Abstract
High dietary salt intake increases risk of stress-related neuropsychiatric disorders. Here, we explored the contribution of high dietary salt intake-induced neuroinflammation in key stress-responsive brain regions, the hypothalamic paraventricular nucleus and basolateral amygdala, in promoting exaggerated neuronal activation and coping behaviors in response to acute psychogenic stress. Mice that underwent high dietary salt intake exhibited increased active stress coping behaviors during and after an acute swim stress, and these were reduced by concurrent administration of minocycline, an inhibitor of microglial activation, without affecting body fluid hyperosmolality caused by high dietary salt intake. Moreover, minocycline attenuated high dietary salt intake-induced increases of paraventricular nucleus tumor necrosis factor-α, activated microglia (ionized calcium-binding adaptor molecule 1), and acute swim stress-induced neuronal activation (c-Fos). In the basolateral amygdala, similar effects were observed on ionized calcium-binding adaptor molecule 1+ and c-Fos+ counts, but not tumor necrosis factor-α levels. These data indicate that high dietary salt intake promotes neuroinflammation, increasing recruitment of neurons in key stress-associated brain regions and augmenting behavioral hyper-responsivity to acute psychological stress.
Keywords: dietary salt, hypothalamic paraventricular nucleus, basolateral amygdala, microglia, minocycline
Introduction
Excessive dietary sodium intake is a worldwide health concern (Whelton, 2015). We recently reported that high dietary NaCl (salt) intake (HDSI) in mice increases activation of stress-sensitive neurons of the hypothalamic paraventricular nucleus (PVN) and basolateral amygdala (BLA) and increases stress coping behaviors during and after acute swim stress (SS) (Mitchell et al., 2018). Blockade of vasopressin 1a receptors in the BLA attenuated active coping behavior independent of salt intake, indicating another central mechanism is likely responsible for the observed HDSI-induced elevations in neuronal activation and active coping behavior (Mitchell et al., 2018).
Sodium-induced neuroinflammation is implicated in development of neuropsychiatric disorders (Dipasquale et al., 2013; Abdoli, 2017), putatively via activation of microglia (Réus et al., 2015). Proinflammatory activation of microglia, which increases production of neuro-active cytokines such as tumor necrosis factor-alpha (TNF-α) (Bardgett et al., 2014), can be blocked by the anti-inflammatory antibiotic minocycline (Kobayashi et al., 2013). Here, we sought to examine how HDSI, alone and concurrent with minocycline administration, affects the state of microglial activation, TNF-α levels, and neuronal transcriptional activation in the PVN and BLA, as well as active coping behaviors, in response to an acute SS. Our findings reveal that even moderately elevated salt intake, insufficient to increase serum osmolality, can induce neuroinflammation and sensitize neuronal and behavioral stress responses.
Methods
Animals
Adult male C57BL/6J mice (3–10 months old) from Jackson Labs were housed in plastic cages (29 cm × 18 cm × 13 cm) containing bedding (Sani-chips, Harlan Teklad) in a temperature-controlled (24°C) vivarium on a 12/12 h-light/dark cycle (lights on at 7:00 am). Animals were group housed and given free access to food (irradiated rodent sterilizable diet, Harlan Teklad) and water except where noted.
Experimental procedures conformed to the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
HDSI Protocol and Minocycline Administration
Mice were randomly assigned to drink tap water (vehicle) or 2% or 4% saline (NaCl) ad libitum for 7 consecutive days. Mice consuming each drinking solution were divided into 2 groups, only one of which had minocycline added to their drinking solution. Mice that drank minocycline-containing solutions had a priming dose added to their tap water 24 hours prior to initiating the HDSI protocol.
Concentrations of minocycline (Sigma-Aldrich) in drinking solutions varied from 0.41 to 0.54 mg/mL to compensate for differences in the volume of each solution that mice drank per day (Mitchell et al., 2018), thereby ensuring that the daily dose of minocycline was consistently approximately 100 mg/kg/d for all HDSI treatments. Estimated daily dosages of minocycline were not different in any HDSI treatment [F(2,15) = 1.6, P = .2]. The oral route of administration and dosing regimen were chosen for their demonstrated capacity to inhibit proinflammatory microglial activation (Kohman et al., 2013).
Behavioral Testing
Experiments were conducted as described in Mitchell et al. (2018). SS was conducted for 5 minutes in an acrylic cylindrical tank (20 cm i.d., 25 cm high) filled to a depth of 18 cm with approximately 25°C tap water. Mobility was defined as active leg movement greater than required to prevent submersion and resulting in forward propulsion. Data were graphed as time (in seconds) mobile to quantify active coping changes, in accord with recent reports (Castagné et al., 2011; Nackenoff et al., 2017; Mitchell et al., 2018). After testing, mice were removed from the tank, dried, and placed inside a transparent cage (29 cm × 18 cm × 13 cm). There, grooming behavior was assessed as time (in seconds) spent stroking or licking the nose, paws, face, or body. Behaviors were recorded with a digital camera and scored by an experimenter binded to treatment conditions.
Serum Osmolality
As previously described (Mitchell et al., 2018), blood samples were drawn via cardiac puncture prior to perfusion fixation while mice were deeply anesthetized with isoflurane (3% for 180 seconds). Blood samples (0.3 mL) were centrifuged for 8 minutes at 5000 rpm, and serum was collected to measure osmolality by freezing point depression (Advanced Instruments, Inc., model 3320). Reports are conflicting whether, and over what time course, isoflurane anesthesia might affect inflammatory markers (Wu et al., 2012; Altay et al., 2014); for the present experiments, all mice were exposed to the same concentration of isoflurane (3% in oxygen) for no longer than 3 minutes immediately prior to perfusion fixation to minimize potential confounds.
Immunohistochemistry
Immunohistochemistry was conducted as described in Mitchell et al. (2018). Brain sections 30 µm thick were incubated in 0.5% sodium borohydride/0.1 M phosphate buffered saline (PBS) (30 min), rinsed (3×, 5 minutes in 0.1 M PBS), and incubated in blocking solution (0.1 M PBS/0.3% Triton-X 100/3% goat serum; 2 hours at 22.5°C). Antigen detection was achieved by incubating adjacent sections in 4°C blocking solution containing polyclonal rabbit anti-ionized calcium-binding adaptor molecule 1 (Iba1; to specifically measure the number of positively stained microglia; Ito et al., 1998) (1:200: Wako Labs) for 12 hours, polyclonal rabbit anti-c-Fos (an indicator of neuronal activation; Mitchell et al., 2018) (1:10 000; Millipore) for 72 hours, or polyclonal rabbit anti-TNF-α (1:250: Abcam) for 12 hours. Sections were rinsed (3×, 5 minutes in 0.1 M PBS), incubated in blocking solution containing biotinylated goat anti-rabbit IgG secondary antibody (1:250; Thermofisher, 2 hours at 22.5°C), rinsed again (3×, 5 minutes in 0.1 M PBS), and immunoreactivity revealed by incubation in streptavidin-AlexaFluor 594 conjugate (1:250; Thermofisher, 10 minutes at 22.5°C). Tissue underwent a final rinse (3×, 5 minutes in 0.1 M PBS) before being mounted on glass slides with ProLong Diamond (Thermo Fisher Scientific).
Iba1 and c-Fos staining was imaged with a confocal microscope (Prairie Technologies) using a Sapphire 561-nm laser (Coherent), and TNF-α immunoreactivity was imaged under mercury illumination epifluorescence (X-cite 120PCXL). A 16-bit Cascade II digital camera (Photometrics, Inc.) captured images, and NIS-Elements Advanced Research 3.2 software was used for analysis. Localization of immunoreactivity was determined according to histological plates 38 (PVN) and 43 (BLA) in the mouse brain atlas of Franklin and Paxinos (1997). Quantification details are described in Mitchell et al. (2018).
Data Analysis
Statistical analyses were performed using GraphPad Prism v7.0 (San Diego, CA). An estimate of the minocycline dose per mouse was calculated daily at approximately 9:00 am as the product of the fractional bodyweight gain of each mouse in a given cage and the volume of solution drunk during the previous 24 hours. A 1-factor ANOVA was used to determine if dosages varied in any HDSI treatment. All other data were analyzed using a 2-factor ANOVA (HDSI condition, minocycline treatment) followed by Dunnett’s or Bonferroni’s multiple comparisons test (see figure legends for details).
Results
Minocycline Attenuates HDSI-Induced Increases in Swimming and Grooming Behavior as Well as Markers of Neuroinflammation and Neuronal Activation in the PVN
A significant HDSI × minocycline interaction was detected for mobility time [F(2,40) = 5.8, P < .01; Figure 1A], indicating minocycline’s behavioral effects were dependent on HDSI. Whereas HDSI with 2% and 4% saline each significantly increased mobility time during SS compared with tap water controls (P < .05, P < .001), time spent mobile in minocycline-treated mice was increased relative to tap water controls only in the 2% saline group (P < .05) (Figure 1A). Most prominent is that the 66% increase in mobility time detected in vehicle-treated mice with 4% HDSI compared with tap water controls was reduced to only 17% when 4% HDSI mice underwent concurrent minocycline treatment. Comparing across minocycline condition within HDSI treatment, only 2% HDSI animals treated with minocycline swam more than vehicle-treated 2% HDSI mice (P < .05). Though no significant interaction between HDSI × minocycline was observed [F(2,39) = 2.3, P = .11; Figure 1B], time spent grooming after SS was affected by HDSI [F(2,39) = 4.1, P < .05], with grooming time significantly increased only in vehicle-treated mice with 4% HDSI compared with tap water controls (P < .001). A main effect of minocycline treatment on grooming time [F(1,39) = 6.4, P < .05] (Figure 1B) was revealed by posthoc tests to have ablated the enhanced grooming elicited by 4% HDSI (P < .05).
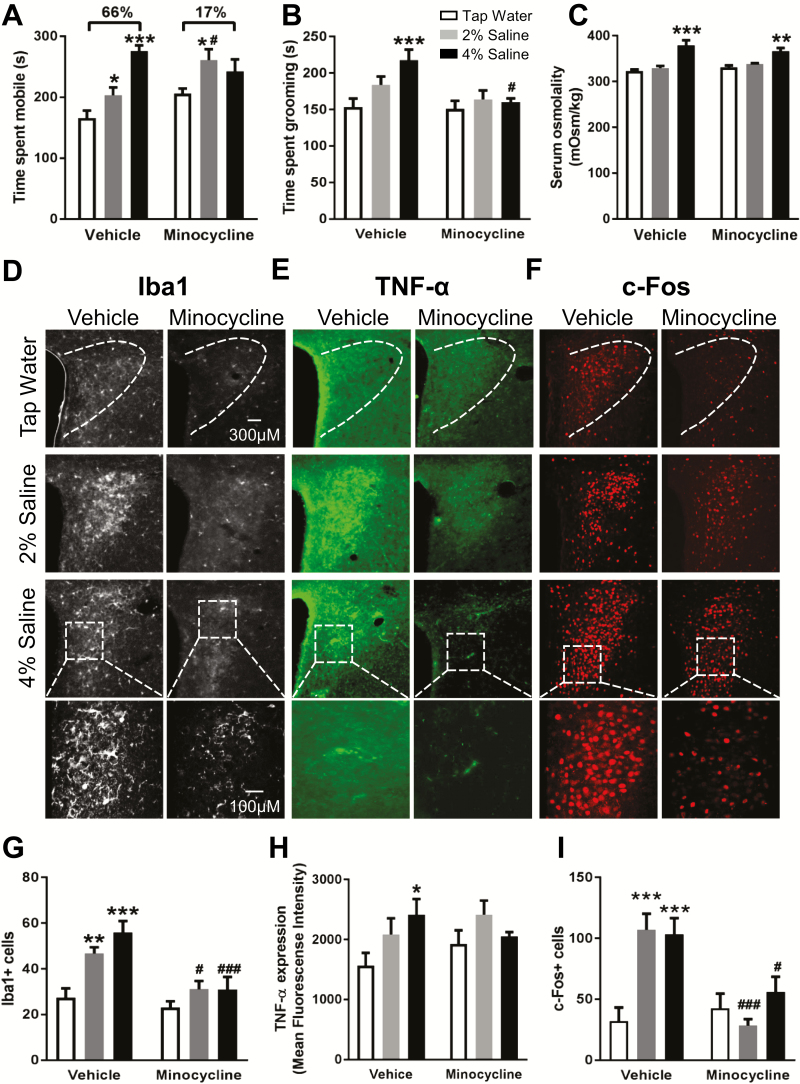
Figure 1.
Concurrent minocycline treatment attenuates high dietary salt intake (HDSI)-induced increases in active stress coping, as well as markers of neuroinflammation and neuronal activation in the paraventricular nucleus (PVN). Summary data for (A) time spent mobile during (n = 6–11), (B) time spent grooming (n = 5–11) immediately after, and (C) serum osmolality (n = 3–9) following an acute swim stress (SS). Representative images of (D) ionized calcium-binding adaptor molecule 1 (Iba1) (white), (E) tumor necrosis factor (TNF)-α (green), and (F) c-Fos (red) immunoreactivity in the PVN of mice after HDSI/minocycline treatment protocols. Summary data for (G) Iba1+ cells (n = 5–6), (H) TNF-α immunofluorescence (n = 4–5), and (I) c-Fos+ cells (n = 5). Values were obtained after the 7-day HDSI protocol with or without concurrent minocycline treatment (approximately 100 mg/kg/d) in drinking solution after acute SS. *P < .05, **P < .01, ***P < .001 difference from tap water treatment within same condition (vehicle/minocycline) by Dunnett’s multiple comparisons test; #P < .05, ###P < .001 difference within HDSI treatment across condition (vehicle/minocycline) by Bonferroni’s multiple comparisons test after 2-factor ANOVA (HDSI, vehicle/minocycline). Data are mean ± SEM.
Serum osmolality was influenced by a main effect of HDSI [F(2,25) = 31.1, P < .001] (Figure 1C) and unaltered by minocycline [F(1,25) = 0.069, P = .8], with no significant interaction [F(2,25) = 2.0, P = .16]. Posthoc tests indicated osmolality was greater in 4% HDSI mice relative to both tap water vehicle and tap water minocycline groups (P < .001, P < .01). These behavioral and osmolality results in vehicle-treated animals align with our previous findings (Mitchell et al., 2018).
Immunohistochemical staining revealed main effects of HDSI [F(2,27) = 10.6, P < .001] and minocycline [F(1,27) = 19.9, P < .001] on numbers of Iba1+ cells in the PVN (Figure 1D,G). A trend toward a HDSI × minocycline interaction suggested that minocycline might preferentially reduce Iba1+ cells in HDSI mice [F(2,27) = 3.2, P = .06] (Figure 1G). Vehicle-treated mice receiving 2% and 4% HDSI had greater Iba1+ cells than tap water controls (P < .01, P < .001), and these increases were attenuated by minocycline treatment (P < .05, P < .001, respectively). Though no significant HDSI × minocycline interaction [F(2,21) = 2.1, P = .15; Figure 1E,H] or main effect of HDSI [F(2,21) = 2.1, P = .15] on PVN TNF-α immunoreactivity was detected, posthoc testing indicated vehicle-treated 4% HDSI mice had elevated levels compared with tap water controls (P < .05) (Figure 1E,H). No main effect of minocycline treatment on TNF-α levels in the PVN was detected (F(1,21) = 1.2, P = .3).
Because microglial activation can result from and promote further release of TNF-α and thereby lead to increased neuronal excitability and excitatory synaptic transmission, c-Fos+ cells were counted to determine if neuronal activation correspondingly increased with HDSI-induced neuroinflammation after acute SS (Figure 1F,I). A significant HDSI × minocycline interaction was detected for c-Fos+ PVN cells [F(2,24) = 7.6, P < .01] (Figure 1F,I). Heightened levels of c-Fos+ nuclei in vehicle-treated mice receiving 2% and 4% HDSI (P < .001, P < .001) were shown by posthoc tests to be significantly reduced by concurrent minocycline treatment (P < .001, P < .05).
Minocycline Attenuates HDSI-Induced Increases of Iba1+ and c-Fos+ Cells in the BLA After SS
In contrast to the PVN, the interaction between HDSI × minocycline on Iba1+ cells in the BLA reached significance [F(2,27) = 6.9, P < .01] (Figure 2A,D). Posthoc tests indicated both 2% and 4% HDSI increased Iba1+ cells in BLA compared with vehicle-treated tap water controls (P < .05, P < .001), and these elevations were blocked by minocycline for both 2% and 4% HDSI mice (P < .05, P < .01) (Figure 2D). Also unlike the PVN, levels of TNF-α in the BLA were unaffected by HDSI or minocycline [F(2,19) = 0.25, P = .8; F(1,19) = 0.66,P = .4] (Figure 2B,E), and no significant interaction was observed [F(2,19) = 0.54, P = .6].
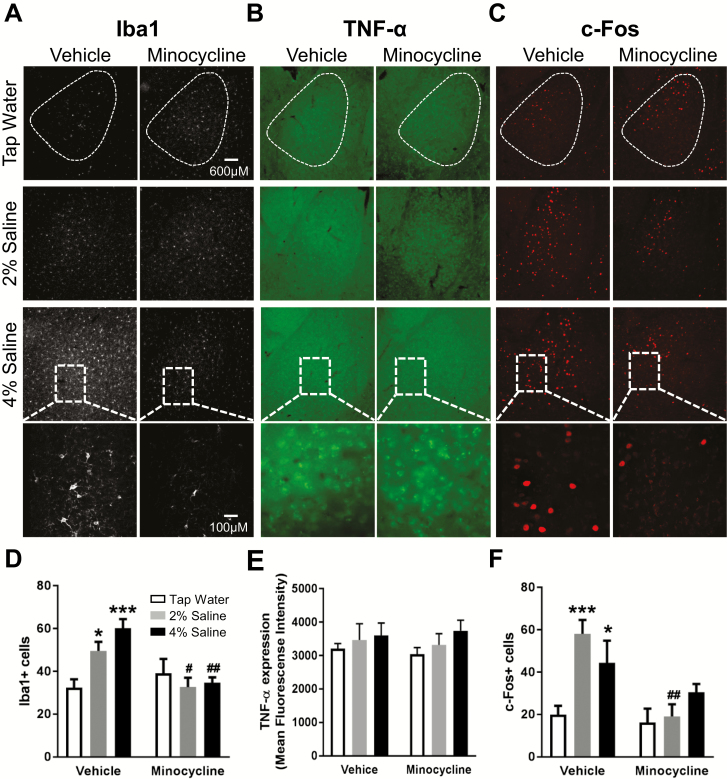
Figure 2.
Concurrent minocycline treatment attenuates high dietary salt intake (HDSI)-induced increases in ionized calcium-binding adaptor molecule 1 (Iba1+) and c-Fos+ cells in the basolateral amygdala (BLA), without affecting tumor necrosis factor (TNF)-α immunoreactivity. Representative images of (A) Iba1 (white), (B) TNF-α (green), and (C) c-Fos (red) immunoreactivity in the BLA after acute swim stress (SS) in mice that underwent the 7-day HDSI protocol with concurrent minocycline treatment (approximately 100 mg/kg/d) in drinking solution. Summary data of (D) Iba1+ cells (n = 5–6), (E) TNF-α immunofluorescence (n = 4–5), and (F) c-Fos+ cells (n = 5). *P < .05, ***P < .001 difference from tap water treatment within condition (vehicle/minocycline) with Dunnett’s multiple comparisons test; #P < .05, ##P < .01 difference within HDSI across condition (vehicle/minocycline) by Bonferroni’s multiple comparisons test after a 2-factor ANOVA (HDSI, vehicle/minocycline). Data are mean ± SEM.
Paralleling c-Fos+ observations in the PVN, c-Fos+ cells in the BLA after acute SS were affected by an interaction between HDSI and minocycline [F(2,24) = 3.8, P < .05]. Relative to vehicle-treated tap water controls, 2% and 4% HDSI mice treated with vehicle had greater c-Fos+ cells (P < .001, P < .05) as indicated by posthoc tests. Minocycline significantly reduced BLA c-Fos+ cells in mice receiving 2% HDSI (P < .01), but not 4% HDSI (P = .4).
Discussion
Our findings suggest that HDSI-induced enhancement of active coping behavior during and after a psychogenic stress likely involves actions of neuroinflammation mediated through activation of microglia in stress-relevant brain regions. This is particularly important considering numbers of microglia and activated neurons were heightened in mice that did not exhibit serum osmolality changes (2% HDSI), suggesting even modest elevations in dietary salt could augment neurophysiological responses to psychological stressors. Moreover, treatment with the microglial inhibitor minocycline largely prevented microglial activation as well as exaggerated neuronal and behavioral SS responses resulting from HDSI without altering serum osmolality. Thus, effects of minocycline appear related to its anti-inflammatory actions in stress-relevant brain regions and are not secondary to any blunting of the disruptive effect of HDSI on body fluid homeostasis.
Though originally developed by Porsolt as an antidepressant screen, prevailing views suggest that activity during SS reflects the animal’s coping strategy during stress exposure (Commons et al., 2017; de Kloet and Molendjik, 2016). Active stress coping associates with increased mobility in SS, aggression, grooming, and social dominance (Cannon, 1915; Engel and Schmale, 1972; Benus et al., 1991). Thus, enhanced behavioral responses to SS resulting from HDSI likely extend from environmental to inter-individual stress stimuli, though this needs to be further characterized. Such interactions are particularly important to examine considering SS or HDSI alone do not substantially elevate c-Fos+ cell counts (Mitchell et al., 2018). The present findings in vehicle-treated animals closely parallel our previous report (Mitchell et al., 2018), demonstrating the reproducibility of this heterotypic stress paradigm with relatively small group sizes. Certainly, HDSI-mediated neuroinflammation as a contributor to heightened stress reactivity and the pathogenesis of anxiety, mood, and psychotic disorders needs to be explored more fully in preclinical studies given growing clinical evidence of an association between salt intake and neuropsychiatric disorders (Dipasquale et al., 2013; Réus et al., 2015; Abdoli, 2017).
Our present data indicate that TNF-α is an HDSI-inducible inflammatory cytokine that is more readily elevated in the hypothalamus compared with the amygdala, which is consistent with literature evidence (Connor et al., 1998; Churchill et al., 2006). HDSI and/or SS might elevate other cytokines in the amygdala not measured here, such as interleukin-1β (Churchill et al., 2006). Alternatively, the brief SS may not have been severe or prolonged enough to have sufficiently increased TNF-α production in the amygdala to reach statistical significance. We have previously shown that locally administered TNF-α increases PVN-driven neuronal output (Bardgett et al., 2014), and this might help explain elevated c-Fos+ numbers in this region following HDSI and SS. The lack of significantly greater TNF-α levels in the BLA despite elevated c-Fos+ nuclei indicates additional or distinct neuronal activation processes are occurring. One possibility is a change in chloride gradients due to HDSI. HDSI in rats reduces active (i.e., phosphorylated) levels of the potassium chloride co-transporter (KCC2) in hypothalamic subregions (Choe et al., 2015), thus increasing intracellular chloride and limiting the inhibitory efficacy of GABAergic inputs. Continued investigations based on these findings will help determine if induction of TNF-α production or KCC2 phosphorylation by HDSI are necessary, separately or collectively, to increase PVN and BLA neuron excitability after SS and to evaluate how such manipulations impact active coping behavior.
Strikingly, HDSI induces neuroinflammation and augments cellular and behavioral responses to a brief, acute SS, without a requisite increase in serum osmolality (i.e., 2% HDSI). A conventional high salt diet may increase an individual’s behavioral reactivity to day-to-day perceived stressors, inadvertently increasing their susceptibility to psychiatric and neuroimmune disorders. The current findings implicate microglial activation and neuroinflammation as important considerations in future studies exploring how HDSI influences neurophysiological and behavioral responses to stress.
Funding
This work was supported by the National Institutes of Health (NIH; grant nos. HL088052 to GMT; MH093320 and MH106978 to LCD); and American Heart Association (grant no. 25710176 to GMT). Stipend support for NCM and TLG was provided by NIH T32 HL07446 and T32 DA031115, respectively. Role of funding sources: none.
Statement of Interest
None.
Acknowledgments
The authors thank Mary Ann Andrade for her excellent technical support.
References
- Abdoli A. (2017) Hypothesis: high salt intake as an inflammation amplifier might be involved in the pathogenesis of neuropsychiatric disorders. Clin Exp Neuroimmunol 8:146–157. [Google Scholar]
- Altay O, Suzuki H, Hasegawa Y, Ostrowski RP, Tang J, Zhang JH (2014) Isoflurane on brain inflammation. Neurobiol Dis 62:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardgett ME, Holbein WW, Herrera-Rosales M, Toney GM (2014) Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-α. Hypertension 63:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA (1991) Heritable variation for aggression as a reflection of individual coping strategies. Experientia 47:1008–1019. [DOI] [PubMed] [Google Scholar]
- Cannon WB. (1915) Bodily changes in pain, hunger, fear and rage: an account of recent researches into the function of emotional excitement. New York: D. Appleton and Company. [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD (2011) Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci Chapter 8:Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Choe KY, Han SY, Gaub P, Shell B, Voisin DL, Knapp BA, Barker PA, Brown CH, Cunningham JT, Bourque CW (2015) High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron 85:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Taishi P, Wang M, Brandt J, Cearley C, Rehman A, Krueger JM (2006) Brain distribution of cytokine mRNA induced by systemic administration of interleukin-1beta or tumor necrosis factor alpha. Brain Res 1120:64–73. [DOI] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG (2017) The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci 8:955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TJ, Song C, Leonard BE, Merali Z, Anisman H (1998) An assessment of the effects of central interleukin-1beta, -2, -6, and tumor necrosis factor-alpha administration on some behavioural, neurochemical, endocrine and immune parameters in the rat. Neuroscience 84:923–933. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Molendijk ML (2016) Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast ID: 6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V (2013) The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res 47:197–207. [DOI] [PubMed] [Google Scholar]
- Engel GL, Schmale AH (1972) Conservation-withdrawal: a primary regulatory process for organismic homeostasis. Ciba Found Symp 8:57–75. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G (1997) The mouse brain in stereotaxic coordinates. San Diego: Academic Press, Inc. [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, iba1. Brain Res Mol Brain Res 57:1–9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Bhattacharya TK, Kilby C, Bucko P, Rhodes JS (2013) Effects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult mice. Behav Brain Res 242:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell NC, Gilman TL, Daws LC, Toney GM (2018) High salt intake enhances swim stress-induced PVN vasopressin cell activation and active stress coping. Psychoneuroendocrinology 93:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nackenoff AG, Simmler LD, Baganz NL, Pehrson AL, Sánchez C, Blakely RD (2017) Serotonin transporter-independent actions of the antidepressant vortioxetine as revealed using the SERT met172 mouse. ACS Chem Neurosci 8:1092–1100. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J (2015) The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300:141–154. [DOI] [PubMed] [Google Scholar]
- Whelton PK. (2015) Dietary sodium intake: scientific basis for public policy. Blood Purif 39:16–20. [DOI] [PubMed] [Google Scholar]
- Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z (2012) The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging 33:1364–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]