular changes in T1D islets. Are the α cell changes the result of the autoimmune attack on the β cells also affecting α cells, the lack of α cell-β cell contact, the diabetic milieu of hyperglycemia, or reduced intra-islet insulin? Have the remnant β cells, which have a number of features of normal β cells, somehow escaped the autoimmunity; do they comprise a specific subset of β cells (Dorrell et al., 2016); or do they represent an incomplete regenerative attempt, arising via de novo neogenesis from facultative pancreas progenitors (Xu et al., 2008), β replication (Brissova et al., 2014; Cano et al., 2008; Nir et al., 2007), and/or transdifferentiation of acinar cells (Zhou et al., 2008) or other islet endocrine cell types, such as α cells (Chera et al., 2014; Thorel et al., 2010)? Additional studies of isolated T1D islets and T1D pancreatic tissue are needed to better understand the phenotype and possible heterogeneity of T1D α and β cells.
EXPERIMENTAL PROCEDURES
Further details and an outline of resources used in this work can be found in Supplemental Experimental Procedures (Resources Table).
Animals
Immunodeficient 10- to 12-week-old NSG male mice were used for human islet transplantation studies (Brissova et al., 2014; Dai et al., 2016). Animals were maintained by Vanderbilt Division of Animal Care in group housing in sterile containers within a pathogen-free barrier facility housed with a 12 hr light/12 hr dark cycle and access to free water and standard rodent chow. All animal procedures were approved from by the Vanderbilt Institutional Animal Care and Use Committees.
Primary Cell Cultures
Primary human islets were cultured in CMRL 1066 media (5.5 mM glucose, 10% FBS, 1% Pen/Strep, and 2 mM L-glutamine) in 5% CO2 at 37°C for 24–72 hr prior to reported studies (Brissova et al., 2014; Dai et al., 2016). No cell lines were used in this study.
Human Subjects
Pancreata and islets from normal and T1D donors were obtained through a partnership with the International Institute for Advancement of Medicine (IIAM), National Disease Research Interchange (NDRI), Integrated Islet Distribution Program (IIDP), and Network for Pancreatic Organ Donors with Diabetes (nPOD). Most pancreata from normal donors were processed either for islet isolation (Balamurugan et al., 2003) or histological analysis (described below and Table S1). In most T1D pancreatic organs, islets and tissue specimens were procured from the same organ. For a number of controls, human islets were obtained through IIDP (Table S1). Donor demographic information and phenotype of T1D donors is summarized in Table 1. The Vanderbilt University Institutional Review Board declared studies on de-identified human pancreatic specimens do not qualify as human subject research.
Human Pancreatic Islet Procurement
Pancreata from normal juvenile and T1D donors were received within 18 hr from cold clamp and maintained in cold preservation solution on ice until processing. Pancreas was then cleaned from connective tissue and fat, measured, and weighed. Prior to islet isolation, multiple cross-sectional slices of pancreas with 2- to 3-mm thickness were obtained from the head, body, and distal tail (Figure S1A). Pancreatic slices were further divided into four quadrants and then either snap frozen or processed for cryosections. Tissue specimens processed for cryosections were fixed in 0.1 M PBS containing 4% paraformaldehyde (Electron Microscopy Sciences) for 3 hr on ice with mild agitation, washed in four changes of 0.1 M PBS over 2 hr, equilibrated in 30% sucrose/0.01 M PBS overnight, and embedded in Tissue-Plus O.C.T. compound (Fisher Scientific). Pancreatic organs were processed for islet isolation using an approach previously described (Balamurugan et al., 2003). Briefly, depending on the size of pancreatic duct, 18G or 22G catheters were inserted into the main pancreatic duct (one catheter toward head and the other one toward tail). Accessory duct and main pancreatic duct were clamped at the points where sections were collected to prevent leakage of collagenase solution during infusion. Collagenase solution consisting of collagenase NB1 (1,600 U/isolation; Crescent Chemical), neutral protease NB1 (200 U/isolation; Crescent Chemical), and DNase I (12,000 U/isolation; Worthington Biochemical Corporation) was pre-warmed to 28°C and delivered intraductally using a Rajotte’s perfusion system and then maintained at 37°C for approximately 20 min. The inflated tissue was then transferred to a Ricordi’s chamber apparatus for combined mechanical and enzymatic digestion, which was maintained at 36°C for 5–15 min prior to warm and cold collection. The digest was incubated in cold RPMI media (Mediatech) supplemented with heat-inactivated 10% fetal calf serum (Life Technologies) for 1 hr on ice. If post-digestion tissue pellet was larger than 2 mL and islets were distinguishable from exocrine tissue by Dithizone staining (Sigma), a purification step consisting of density gradient (Biocoll; Cedarlane) centrifugation on a COBE 2991 Cell Processor (Gambro-Terumo) was used to separate islets from exocrine tissue. Islets were re-suspended in CMRL 1066 medium (Mediatech) supplemented with 10% heat-inactivated fetal calf serum (Life Technologies), 100 units/mL penicillin/0.1 mg/mL streptomycin (Life Technologies), and 2 mmol/L L-glutamine (Life Technologies). On average, islet-enriched fraction contained from 30,000 (T1D pancreas) to 90,000 IEQs (normal pancreas) with 25%–50% purity. Islets were cultured for 12–24 hr and then shipped from Pittsburgh to Vanderbilt University and/or University of Massachusetts for further analysis following shipping protocols developed by the Integrated Islet Distribution Program (IIDP). Subsequent assays with isolated islets were set up within 24–48 hr of islet arrival.
Statistical Analysis
To compare global differences in perifusion outcomes in T1D donors and controls, two-way ANOVA with Sidak’s multiple comparisons test was used. Data were expressed as mean ± SEM. Two-tailed Student’s t test was used for analysis of statistical significance for two-group comparisons between T1D donors and controls. A p value less than 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism software. Statistical details of experiments are described in the figure legends, Results section, and Supplemental Experimental Procedures.
Supplementary Material
Highlights.
T1D β cells appear to maintain several aspects of regulated insulin secretion
T1D α cells have impaired glucagon secretion and altered gene expression
Unlike in rodents, α-to-β cell conversion in human T1D is a very rare event
T1D α cell identity factors improve in a non-autoimmune, normoglycemic environment
Acknowledgments
We thank Dr. Seung Kim for providing critical comments on the manuscript. We are very grateful to Drs. Raphael Scharfmann, Michael German, and Gérard Gradwohl for helpful discussions and sharing reagents. Special thanks to Mr. David Scheel for experimental advice. This research was performed using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (HIRN, RRID:SCR_014393; https://hirnetwork.org; UC4 DK104211, DK108120, and DK112232), by DK106755, DK72473, DK89572, DK97829, DK94199, the Vanderbilt Diabetes Research and Training Center (DK20593), and by grants from the JDRF (2-SRA-2015-68-Q-R, 2-SRA-2016-149-Q-R, 17-2013-321, 17-2013-324), the Leona M. and Harry B. Helmsley Charitable Trust, and the Department of Veterans Affairs (BX000666). Islet imaging and functional analysis were performed with the support of the Cell Imaging Shared Resource and Islet Procurement and Analysis Core of the Vanderbilt Diabetes Research and Training Center (DK20593). Flow cytometry analysis was performed in the Vanderbilt Flow Cytometry Shared Resource (P30 CA68485 and DK058404). C-peptide and autoantibodies were analyzed at the Northwest Lipid Metabolism and Diabetes Research Laboratories and Barbara Davis Center for Childhood Diabetes, respectively. This research was performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD; www.jdrfnpod.org) and the Integrated Islet Distribution Program (IIDP; https://iidp.coh.org/). We are grateful to Organ Procurement Organizations partnering with the International Institute for Advancement of Medicine and National Disease Research Interchange. We are especially thankful to organ donors and their families.
Footnotes
DATA AND SOFTWARE AVAILABILITY
The accession number for the sequencing data reported in this paper is GEO: GSE106148.
Supplemental information includes Supplemental Experimental Procedures, four figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.02.032.
AUTHOR CONTRIBUTIONS
Conceived and Designed the Experiments, M.B., R.H., D.S., S.S., N.P., R.B., M.C.-T., M.A., D.M.H., R.S., and A.C.P.; Conducted the Experiments, M.B., R.H., D.S., S.S., N.P., C.D., D.M.B., R.B., R.A., G.P., J.L., F.C.P., and M.S.; Analyzed and Interpreted the Data, M.B., R.H., D.S., S.S., C.D., D.M.B., R.B., R.A., G.P., M.S., L.H.P., D.M.H., R.S., S.E.L., and A.C.P.; Wrote the Manuscript, M.B., R.H., D.S., S.S., and A.C.P.; Reviewed/Edited the Manuscript, M.B., R.H., D.S., S.S., C.D., D.M.B., R.B., M.C.-T., R.A., G.P., J.L., F.C.P., M.G.v.H., D.L.G., L.D.S., M.S., L.H.P., M.A., D.M.H., S.E.L., N.P., R.S., and A.C.P.
DECLARATION OF INTERESTS
M.G.v.H. is an employee of Novo Nordisk.
References
- Alkorta-Aranburu G, Sukhanova M, Carmody D, Hoffman T, Wysinger L, Keller-Ramey J, Li Z, Johnson AK, Kobiernicki F, Botes S, et al. Improved molecular diagnosis of patients with neonatal diabetes using a combined next-generation sequencing and MS-MLPA approach. J. Pediatr. Endocrinol. Metab. 2016;29:523–531. doi: 10.1515/jpem-2015-0341. [DOI] [PubMed] [Google Scholar]
- Balamurugan AN, Chang Y, Fung JJ, Trucco M, Bottino R. Flexible management of enzymatic digestion improves human islet isolation outcome from sub-optimal donor pancreata. Am. J. Transplant. 2003;3:1135–1142. doi: 10.1046/j.1600-6143.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ, Kim S, Kucukural A, Davis RJ, Kent SC, et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes. 2015;64:3172–3181. doi: 10.2337/db15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G, de Feo P, Compagnucci P, Cartechini MG, Angeletti G, Santeusanio F, Brunetti P, Gerich JE. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983;32:134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- Brissova M, Aamodt K, Brahmachary P, Prasad N, Hong J-Y, Dai C, Mellati M, Shostak A, Poffenberger G, Aramandla R, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014;19:498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Rulifson IC, Heiser PW, Swigart LB, Pelengaris S, German M, Evan GI, Bluestone JA, Hebrok M. Regulated beta-cell regeneration in the adult mouse pancreas. Diabetes. 2008;57:958–966. doi: 10.2337/db07-0913. [DOI] [PubMed] [Google Scholar]
- Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, Downie C, Liu K, Wang J, Xing Y, et al. Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metab. 2017;25:622–634. doi: 10.1016/j.cmet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra V, Albagli-Curiel O, Hastoy B, Piccand J, Randriamampita C, Vaillant E, Cavé H, Busiah K, Froguel P, Vaxillaire M, et al. RFX6 regulates insulin secretion by modulating Ca2+ homeostasis in human β cells. Cell Rep. 2014;9:2206–2218. doi: 10.1016/j.celrep.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney M, Gjernes E, Druelle N, Ravaud C, Vieira A, Ben-Othman N, Pfeifer A, Avolio F, Leuckx G, Lacas-Gervais S, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet. 2013;9:e1003934. doi: 10.1371/journal.pgen.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Kayton NS, Shostak A, Poffenberger G, Cyphert HA, Aramandla R, Thompson C, Papagiannis IG, Emfinger C, Shiota M, et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest. 2016;126:1857–1870. doi: 10.1172/JCI83657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, Phillips N, Levy SE, Greiner DL, Shultz LD, et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J. Clin. Invest. 2017;127:3835–3844. doi: 10.1172/JCI91761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, Grompe M. Human islets contain four distinct subtypes of β cells. Nat. Commun. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T, et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014;19:259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973;182:171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayton NS, Poffenberger G, Henske J, Dai C, Thompson C, Aramandla R, Shostak A, Nicholson W, Brissova M, Bush WS, Powers AC. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am. J. Physiol. Endocrinol. Metab. 2015;308:E592–E602. doi: 10.1152/ajpendo.00437.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogvold L, Skog O, Sundström G, Edwin B, Buanes T, Hanssen KF, Ludvigsson J, Grabherr M, Korsgren O, Dahl-Jørgensen K. Function of isolated pancreatic islets from patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment in vitro: results from the DiViD study. Diabetes. 2015;64:2506–2512. doi: 10.2337/db14-1911. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Dotta F, Ling Z, Lupi R, Del Guerra S, Santangelo C, Realacci M, Marselli L, Di Mario U, Navalesi R. Function of pancreatic islets isolated from a type 1 diabetic patient. Diabetes Care. 2000;23:701–703. doi: 10.2337/diacare.23.5.701. [DOI] [PubMed] [Google Scholar]
- Mundinger TO, Mei Q, Foulis AK, Fligner CL, Hull RL, Taborsky GJ., Jr Human type 1 diabetes is characterized by an early, marked, sustained, and islet-selective loss of sympathetic nerves. Diabetes. 2016;65:2322–2330. doi: 10.2337/db16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro MJ, Dharmadhikari G, Grün D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJP, van Oudenaarden A. A single-cell transcriptome atlas of the human pancreas. Cell Syst. 2016;3:385–394.e3. doi: 10.1016/j.cels.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram RA, Jones AG, Besser REJ, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccand J, Strasser P, Hodson DJ, Meunier A, Ye T, Keime C, Birling M-C, Rutter GA, Gradwohl G. Rfx6 maintains the functional identity of adult pancreatic β cells. Cell Rep. 2014;9:2219–2232. doi: 10.1016/j.celrep.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyoura M, Jacobsen L, Carmody D, Del Gaudio D, Alkorta-Aranburu G, Arndt K, Hu Y, Kobiernicki F, Kusmartseva I, Atkinson MA, et al. Pancreatic histopathology of human monogenic diabetes due to causal variants in KCNJ11, HNF1A, GATA6, and LMNA. J. Clin. Endocrinol. Metab. 2018;103:35–45. doi: 10.1210/jc.2017-01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstolpe Å, Palasantza A, Eliasson P, Andersson E-M, Andréasson A-C, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ, et al. Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care. 2014;37:1741–1744. doi: 10.2337/dc13-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Qu H-Q, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch A-M, Grabs R, Wang J, Lynn FC, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Liu F-F, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4:1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M, Lutz R, Russ U, Quast U, Bryan J. Analysis of two KCNJ11 neonatal diabetes mutations, V59G and V59A, and the analogous KCNJ8 I60G substitution: differences between the channel subtypes formed with SUR1. J. Biol. Chem. 2009;284:6752–6762. doi: 10.1074/jbc.M805435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.