Abstract
The underlying neurobiological basis of major depressive disorder remains elusive due to the severity, complexity, and heterogeneity of the disorder. While the traditional monoaminergic hypothesis has largely fallen short in its ability to provide a complete picture of major depressive disorder, emerging preclinical and clinical findings suggest that dysfunctional glutamatergic neurotransmission may underlie the pathophysiology of both major depressive disorder and bipolar depression. In particular, recent studies showing that a single intravenous infusion of the glutamatergic modulator ketamine elicits fast-acting, robust, and relatively sustained antidepressant, antisuicidal, and antianhedonic effects in individuals with treatment-resistant depression have prompted tremendous interest in understanding the mechanisms responsible for ketamine’s clinical efficacy. These results, coupled with new evidence of the mechanistic processes underlying ketamine’s effects, have led to inventive ways of investigating, repurposing, and expanding research into novel glutamate-based therapeutic targets with superior antidepressant effects but devoid of dissociative side effects. Ketamine’s targets include noncompetitive N-methyl-D-aspartate receptor inhibition, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid throughput potentiation coupled with downstream signaling changes, and N-methyl-D-aspartate receptor targets localized on gamma-aminobutyric acid-ergic interneurons. Here, we review ketamine and other potentially novel glutamate-based treatments for treatment-resistant depression, including N-methyl-D-aspartate receptor antagonists, glycine binding site ligands, metabotropic glutamate receptor modulators, and other glutamatergic modulators. Both the putative mechanisms of action of these agents and clinically relevant studies are described.
Keywords: antidepressants, glutamate receptors, ketamine, treatment-resistant depression
Introduction
Major depressive disorder (MDD) is associated with global morbidity leading to costly psychiatric care and significant societal burden, with a commensurate impact on public health (Whiteford et al., 2013). Worldwide, more than 300 million people now live with depression, a staggering increase of over 18% between 2005 and 2015. Workplace costs associated with depression-related disability further add to the global burden of the disease, as do suicide-related expenditures (Greenberg et al., 2015). Indeed, MDD is the leading cause of suicide in the United States.
Standard pharmacological treatments for depression presently revolve around monoaminergic-based targets due to the early serendipitous discovery that drugs inhibiting the reuptake or metabolism of monoaminergic neurotransmitters (serotonin, noradrenaline, dopamine) possess antidepressant effects (Bunney and Davis, 1965; Schildkraut, 1965; Heninger et al., 1996). However, despite their widespread use, the efficacy of these agents is limited by delayed therapeutic onset, low remission rates, and increased treatment refractoriness (Rush et al., 2006; McIntyre et al., 2014). For instance, the large NIMH-funded Sequenced Treatment Alternatives to Relieve Depression study found that only one-third of MDD patients achieved remission after an adequate trial with a traditional antidepressant. Furthermore, even after 2 additional levels of antidepressant switch therapy or augmentation treatment, only two-thirds of patients achieved remission; thus, roughly 35% of patients in the Sequenced Treatment Alternatives to Relieve Depression study were nonremitters, a group described as having treatment-resistant depression (TRD) (Trivedi et al., 2006). Similarly, the remission rate in the recently completed Prolonging Remission in Depressed Elderly study was only 61%, despite the fact that the study used additional effective interventions such as electroconvulsive therapy (Kellner et al., 2016a, b; Rasmussen, 2017).
A large proportion of the global burden of depression described above is attributable to TRD, which accounts for roughly one-third of depressed subjects. While a number of methods for assessing TRD exist (Malhi and Byrow, 2016), it is loosely defined as failure to respond to one or more FDA-approved antidepressant treatments as measured via various criteria, most often the Antidepressant Treatment History Form (Sackeim, 2001); it should be noted that in the context of this paper, TRD refers to treatment resistance to standard antidepressants only. TRD is associated with poorer overall clinical outcomes, significant physical and mental comorbidities, a substantial burden on individuals and their families, high healthcare costs, and marked and protracted functional impairment (Fekadu et al., 2009; Culpepper, 2011). Lack of response to antidepressant therapy in general—and for TRD patients in particular—is also associated with increased suicide risk (Machado-Vieira et al., 2009). Current therapeutics have limited efficacy in treating suicidal ideation, and no FDA-approved medications exist for that purpose (Griffiths et al., 2014). As a result, new trends in polypharmacy for MDD have been conceived in the last few years, particularly for treating chronic and severe forms of TRD, underscoring the critical unmet medical need for new agents with novel mechanisms of action that show rapid antidepressant efficacy (Olin et al., 2012).
In recent years, compelling evidence has accrued in favor of the glutamatergic system as a primary mediator of psychiatric pathology and a target for the therapeutic action of drugs, particularly rapid-acting antidepressants (Sanacora et al., 2008, 2012; Duman and Aghajanian, 2012; Musazzi et al., 2013; Duman et al., 2016; Lener et al., 2017; Murrough et al., 2017a). Although glutamate was not recognized as a neurotransmitter until the 1980s—when monoaminergic pathways had already been mapped in the brain—this excitatory amino acid is the most abundant neurotransmitter in the brain. Early clinical findings suggested that glutamate plasma levels were significantly higher in patients with mood disorders (Altamura et al., 1993; Sanacora et al., 2008). In this context, emerging preclinical and clinical evidence of the impaired relationship between glutamatergic neurotransmission and synaptic plasticity in mood disorders has guided the search for novel pharmacotherapeutic strategies.
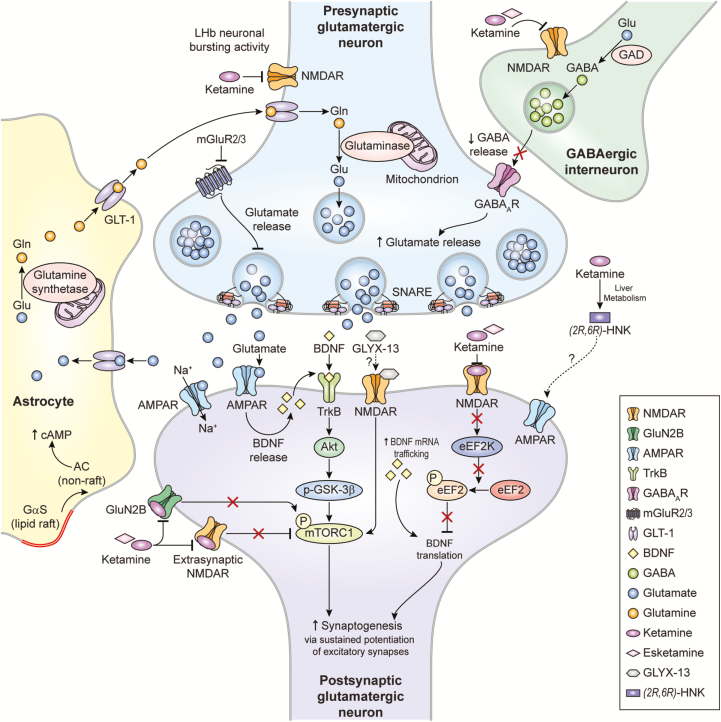
Towards this end, the glutamatergic agent ketamine has been intensely investigated over the past 2 decades. Ketamine’s antidepressant effects are hypothesized to be mediated by direct and indirect N-methyl-D-aspartate receptor (NMDAR) inhibition, gamma aminobutyric acid (GABA)-ergic interneuron disinhibition, and conversion to hydroxynorketamine (HNK) metabolites; together, these processes increase presynaptic glutamate release, with the net effect of enhancing glutamatergic throughput at the α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) relative to the NMDAR (Zanos et al., 2016, 2018a). These presumed mechanisms are not mutually exclusive and may in fact work in a complementary fashion to exert sustained potentiation of excitatory synapses to maintain antidepressant response (Figure 1).
Figure 1.
Proposed mechanisms of action of glutamatergic modulators and other putative rapid-acting antidepressants. Disinhibition hypothesis: Ketamine and esketamine selectively block N-methyl-D-aspartate receptors (NMDARs). These are expressed on gamma-aminobutyric acid (GABA)-ergic inhibitory interneurons, and their blockade decreases interneuron activity that, in turn, leads to disinhibition of pyramidal neurons and enhanced glutamatergic firing. Glutamate then binds to and activates post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). Inhibition of spontaneous NMDAR-mediated transmission: Alternatively, ketamine may induce rapid brain-derived neurotrophic factor (BDNF) translation in the hippocampus, reduce phosphorylation, and activate eukaryotic elongation factor 2 (eEF2). Ketamine may also preferentially bind to NMDARs and affect neuronal NMDAR-mediating spontaneous excitatory transmission, which at rest keeps eEF2 phosphorylated and inhibits BDNF synaptic translation. De-suppression of BDNF translation then contributes to changes in synaptic plasticity that mediate ketamine’s antidepressant effects. AMPAR activation is also necessary for these effects. Inhibition of extra-synaptic NMDARs: Ketamine selectively blocks extra-synaptic GluN2B-containing NMDARs, which are tonically activated by low levels of ambient glutamate regulated by the excitatory amino acid transporter 2 (EAAT2) located on astrocytes. Inhibition of the extra-synaptic GluN2B-NMDARs de-suppresses mammalian target of rapamycin complex 1 (mTORC1) function, which in turn induces protein synthesis. Blockade of spontaneous NMDAR activation inhibits eEF2 kinase (eEF2K) activity, thus preventing phosphorylation of its eEF2 substrate. This effect subsequently enhances BDNF translation and, ultimately, protein synthesis. Inhibition of lateral habenula (LHb) neurons: In animal models, local neuronal firing in a single brain region known as the lateral habenula (LHb) drives significant depressive-like behaviors. Ketamine decreases burst activity in the LHb by blocking NMDAR-dependent burst activity in the LHb and disinhibiting the downstream activity of midbrain dopaminergic neurons and serotoninergic neurons, which are responsible for activating the reward centers in the brain. Local blockade of NMDARs or low-voltage-sensitive T-type voltage sensitive calcium channels (T-VSCCs) in the LHb sufficed to induce rapid antidepressant effects. The role of ketamine metabolites: (2R,6R)-hydroxynorketamine (HNK) exerts NMDAR inhibition-independent antidepressant actions by activating AMPAR-mediated synaptic potentiation.
Other glutamatergic modulators: Metabotropic glutamate receptor (mGluR) 2/3 antagonists are thought to enhance synaptic glutamate levels, thereby boosting AMPAR transmission and firing rates and extracellular monoamine levels. GLYX-13, which has partial agonist properties at NMDARs, is hypothesized to activate mTORC and subsequently induce protein synthesis. GLYX-13 requires AMPA and activity-dependent BDNF release, but unlike ketamine does not produce glutamate bursts. AMPAR activation enhances BDNF release, activates the tropomyosin receptor kinase B (TrkB) receptor, and subsequently promotes protein synthesis by activating the mTORC complex. AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; GAD, glutamate decarboxylase; Gαs, G alpha subunits; Gln, glutamine; GLT-1, glutamate transporter 1; Glu, glutamate; GSK-3, glycogen synthase kinase 3; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor.
Adapted with permission from (Lener et al., 2017; Zanos et al., 2018a).
This article reviews the proposed mechanism of action of ketamine and other glutamatergic-based drugs used in TRD as well as the clinical evidence supporting their use in depressive disorders. It focuses on proof-of-concept evidence drawn from clinical studies of novel glutamate-modulating agents for TRD, including NMDAR antagonists, glycine site ligands, metabotropic glutamate receptor modulators, and other glutamatergic-modulating treatments.
NMDAR Antagonists
Ketamine’s Mechanism of Action: Overview
In recent years, ketamine’s mechanism of action has been widely studied not only as a tool to understand the nature of rapid antidepressant action and identify new therapeutic targets, but also in an attempt to better understand the basic neurobiological underpinnings of stress-related psychopathology (Monteggia et al., 2014; Murrough et al., 2017a; Zanos and Gould, 2018). Preclinical studies have been carried out using rodent stress models, most often using chronic stress protocols but occasionally using acute stress. Since the early hypothesis of NMDAR inhibition of GABA interneurons (see below) was first formed, several different mechanisms have been proposed to explain ketamine’s antidepressant effects, not all of which are mutually exclusive (see Figure 1) (Li et al., 2010; Zanos and Gould, 2018). One of ketamine’s striking effects in rodents is its ability to rapidly (within 24 hours of administration) restore dendritic arborization and the density of synaptic spines reduced by chronic stress, an effect observed with traditional antidepressants after only weeks of treatment (Bessa et al., 2009; Morais et al., 2017). This effect coincides with the peak of ketamine’s antidepressant effects (24 hours) and is considered essential for its therapeutic actions (Duman and Aghajanian, 2012; Musazzi et al., 2017). In addition, this finding further validates the neuroplasticity/glutamatergic hypothesis of depression, which posits that structural and functional disruption of glutamate synapses/circuitry is associated with stress-related psychopathology (Duman and Aghajanian, 2012; Sanacora et al., 2012; Duman et al., 2016; Murrough et al., 2017a). While a detailed analysis of all of ketamine’s proposed mechanisms of action is beyond the scope of this article, below we briefly review a number of different hypotheses and model mechanisms (Figure 1).
Ketamine’s Mechanisms of Action: NMDAR-Dependent Mechanisms
Inhibition of NMDARs on GABA Interneurons
It has been postulated that, in the prefrontal cortex (PFC), ketamine preferentially blocks NMDARs on GABAergic interneurons, which, in turn, increases the firing of pyramidal neurons. This induces rapid and transient activation of mammalian target of rapamycin complex (mTORC) signaling and, in turn, increases the local expression of synaptic proteins (Arc, PSD-95, GluR1, and synapsin I), consistent with the timing observed for inducing new spine synapses (Li et al., 2010). mTORC inhibition with rapamycin blocks ketamine’s effects. An essential effector of this rapid response is assumed to be brain derived neurotrophic factor (BDNF), a neurotrophin abundant in the adult brain that is produced and released at synapses in an activity-dependent fashion (Lu, 2003; Leal et al., 2014; Song et al., 2017). According to this hypothesis, the glutamate burst induced by ketamine increases AMPAR-mediated excitatory transmission, which, in turn, increases the release of BDNF at synapses and activates mTORC. Studies found that knockin mice carrying the human BDNF Val66Met polymorphism show impaired processing and activity-dependent release of BDNF, resulting in synaptic deficits and impeded synaptogenesis that ultimately abolish the antidepressant behavioral response to ketamine (Liu et al., 2012).
Inhibition of Spontaneous NMDAR-Mediated Transmission
Via a different—though not necessarily alternative—mechanism, ketamine has been shown to induce rapid BDNF translation in the hippocampus, which depends on reduced phosphorylation and activation of eukaryotic elongation factor 2 (eEF2) (Autry et al., 2011). Notably, eEF2 kinase knockout animals are not sensitive to the acute effects of ketamine administration, despite the fact that ketamine would be expected to bind preferentially to NMDARs and affect neuronal NMDAR-mediating spontaneous excitatory transmission; at rest, this mechanism keeps eEF2 phosphorylated and inhibits BDNF synaptic translation (Monteggia et al., 2013). De-suppression of BDNF translation then contributes to changes in synaptic plasticity that mediate ketamine’s antidepressant effects, and AMPAR activation is also necessary for these effects. Both mechanisms described above could be involved simultaneously and could also be interconnected (for instance, because BDNF stimulates mTORC signaling).
Inhibition of Extrasynaptic NMDARs
Extrasynaptic NMDARs, primarily comprising heterotetramers containing GluN2B subunits, are tonically activated by low levels of ambient glutamate. Under baseline conditions, activation of cortical extra-synaptic GluN2B-containing NMDARs inhibits mTOR-dependent signaling, which suppresses protein synthesis, thereby maintaining synaptic homeostasis (Gray et al., 2011). Genetic deletion of GluN2B from principal cortical neurons in 2BΔCtx knockout mice was shown to mimic and occlude the effects of ketamine in suppressing depressive-like behaviors and increased the frequency of individual excitatory synaptic events onto pyramidal neurons in layers II/III of the PFC (Miller et al., 2014). Ketamine rapidly and transiently increased mTOR phosphorylation, which is occluded in 2BΔCtx mice (Miller et al., 2014). These data suggest that GluN2B-containing NMDARs may play a role in ketamine’s rapid antidepressant effects due to their ability to directly suppress mTOR signaling and limit protein synthesis in principal cortical neurons. Miller and colleagues found that GluN2B-containing NMDARs are enriched at synapses between the medial dorsal thalamus and medial prefrontal cortex (mPFC) (Miller et al., 2017). In mice, postdevelopmental deletion of GluN2B from pyramidal neurons in the mPFC via optogenetic manipulation induced strong antidepressant-like behavior. The same study found that, interestingly, GluN2B deletion had negligible effects on mPFC synaptic inputs from the ventral hippocampus. The notion that these networks are involved in the action of ketamine and other NMDAR antagonists is supported by human (Vollenweider and Kometer, 2010), primate (Lv et al., 2016; Maltbie et al., 2016), and rodent studies (Dawson et al., 2014; Amat-Foraster et al., 2018; Shen et al., 2018).
Inhibition of Lateral Habenula Neurons
The inhibition of lateral habenula (LHb) glutamatergic neurons was recently proposed as an additional NMDAR-dependent mechanism. LHb neuronal activity is significantly increased in animal models of depression (Yang et al., 2018b) as well as in MDD patients (Lawson et al., 2017; Yang et al., 2018a). Activation of LHb glutamatergic neurons inhibits the activity of midbrain dopaminergic neurons, and ketamine’s rapid antidepressant effects are mediated by blockade of NMDAR-dependent burst activity in the LHb (Li et al., 2011a). Moreover, local blockade of NMDARs or low-voltage-sensitive T-type voltage-sensitive calcium channels in the LHb sufficed to induce rapid antidepressant effects (Yang et al., 2018a). These results suggest a simple model whereby ketamine quickly elevates mood by blocking the NMDAR-dependent burst activity of LHb neurons and, in turn, disinhibits downstream monoaminergic reward centers (Yang et al., 2018b). It should be noted, however, that this putative mechanism of action has only been assessed acutely, at 1 hour post-ketamine infusion in the LHb; whether this mechanism is active at the peak of ketamine’s sustained antidepressant effects (after 24 hours) remains unknown.
GABAB Receptor Expression/Function
GABAB receptors (GABABR) are inhibitory G protein-coupled receptors found at both pre- and postsynaptic sites in most neurons and glial cells (Padgett and Slesinger, 2010). In a study that sheds considerable light onto how NMDAR blockade may activate mTOR signaling and downstream pathways, NMDAR blockade with AP5 for 90 minutes in cultured hippocampal neurons increased GABABRs on the surface of the dendritic membranes and shifted postsynaptic GABABR function from reducing to increasing dendritic resting calcium levels, requiring L-type calcium channels in both normal and reduced states of synaptic activity (Workman et al., 2013, 2018). Mice treated with Ro-25-6891, a selective NR2B antagonist, demonstrated that this activation of GABABRs was required to stimulate mTOR kinase activity and promote the synthesis of synaptic plasticity-related proteins (Workman et al., 2013), a typical outcome of ketamine treatment. However, there was no direct demonstration that ketamine treatment itself engaged this mechanism.
Ketamine’s Mechanisms of Action: NMDAR-Independent Mechanisms
Ketamine is a mixture of the 2 enantiomers: (R)- and (S)-ketamine. While (S)-ketamine has greater affinity for NMDARs, (R)-ketamine has better and longer-lasting antidepressant efficacy in rodent models (Zhang et al., 2014; Yang et al., 2015; Zanos et al., 2016). This discrepancy between affinity and potency of the 2 enantiomers suggests that ketamine’s antidepressant effects do not entirely depend on NMDAR antagonism (Zanos and Gould, 2018).
Hydroxynorketamine
After ketamine administration, (2S,6S)- and (2R,6R)-HNK are the major metabolites found in the brain and plasma of rodents (Zanos et al., 2016) and in the plasma of humans (Moaddel et al., 2010; Zarate et al., 2012a). To determine whether the metabolism of ketamine to HNK is required for its antidepressant actions, ketamine was deuterated at the C6 position (6,6-dideuteroketamine), a modification that does not change the affinity of unmetabolized ketamine for NMDARs but does slow its metabolism. Unlike ketamine, 6,6-dideuteroketamine had no antidepressant effects in the forced swim test (FST) or learned helplessness test (LHT) at 24 hours (Zanos et al. 2016). To understand whether (2S,6S)-HNK or (2R,6R)-HNK exerted antidepressant effects independently of ketamine, their behavioral effects were compared in the FST and LHT. Both metabolites had potent antidepressant effects, with (2R,6R)-HNK showing greater effects than (2S,6S)-HNK, in line with previous findings showing that (R)-ketamine has greater antidepressant efficacy (Zhang et al., 2014). In addition, while ketamine and (2S,6S)-HNK were both associated with increased locomotor activity in the open field test and lack of motor coordination in the rotarod test, (2R,6R)-HNK did not induce these NMDAR inhibition-mediated side effects in mice (Zanos et al., 2016).
These findings demonstrate that a distinct metabolite of ketamine was both necessary and sufficient to produce ketamine’s antidepressant effects. Interestingly, at relevant concentrations (10 mg/kg), (2R,6R)-HNK did not appear to inhibit NMDARs (Zanos et al., 2016; Suzuki et al., 2017), implying that HNK’s antidepressant effects (and those of ketamine itself) do not completely depend on binding to NMDARs. Moreover, (2R,6R)-HNK may not inhibit NMDARs; however, this possibility was questioned by Suzuki and colleagues (2017), who showed that, at concentrations of 50 µM (but not 10 µM), (2R,6R)-HNK partially (40%) inhibited NMDAR-dependent currents at rest in hippocampal neurons (Suzuki et al., 2017). The authors argued that after i.p. administration in mice, a larger proportion of ketamine could be metabolized in the liver, thus increasing the metabolite’s contribution to antidepressant effects. They further suggested that while the initial effects of ketamine could be due to the unmetabolized drug, the 24-hour and longer antidepressant effects could be due to continued NMDAR inhibition by HNK. In contrast, Zanos and colleagues found that, at relevant antidepressant concentrations (10 µM), (2R,6R)-HNK neither inhibited NMDARs nor induced any of the side effects typically associated with ketamine (Zanos et al., 2016). Clinical trials will be required to assess the antidepressant properties of HNK in humans.
Stabilization of Glutamate Release/Excitatory Transmission
Preclinical evidence suggests that ketamine stabilizes dysfunction in excitatory (but also inhibitory) transmission within and between relevant brain areas involved in the pathophysiology of MDD (Thompson et al., 2015; Duman et al., 2016; Musazzi et al., 2017; Workman et al., 2018; Zanos et al., 2018b). In particular, one net effect of ketamine (and its metabolites) seems to be sustained activation of AMPAR-mediated excitatory transmission within the mPFC, which results in increased input to subcortical areas, thereby impacting stress reactivity and mood.
Early work with classical microdialysis explored the actual impact of ketamine on glutamate release in the mPFC (Moghaddam et al., 1997), but 2 more recent studies have also investigated this phenomenon. One study observed that, 24 hours after administration of 10 mg/kg (R)-ketamine or (2R,6R)-HNK, basal glutamate release measured via zero-net-flux quantitative microdialysis was significantly increased (Pham et al., 2018). Glutamate reuptake was not affected by either ketamine or (2R,6R)-HNK. At the same timepoint, the mice showed behavioral improvement in the FST. The authors concluded that mPFC activation by ketamine had an excitatory effect and increased glutamate release by pyramidal neurons without affecting its reuptake, and that the (2R,6R)-HNK metabolite significantly contributed to this effect.
Another study used freshly purified synaptosomes in superfusion to explore the effects of racemic ketamine on glutamate release in rats vulnerable to the maladaptive effects of chronic mild stress (CMS) (Tornese et al., 2017). Different versions of the CMS protocol, one of the most popular animal models of depression, have been used by several groups to investigate ketamine’s rapid antidepressant effects (Li et al., 2011b; Ma et al., 2013; Papp et al., 2017); such studies typically assess ketamine’s effects in a group of stressed animals as a whole. A recent study used the sucrose consumption test for anhedonia to separate vulnerable from resilient rats during 5-week CMS (Tornese et al., 2017). The investigators found that most stress-induced maladaptive changes in the hippocampus were observed only in vulnerable rats. CMS markedly and significantly reduced both basal and depolarization-evoked glutamate release. Ketamine administration (10 mg/kg) 24 hours before the end of CMS in vulnerable rats restored basal glutamate release. Ketamine concomitantly restored anhedonic behavior, dendritic atrophy, and BDNF mRNA dendritic trafficking (but not BDNF expression) (Tornese et al., 2017). These results suggest that one of ketamine’s major effects is to restore the basal release of glutamate, which is needed to maintain homeostatic synaptic plasticity (Reese and Kavalali, 2015). Ketamine did not restore BDNF expression reduced by CMS but did restore BDNF mRNA dendritic trafficking, a process known to be stimulated by chronic use of traditional antidepressants as well as physical exercise (Baj et al., 2012). The same process was found to be impaired in knockin mice carrying the human Val66Met mutation of BDNF (Baj et al., 2012; Mallei et al., 2015). It is possible that increased BDNF mRNA dendritic trafficking and local translation of BDNF protein is crucial for restoring homeostatic synaptic plasticity as well as neuroarchitecture (Tornese et al., 2017). These findings are in line with the notion that rapid BDNF release is required for ketamine’s antidepressant effects (Lepack et al., 2014).
Regulation of the Dopaminergic System
Dysregulation of the dopaminergic system is known to underlie the pathophysiology of MDD (Belujon and Grace, 2014, 2017; Han and Nestler, 2017). Anhedonia—loss of the ability to experience pleasure and 1 of the 2 core symptoms of MDD—is related to dysregulation of reward-related circuitry that is made up of the mesolimbic dopaminergic pathway from the ventral tegmental area to the nucleus accumbens as well as multiple regulatory pathways. In rodents, these include the infralimbic PFC-basolateral amygdala-ventral pallidum (VP) circuit and the infralimbic PFC-hippocampus ventral subiculum (Vsub)-nucleus accumbens-VP circuit (Belujon and Grace, 2014, 2017). The first circuit reinforces the inhibition of dopaminergic neurons by the VP, while the second blocks VP inhibition, increasing dopaminergic neuronal activation. Both single and repeated administration of subanesthetic ketamine (5 mg/kg) rapidly restored activation of dopaminergic neurons, which was reduced in rats subjected to the LHT. Ketamine administration also improved depressive-like behaviors induced by the LHT, and this restorative action was maintained for 24 hours post-ketamine administration (Belujon and Grace, 2014). Moreover, the rapid and sustained antidepressant-like effects of ketamine in the LHT also stimulate AMPARs (Koike et al., 2011). It is not clear whether ketamine’s regulatory effects on dopaminergic neurons also involve NMDAR antagonism. However, these studies suggest that an essential restorative action of the dopaminergic system also underlies ketamine’s mechanism of action (Kokkinou et al., 2018).
G Alpha Subunit Translocation-Cyclic Adenosine Monophosphate Production
Treatment with different classes of antidepressants has been found to increase cellular cyclic adenosine monophosphate levels by augmenting the functional coupling of G alpha subunits (Gαs) and adenylyl cyclase after translocation of Gαs from lipid raft domains into non-raft regions (Czysz et al., 2015). This hypothesis of antidepressant action has been corroborated by the finding that the human postmortem brain tissue of depressed individuals who committed suicide showed Gαs preferentially localized to lipid rafts (Donati et al., 2008). The same authors recently found that treatment of C6 glioma cells or primary astrocytes with 10 µM of ketamine for 15 minutes induced selective redistribution of Gαs. Moreover, and similar to traditional antidepressants, exposure to ketamine elicited a sustained increase in cellular cyclic adenosine monophosphate attributable to Gαs translocation from lipids rafts (Wray et al., 2018). The ketamine metabolite (2R,6R)-HNK—which, as noted above, has antidepressant effects similar to those of ketamine but does not bind to NMDARs—exerted similar effects in cells where NMDARs were knocked down to undetectable levels (Wray et al., 2018). The authors also found a significant increase in BDNF expression in C6 cells 24 hours, but not 1 hour, after ketamine treatment (Wray et al., 2018). Taken together, these results suggest that acute ketamine treatment exerts effects similar to those of chronic antidepressants via a mechanism seemingly independent of NMDAR antagonism.
Reconfiguration of Brain Homeostasis
Both preclinical and clinical studies have demonstrated that ketamine administration helps the overall network reconfiguration of disrupted prefrontal connectivity, restoring metabolic homeostasis and synchronizing gamma oscillatory activity in the brain (Arnsten et al., 2016; Lv et al., 2016; Nugent et al., 2018). For example, MDD subjects show enhanced metabolic activity in the subgenual cingulate cortex, an area highly associated with negative emotions (Mayberg et al., 2005). This symptomatology constellation is likely driven by excessive excitatory neurotransmission; treatments, like ketamine, that decrease NMDAR-dependent firing may have a salutary effect at this relevant site (Wang and Arnsten, 2015; Arnsten et al., 2016).
Various abnormalities in functional connectivity within the PFC and other cortical and subcortical regions have also been observed in individuals with MDD (Kaiser et al., 2015), and ketamine administration has been found to enhance normalization of desynchronized global brain connectivity in depressed subjects (Abdallah et al., 2017a, 2017b). In vivo evidence of glutamate dysfunction in mood disorders has also been shown using proton magnetic resonance spectroscopy, including reduced glutamate levels in the dorsolateral PFC of individuals with MDD (Yildiz-Yesiloglu and Ankerst, 2006) as well as the dorsomedial and dorsoanterolateral PFC (Hasler et al., 2007) and the anterior cingulate cortex (Auer et al., 2000).
In addition, studies have suggested that ketamine and other glutamatergic modulators exert antidepressant effects by increasing synchronization of gamma oscillatory activity in the brain (Lazarewicz et al., 2010; Ahnaou et al., 2014; Sanacora et al., 2014; Zanos et al., 2016). Although this complex process is not entirely understood, multiple mechanisms appear to be involved in regulating gamma oscillation, including silencing GABAergic inhibition at the synapses and increasing glutamate release, thereby increasing AMPAR activation (Ren et al., 2016; Zanos et al., 2018a). This, in turn, induces activity-dependent plasticity (Buzsaki and Wang, 2012; Duman and Aghajanian, 2012). Interestingly, a recent animal study found that the ketamine metabolite (2R,6R)-HNK increases gamma oscillations and exerts long-lasting antidepressant effects (Zanos et al., 2016). Pretreatment with an AMPAR blocker abolished (2R,6R)-HNK-induced gamma oscillations and the concomitant antidepressant effects of this metabolite. Relatedly, a recent replication study of MDD subjects found that ketamine infusion increased gamma oscillatory activity even 6 to 9 hours post-ketamine infusion (Nugent et al., 2018). Interestingly, baseline gamma levels appeared to moderate the relationship between increases in gamma power post-ketamine and antidepressant response, suggesting that resting gamma oscillations may be a proxy measure of inhibition/excitation balance and homeostasis.
The various mechanisms detailed above are not an exhaustive catalog of ketamine’s proposed mechanisms of action. For additional data and hypotheses, we direct the interested reader to several recent reviews on the subject (Miller et al., 2016; Lener et al., 2017; Strasburger et al., 2017; Abdallah et al., 2018; Zanos and Gould, 2018). It should also be noted that, despite all the research recently done, the mechanisms underlying ketamine’s antidepressant effects are not yet entirely clear and could be even more complex than those of traditional antidepressants.
Ketamine: Clinical Evidence
The discovery that the prototypic glutamatergic modulator ketamine has antidepressant effects was hailed as arguably one of the most important recent discoveries in psychiatry (Insel, 2014a). In addition to the putative mechanisms of actions described above, ketamine’s mechanistic processes also include potential sigma-1 and mu-opioid receptor activation. As noted above, ketamine exists as a mixture of 2 enantiomers—(R) and (S)—that show different affinity for glutamate receptors (Mion and Villevieille, 2013). (S)-ketamine has been studied in a number of clinical trials (described below), but no clinical trials to date have investigated the efficacy of (R)- vs (S)-ketamine.
Berman and colleagues initially found that a single, subanesthetic-dose ketamine infusion had rapid antidepressant effects (Berman et al., 2000). Subsequently, Zarate and colleagues conducted a series of randomized controlled studies establishing that subanesthetic-dose ketamine (0.5 mg/kg, i.v.) administered over 40 minutes led to rapid, robust, and relatively sustained antidepressant effects in TRD—both MDD (Zarate et al., 2006; Diazgranados et al., 2010a; Ibrahim et al., 2011; Iadarola et al., 2015) and bipolar depression (Diazgranados et al., 2010b; Zarate et al., 2012b). In research settings, studies of TRD patients found a response rate to this ketamine dose of more than 70% within 24 hours post-infusion (Zarate et al., 2006), with about 50% to 70% exhibiting a variable duration of response (aan het Rot et al., 2010; Wan et al., 2015). At this dose, ketamine was also shown to have superior antidepressant effectiveness over the benzodiazepine midazolam, which was used as an active placebo comparator to mimic ketamine’s sedative and anxiolytic effects; roughly 65% of patients achieved a ≥50% reduction in Montgomery-Asberg Depression Rating Scale (MADRS) score in response to ketamine compared with approximately 28% of patients receiving midazolam (Murrough et al., 2013b). Building on this work, numerous meta-analyses have corroborated the antidepressant effects of ketamine in both MDD and bipolar depression (Caddy et al., 2014; McGirr et al., 2015; Newport et al., 2015; Romeo et al., 2015; Kishimoto et al., 2016).
Further studies established that off-label ketamine use is also associated with significant and rapid (1–4 hours) antisuicidal effects (Price et al., 2009; Diazgranados et al., 2010a; Murrough et al., 2015). Indeed, a recent meta-analysis of 167 patients with a range of mood disorder diagnoses found that ketamine was associated with reduced suicidal thoughts compared with placebo; these effects occurred as rapidly as within a few hours and lasted up to 7 days (Wilkinson et al., 2018). Ketamine also appears to have pan-therapeutic effects, improving mood symptoms across a variety of diagnoses, including anhedonia (Lally et al., 2014), fatigue (Saligan et al., 2016), obsessive-compulsive disorder (Bloch et al., 2012; Rodriguez et al., 2013), and post-traumatic stress disorder (Feder et al., 2014; Hartberg et al., 2018). While a full review of ketamine’s antidepressant effects is beyond the scope of this article, the interested reader is referred to several recent reviews of this subject (Abdallah et al., 2018; Molero et al., 2018).
Despite ketamine’s robust antidepressant effects, response is transient and the remission period after a single infusion is relatively short (days to weeks). To prolong ketamine’s antidepressant efficacy, multiple doses have been clinically studied to determine the safety, tolerability, and efficacy of repeat-dose i.v. ketamine. To date, the preliminary data suggest that up to two to three 0.5-mg/kg i.v. infusions per week for 2 weeks are well-tolerated and effective (aan het Rot et al., 2010; Rasmussen et al., 2013; Diamond et al., 2014). In one study, 10 medication-free TRD patients who had previously responded to ketamine were given 6 infusions (0.5 mg/kg, i.v. over 40 minutes) for 12 days (aan het Rot et al., 2010). Minor adverse effects were associated with repeat dosing. Interestingly, 9 of the 10 patients met response criteria after the first as well as the sixth infusion; however, 8 of those 9 patients relapsed, on average, 19 days after the sixth infusion. Other preliminary studies have similarly established that repeated ketamine infusions are superior to a single dose and may be a potential avenue for extending ketamine’s antidepressant actions for up to several months (Murrough et al., 2013a; Rasmussen et al., 2013; Shiroma et al., 2014; Singh et al., 2016b). For instance, a recent, randomized, multicenter, double-blind study assessed change in MADRS score in 68 MDD patients (Singh et al., 2016b); MADRS scores improved from baseline to day 15 in those receiving ketamine vs placebo: −18.4 (SD = 12.0) for ketamine administered twice weekly for 2 weeks vs −5.7 (SD = 10.2, P < .001) for placebo, and −17.7 (SD = 7.3) for ketamine administered thrice weekly vs −3.1 (SD = 5.7, P < .001) for placebo. No difference in MADRS score was observed between the 2 dosing frequencies, and no difference in the number of treatment-based adverse effects was reported. Transient dissociative symptoms appeared to lessen with repeated dosing (Singh et al., 2016b). Another study reported similar decreases in MADRS scores (18.9, SD = 6.6, P < .001) when assessing the effects of open-label ketamine infusion 6 times over 12 days in a group of 24 individuals with TRD (Murrough et al., 2013b). Another recent, double-blind, crossover trial using active placebo (midazolam) in 40 TRD subjects found that repeated (twice to thrice weekly) ketamine (0.5 mg/kg) infusions maintained and prolonged initial antidepressant response to a single infusion (Phillips et al., 2018). However, a recent, small, double-blind, placebo-controlled, add-on therapy study in outpatient TRD subjects with chronic, recurrent suicidal ideation found that ketamine did not separate from placebo in ameliorating depressive symptoms (P = .47) or suicidal ideation (P = .32) (Ionescu et al., 2019). Although the underlying strategy for maintaining this response is still lacking, it is worth noting that several studies are currently underway that may further inform efforts to prolong ketamine’s effects; results are not yet publicly available.
To address ketamine’s potential adverse effects, investigators are increasingly exploring other potent and effective alternative—and largely more convenient—means of ketamine administration. These include intranasal (Lapidus et al., 2014), intramuscular (Glue et al., 2011), oral (Irwin et al., 2013), and sublingual (Lara et al., 2013) routes. One study found that sublingual ketamine had antidepressant effects in 20 of 27 patients (77%), with only mild adverse effects (Lara et al., 2013). Evidence from small case studies also suggests that oral ketamine may be effective. In one such study, 14 patients receiving hospice care demonstrated improvements in both mood and anxiety symptoms after 28 days of open-label oral ketamine (Irwin et al., 2013). Intramuscular ketamine—which does not require specialized equipment to administer—has also been studied. This route of administration has similar bioavailability to i.v. ketamine, in contrast to sublingual and oral ketamine, which have bioavailability of 30% and 20%, respectively. A small study of 2 female TRD subjects who received open-label intramuscular ketamine found that the patients responded in a dose-dependent manner (Glue et al., 2011). Finally, a randomized, double-blind, crossover, placebo-controlled trial of 50 mg of intranasal ketamine found that, at this dose, intranasal ketamine had antidepressant effects that appeared within 24 hours; minimal dissociative and psychotomimetic effects were observed (Lapidus et al., 2014). Despite these promising preliminary results, larger, controlled studies are needed to assess whether these alternative routes of administration will exert the same robust antidepressant effects as i.v. ketamine.
Taken together, the results reviewed above suggest that ketamine may not only ameliorate depressive symptoms within hours of administration but that its effects may be sustained when administered over multiple sessions. While these data are promising, ketamine-induced side effects—though transient in nature—must be seriously considered, especially in the absence of well-controlled longitudinal studies that assess this question.
(S)-Ketamine Hydrochloride
Clinical trials of the (S)-ketamine hydrochloride (esketamine) enantiomer of ketamine to treat TRD are underway. A multicenter, randomized, placebo-controlled trial (NCT0160080) with 30 TRD patients receiving i.v. infusions of 0.2 mg/kg and 0.4 mg/kg of esketamine, respectively, found that subjects experienced significant relief in their depressive symptoms (as evidenced by a decrease in MADRS score) within 2 hours of administration; those effects were sustained for three days and, in some patients, lasted for up to 2 weeks following a single infusion (Singh et al., 2016a). In that study, esketamine’s side effects—which include nausea, headache, and dissociation—appeared to be milder than those observed in response to i.v. ketamine. Another recent multisite, randomized, phase 2 study (SYNAPSE, NCT01998958) examined the antidepressant efficacy of intranasal esketamine (28 mg, 56 mg, or 84 mg) in 67 patients with TRD. Those who received intranasal esketamine at doses of 56 or 84 mg plus an oral antidepressant showed significant improvement in depressive symptoms (as measured by change in MADRS total score from baseline to day 8, the primary endpoint) compared with those who received matching placebo (Daly et al., 2018). Interestingly, esketamine was superior to placebo in all 3 esketamine groups (28 mg, 56 mg, and 84 mg), with a significant ascending dose-response relationship. Another similarly designed proof-of-concept, multisite, 4-week, double-blind study compared standard treatment plus intranasal esketamine (84 mg) with standard treatment plus placebo in individuals with MDD at imminent risk of suicide. The study involved 68 participants randomly assigned to 1 of 2 groups who received either esketamine or placebo twice a week for 4 weeks in addition to standard-of-care treatment; all participants continued to receive standard antidepressant treatment throughout the study. The researchers assessed the effects of intranasal esketamine at 4 hours, 24 hours, and 25 days post-treatment. The study found that, compared with the placebo group, depression rating scale scores were significantly improved post-esketamine at 4 hours and at 24 hours, and that suicidal ideation was reduced; esketamine’s effects did not differentiate from placebo at 25 days (Canuso et al., 2018).
Based on this evidence, esketamine is currently in phase 3 clinical trials for the treatment of TRD (NCT02417064, NCT02497287, NCT02418585) and received breakthrough therapy designation from the FDA. If ongoing studies are positive, approval will likely be fast-tracked.
Additional Agents Targeting NMDARs
Dextromethorphan, Dextromethorphan-Quinidine, and Dextromethorphan-Bupropion
The cough suppressant dextromethorphan is a noncompetitive, nonselective NMDAR antagonist that shares some of ketamine’s rapid-acting antidepressant properties (Lauterbach, 2011, 2012). Its mechanism of action is largely unknown, though preclinical mouse studies showed that sigma-1 receptor activation is required for the antidepressant-like effects of dextromethorphan in the FST (Nguyen et al., 2014). The same group recently reported that dextromethorphan’s antidepressant effects also depend on AMPARs (Nguyen and Matsumoto, 2015) as well as the nitric oxide/cyclic guanosine monophosphate pathway (Sakhaee et al., 2017).
A randomized, placebo-controlled trial of dextromethorphan explored its use as an adjunct to valproate in bipolar depression. No significant differences were observed between groups on the outcome measures of interest (mean Hamilton Depression Rating Scale [HAM-D] and Young Mania Rating Scale scores), potentially due to metabolism-related reductions in drug concentration (Lee et al., 2012). To date, no randomized controlled trials have explored the use of dextromethorphan as monotherapy for the treatment of depression.
A dextromethorphan-quinidine combination is also being studied for TRD under the name Nuedexta (AVP-923), which is FDA approved for the treatment of pseudobulbar affect. The addition of low-dose quinidine (a CYP2D6 enzyme inhibitor) to dextromethorphan increases the bioavailability of this agent. A retrospective chart review of 22 subjects with bipolar disorder-I or bipolar disorder not otherwise specified found that 20 mg of dextromethorphan and 10 mg of quinidine once or twice daily, when added to a current medication regimen over a 90-day treatment period, significantly improved Clinical Global Impression scale scores (Kelly and Lieberman, 2014). Another case report found that Nuedexta had antidepressant effects in a depressed patient with emotional lability (Messias and Everett, 2012). Finally, an open-label, one-arm trial of 20 patients with TRD who received oral doses of Nuedexta (45 mg and 10 mg, respectively) every 12 hours found that, after 10 weeks, 45% of subjects had experienced remission and 35% had experienced response, results that can be considered modest for an open-label trial; 6 subjects discontinued before study completion (Murrough et al., 2017b). Mild side effects have been reported with Nuedexta, including gastrointestinal complaints, dizziness, and sedation, and one patient experienced severe insomnia (Murrough et al., 2017b).
Another related compound is AVP-786, an experimental compound formed by combining deuterium-modified dextromethorphan hydrobromide (to reduce first pass metabolism) and ultra-low dose quinidine sulfate (an inhibitor of the enzyme CYP 2D6 used to increase bioavailability). A recent 10-week, multicenter, randomized, double-blind, placebo-controlled clinical trial (NCT02153502) evaluated the efficacy, safety, and tolerability of AVP-786 as adjunctive therapy in patients with TRD, but results have not yet been released.
Finally, the related compound AXS-05 contains both dextromethorphan and bupropion (a norepinephrine and dopamine reuptake inhibitor currently approved for the treatment of depression). Bupropion may work to increase the bioavailability of dextromethorphan in the brain, and AXS-05 may offer a theoretical advantage in patients at risk of overdose or with cardiac conduction concerns. AXS-05 is currently in phase 3 trials (NCT02741791). This randomized, double-blind, placebo-controlled, 12-week study comprises a 6-week, open-label bupropion lead-in period followed by a 6-week, double-blind treatment period. This ongoing study seeks to assess the efficacy of AXS-05 augmentation with bupropion vs bupropion monotherapy in patients with TRD, but no results have yet been posted.
Lanicemine (AZD6765)
Lanicemine (formerly known as AZD6765) possesses rapid antidepressant effects. It is a moderate-affinity, low-trapping NMDAR antagonist, but no preclinical mechanistic studies are available to confirm its mechanism of action. An initial study of 22 medication-free subjects with TRD showed that a single lanicemine infusion (150 mg) was more effective than placebo (Zarate et al., 2013). Lanicemine’s antidepressant effects occurred within 80 minutes of a single administration and lasted for approximately 2 hours, with no dissociative symptoms noted. However, the study concluded that symptom improvement was transient and short-lived. A subsequent 3-week, placebo-controlled trial studied repeated-dose adjunctive lanicemine infusions in subjects with TRD. Lanicemine, which was given at 2 different doses (100 mg and 150 mg), again demonstrated antidepressant effects without ketamine-like side effects, but these were not rapid-acting (Sanacora et al., 2014). Finally, in a larger, 6-week, phase 2b study, adjunctive repeated-dose (50 mg and 100 mg) lanicemine did not separate from placebo, possibly due to the large placebo effect (39% at trial endpoint) (Sanacora et al., 2017). Thus, despite the fact that lanicemine was well tolerated and caused no psychotomimetic or dissociative side effects, the evidence from large clinical trials suggests that this agent did not separate from placebo (Sanacora and Schatzberg, 2015; Sanacora et al., 2017). Based on this evidence, clinical development of lanicemine was discontinued.
Subunit NR2B-Specific NMDAR Antagonists
The mechanism of action of NR2B-specific NMDA antagonists is thought to be similar to that of ketamine (Miller et al., 2014); however, few preclinical studies have been conducted to confirm this hypothesis.
CP-101,606/Traxoprodil
CP-101,606 is a nonselective, NR2B-specific, NMDAR antagonist with some sigma receptor effects. In a randomized, double-blind, placebo-controlled study of 30 TRD patients, monotherapy with CP-101,606 had significant antidepressant effects, as evidenced by a 50% reduction in HAM-D scores at day 5. Notably, investigators found a 60% response rate in patients receiving CP-101,606 compared with 20% in the matched placebo group; this effect lasted at least a week in 78% of treatment responders (Preskorn et al., 2008). However, during the trial, dissociative side effects were prominent and required both dose reductions and slower infusions, underscoring that NR2B antagonists also have dissociative side effects. Ultimately, development of this compound was discontinued due to cardiotoxicity (specifically, QTc prolongation) in certain patients.
MK-0657 (CERC-301)
In a 12-day, double-blind, randomized, placebo-controlled, crossover pilot study of 5 TRD patients, the oral NR2B antagonist MK-0657 showed no antidepressant effects over placebo on the primary efficacy measure; however some improvement in antidepressant symptoms was observed when other depression rating scales—specifically the HAM-D and the self-reported Beck Depression Inventory—were used (Ibrahim et al., 2012a). No serious or dissociative adverse effects were noted.
MK-0657, subsequently known as CERC-301, received fast-track designation for the treatment of MDD. Results from a phase 2 trial (NCT02459236) using 12 or 20 mg of CERC-301 found that this agent did not achieve primary endpoint efficacy due to its lack of significant antidepressant effects. However, it is worth noting that the 20 mg dose at day 2 did show some clinically meaningful antidepressant effects, though these did not reach statistical significance (https://ir.cerecor.com/press-releases/detail/30/cerecor-reports-top-line-data-from-cerc-301-phase-2-study). Another 28-day phase 2 study of adjunctive CERC-301 in patients with severe MDD with suicidal ideation (NCT01941043) found that 8 mg of CERC-301 had no significant antidepressant effects (Paterson et al., 2015).
NMDAR And/Or Glycine Site Modulators
D-Cycloserine
D-cycloserine (DCS) is a partial agonist at NMDARs containing GluN2A and GluN2B subunits, and a full agonist at the GluN2C and GluN2D subunits (Sheinin et al., 2001). It is thought to exert its antidepressant effects by attenuating the function of NMDAR-bearing GluN2A or GluN2B subunits, though it remains unknown whether DCS’s agonist or antagonist properties account for its antidepressant efficacy; no relevant preclinical mechanistic studies are available.
In a placebo-controlled trial, 250 mg/day of adjunctive DCS had no significant antidepressant effects in 22 patients with TRD (Heresco-Levy et al., 2006). Interestingly, a second study of 26 TRD patients explored escalating-dose adjunctive DCS (up to 1000 mg/day) and found that one-half of the participants had a significant antidepressant response to DCS, as measured by the HAM-D and Beck Depression Inventory (Heresco-Levy et al., 2013).
A subsequent open-label study investigated adjunctive DCS as a maintenance treatment after a single ketamine infusion in 12 patients with bipolar depression (n = 7 completers) (Kantrowitz et al., 2015). Depressive symptoms improved from baseline through week 8 (except at the 2-week timepoint), and a large effect size was seen at day 1. Four of the 7 patients who completed the study remained in remission after 8 weeks. Furthermore, clinical improvement at the 8-week timepoint correlated with magnitude of improvement 24 hours post-ketamine. However, interpretation of the efficacy of this agent is limited by the fact that this study had no control group.
In a recent meta-analysis that drew its data from the studies cited above, DCS was linked to acute antidepressant response at high doses (1000 mg) but not at low doses (250 mg) (Newport et al., 2015).
GLYX-13 (Rapastinel)
GLYX-13 is a glycineB-like functional partial agonist. In preclinical studies, GLYX-13 was found to exert rapid antidepressant-like effects without the dissociative and sedative side effects of ketamine (Burgdorf et al., 2013). Proposed mechanisms of action for this agent include directly modulating NR2B-containing NMDARs (Burgdorf et al., 2013), improving hippocampal long-term potentiation (Burgdorf et al., 2015), and enhancing BDNF exocytosis via voltage-gated calcium channels and TrkB signaling (Kato et al., 2017) as well as via the mTORC1 pathway (Liu et al., 2017). In addition, recent theories regarding the mechanism of action of GLYX-13 have evolved and implicate NMDAR activation (Zanos et al. 2018a).
Clinically, one large phase 2b study randomized 116 unmedicated TRD patients to receive GLYX-13 i.v. at doses of either 1, 5, 10, or 30 mg/kg or placebo over 3 to 15 minutes. Patients in the 5-mg/kg and 10-mg/kg groups had the most significant antidepressant response, and no serious adverse effects were reported (Moskal et al., 2014). Building on this work, the same investigators then studied adjunctive GLYX-13 in a randomized, double-blind study of 116 TRD patients (Preskorn et al., 2015). Subjects were randomized to weekly infusions of i.v. GLYX-13 at doses of 1, 5, or 10 mg/kg or placebo. After an interim safety and efficacy analysis, an additional cohort was added who received i.v. GLYX-13 (30 mg/kg) or placebo. As with the first study, those who received 5 or 10 mg/kg of i.v. GLYX-13 had significantly reduced HAM-D scores compared with placebo. No antidepressant effects were observed after day 7. Due to these promising preliminary findings, GLYX-13 received breakthrough therapy designation from the FDA for adjunctive treatment of MDD. A number of clinical trials looking at the safety and efficacy of GLYX-13 as monotherapy or adjunctive treatment in subjects with MDD and suicidality are underway (active trials available at https://clinicaltrials.gov/ct2/results?cond=&term=rapastinel&cntry=&state=&city=&dist=).
Sarcosine
Sarcosine is a glycine transporter-1 inhibitor whose mechanism of action is thought to depend on NMDAR modulation, though no mechanistic studies are available to confirm this hypothesis.
In a 6-week, randomized, double-blind, citalopram-controlled clinical trial of 40 patients with MDD, sarcosine was associated with higher remission and response rates than the selective serotonin reuptake inhibitor citalopram, as assessed by the HAM-D and other rating scales. Furthermore, those treated with sarcosine were less likely to drop out of the study. No significant adverse effects were reported (Huang et al., 2013). No active studies are presently investigating use of this agent for depression.
AV-101
AV-101 is a novel prodrug that crosses the blood brain barrier, where it is converted to 7-Chlorokynurenic acid, a potent and highly selective competitive glycine binding site antagonist at the NMDAR (Wallace et al., 2017); no preclinical mechanistic studies have explored its mechanism of action.
Two independent phase 2 trials are underway. The first is an NIMH-funded, randomized, controlled trial studying the efficacy of AV-101 in TRD individuals who will receive 2 weeks of either 1080 mg/day or 1440 mg/day of AV-101. The primary outcome measure is change in HAM-D total score (NCT02484456). Another larger, multicenter, randomized, placebo-controlled clinical trial is examining the efficacy and safety of adjunctive-use oral AV-101 in 180 patients with TRD. Patients will concomitantly receive either a selective serotonin reuptake inhibitor or a serotonin norepinephrine reuptake inhibitor. The primary outcome measure is change from baseline to study endpoint as measured by the MADRS (NCT03078322).
Metabotropic Glutamate Receptors
The metabotropic glutamate receptors (mGluRs) are expressed widely throughout the brain and modulate the glutamate signaling pathway outside of the NMDAR and AMPAR pathways.
Several preclinical studies have explored the antidepressant effects of mGluR modulators. In this context, AMPAR stimulation was consistently found to be necessary for the antidepressant effects of mGluR2/3 antagonists (Karasawa et al., 2005; Witkin et al., 2016). At the synaptic level, both BDNF/TrkB signaling and the mTORC1 pathway were also found to be key to the antidepressant effects of mGluR2/3 antagonists, echoing the antidepressant effects of other glutamatergic agents (Koike et al., 2011). The antidepressant effects of presynaptic mGluR2 agonists would hypothetically occur by reducing excessive glutamate release; in contrast, GluR2/3 antagonists are thought to enhance synaptic glutamate levels, thereby boosting AMPAR transmission and firing rates and extracellular monoamine levels. Interestingly, the antidepressant effects of mGluR5 negative allosteric modulators (NAMs) were found to not depend on AMPA, TrkB, or mTOR activation, suggesting that these agents act through mechanisms distinct from those of ketamine (Iijima and Fukumoto, 2012; Palucha-Poniewiera et al., 2014).
Several agents that act as positive allosteric modulators or NAMs have been studied in TRD.
AZD2066
The mGluR5 NAM AZD2066 was studied in a randomized controlled trial that compared its efficacy with both placebo and duloxetine, a serotonin norepinephrine reuptake inhibitor (NCT01145755). No significant difference was observed between the 3 arms of the study (results available at https://clinicaltrials.gov/ct2/results?cond=depression&term=AZD2066&cntry=&state=&city=&dist=). No active studies are presently investigating use of this agent for depression.
RO4917523/Basimglurant
The potential antidepressant effects of the mGluR5 NAM basimglurant were studied in a 9-week, phase 2, randomized, multicenter trial of 333 TRD patients (Quiroz et al., 2016). No significant difference in MADRS score was observed between the 3 treatment arms (placebo, adjunctive basimglurant 0.5 mg, or adjunctive basimglurant 1.5 mg) (NCT00809562). No active studies are presently investigating use of this agent for depression.
JNJ40411813/ADX71149
The potential antidepressant effects of JNJ40411813, a novel mGluR2 PAM, were investigated in a phase 2a, randomized, multicenter, double-blind, proof-of-concept study. JNJ40411813 was administered as an adjunctive treatment to 121 MDD subjects who also had significant anxiety symptoms. No significant change in MADRS score was observed between the different treatment arms of the study (NCT01582815) (Kent et al., 2016). No active studies are presently investigating use of this agent for depression.
RO4995819
RO4995819 is an mGluR2/mGluR3 antagonist and a negative allosteric modulator. In a randomized, double-blind, placebo-controlled study of 357 TRD patients, RO4995819 (at doses of 5, 15, or 30 mg) had no antidepressant effects compared with placebo, as measured by change in MADRS score (NCT01457677; results available at https://www.roche.com/dam/jcr:75711f62-3d1f-46b5-a7b1-87b92fd40e7b/en/irp150128.pdf). No active studies are presently investigating use of this agent for depression.
Other Glutamatergic Modulators
Riluzole
The pharmacological profile of riluzole includes inhibiting voltage-dependent sodium channels in neurons (Urbani and Belluzzi, 2000). It also exerts a range of effects on the glutamatergic system, such as increasing synaptic AMPAR trafficking (Du et al., 2007), stimulating neurotrophic factor synthesis (Mizuta et al., 2001), enhancing synaptic glutamate reuptake (Frizzo et al., 2004), and reversing dysfunctional metabolism in glia (Banasr et al., 2010).
Riluzole, which is FDA-approved for the treatment of amyotrophic lateral sclerosis, has also been used to treat both refractory obsessive-compulsive disorder and depression. While initial results of riluzole use in TRD were promising (Zarate et al., 2004), many subsequent randomized, placebo-controlled trials found that antidepressant response to this agent failed to separate from placebo. This includes trials of riluzole as monotherapy in bipolar depression (Park et al., 2017), as an add-on to potentially extend ketamine’s antidepressant effects in TRD (Ibrahim et al., 2012b), and as augmentation treatment in a parallel comparison design study of TRD (Mathew et al., 2017).
Diazoxide
Diazoxide is known to increase glutamate uptake from the synaptic cleft by activating the KATP channel to chronically increase expression of the excitatory amino acid transporter-2 system in glial cells. The excitatory amino acid transporter-2 transporter, which is predominantly expressed on astroglial cells and is responsible for 90% of the total glutamate uptake in the synaptic cleft, is one of the major glutamate transporter systems (Kim et al., 2011). However, a recent phase 2, randomized, placebo-controlled trial of diazoxide in TRD was halted due to major side effects and intolerability (NCT02049385) (Kadriu et al., 2018).
Conclusion
Here, we have reviewed the clinical evidence supporting the use of novel glutamatergic agents for the treatment of TRD and also discussed their putative mechanisms of action. Over the past 2 decades, the findings reviewed above have fundamentally shifted our understanding of the pathophysiology of depression and are informing the development of novel glutamatergic-based agents that act more rapidly than currently available monoaminergic-based agents (Sanacora et al., 2008, 2012; aan het Rot et al., 2010; aan het Rot et al., 2012; Musazzi et al., 2013; Insel, 2014b; Park et al., 2015)—indeed, a number of these therapies act within hours to days instead of weeks to months. As a result of these promising findings, novel paradigms of rapid antidepressant response are currently being adapted in nonexperimental clinical settings to treat acute depressive symptoms, suicidality, and TRD. In addition, some of these agents have been awarded breakthrough therapy status by the FDA, including esketamine, GLYX-13, and AV-101.
Given the complexity of mood disorders, many questions nevertheless remain unanswered. For instance, what populations are these agents best suited for? Can ideal candidates for novel therapeutics be identified by genotype, serum markers, or neuroimaging modalities? What neural perturbations are specific to those who respond—as opposed to those who do not respond—to ketamine (or esketamine, GLYX-13, AV-101, etc)? Do different treatment modalities share common underlying mechanisms? How can we reduce the high relapse rates associated with these rapid-acting treatments? Despite these questions, the future of novel, rapid-acting, glutamate-based antidepressants is bright. Continued investigation into this rapidly growing research area will improve our understanding of the utility and safety of the multiple agents reviewed above in order to confirm their antidepressant efficacy in larger samples and further clarify their underlying mechanisms of action.
Funding
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002857), by a NARSAD Independent Investigator Award to Dr Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr Zarate. This work was also supported by grants from MIUR (PRIN 2015 prot-2015HRE757) to Dr Popoli and Fondazione Cariplo (Prog. 2014-1133) to Dr Musazzi.
Statement of Interest
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the US government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Acknowledgments
The authors thank the NIH 7SE research unit and staff for their support.
References
- Aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ(2010)Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145. [DOI] [PubMed] [Google Scholar]
- Aan Het Rot M, Zarate CA Jr, Charney DS, Mathew SJ(2012)Ketamine for depression: where do we go from here?Biol Psychiatry 72:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill CL, Salas R, Averill LA, Baldwin PR, Krystal JH, Mathew SJ, Mathalon DH (2017a) Prefrontal connectivity and glutamate transmission: relevance to depression pathophysiology and ketamine treatment. Biol Psychiatry Cogn Neurosci Neuroimaging 2:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, DeWilde KE, Wong E, Anticevic A, Tang CY, Iosifescu DV, Charney DS, Murrough JW (2017b) Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH(2018)The neurobiology of depression, ketamine and rapid-acting antidepressants: is it glutamate inhibition or activation?Pharmacol Ther 190:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnaou A, Ver Donck L, Drinkenburg WH(2014)Blockade of the metabotropic glutamate (mglur2) modulates arousal through vigilance states transitions: evidence from sleep-wake EEG in rodents. Behav Brain Res 270:56–67. [DOI] [PubMed] [Google Scholar]
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D’Andrea G, Zamberlan F(1993)Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry 150:1731–1733. [DOI] [PubMed] [Google Scholar]
- Amat-Foraster M, Jensen AA, Plath N, Herrik KF, Celada P, Artigas F(2018)Temporally dissociable effects of ketamine on neuronal discharge and gamma oscillations in rat thalamo-cortical networks. Neuropharmacology 137:13–23. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Murray JD, Seo H, Lee D(2016)Ketamine’s antidepressant actions: potential mechanisms in the primate medial prefrontal circuits that represent aversive experience. Biol Psychiatry 79:713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F(2000)Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry 47:305–313. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM(2011)NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baj G, D’Alessandro V, Musazzi L, Mallei A, Sartori CR, Sciancalepore M, Tardito D, Langone F, Popoli M, Tongiorgi E(2012)Physical exercise and antidepressants enhance BDNF targeting in hippocampal CA3 dendrites: further evidence of a spatial code for BDNF splice variants. Neuropsychopharmacology 37:1600–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G(2010)Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA(2014)Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76:927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA(2017)Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol 20:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH(2000)Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N(2009)The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773, 739. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, Krystal JH, Bhagwagar Z, Sanacora G, Pittenger C(2012)Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry 72:964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney WE Jr, Davis JM(1965)Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry 13:483–494. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR(2013)GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38:729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Weiss C, Gross A, Boikess SR, Kroes RA, Khan MA, Burch RM, Rex CS, Disterhoft JF, Stanton PK, Moskal JR(2015)The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience 308:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang XJ(2012)Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK(2014)Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol 4:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC(2018)Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175:620–630. [DOI] [PubMed] [Google Scholar]
- Culpepper L.(2011)Understanding the burden of depression. J Clin Psychiatry 72:e19. [DOI] [PubMed] [Google Scholar]
- Czysz AH, Schappi JM, Rasenick MM(2015)Lateral diffusion of gαs in the plasma membrane is decreased after chronic but not acute antidepressant treatment: role of lipid raft and non-raft membrane microdomains. Neuropsychopharmacology 40:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC(2018)Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry 75:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N, McDonald M, Higham DJ, Morris BJ, Pratt JA(2014)Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks. Neuropsychopharmacology 39:1786–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R(2014)Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol 28:536–544. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr (2010a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr (2010b) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN, Rasenick MM(2008)Postmortem brain tissue of depressed suicides reveals increased gs alpha localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J Neurosci 28:3042–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, Falke C, Zarate CA Jr, Manji HK(2007)The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology 32:793–802. [DOI] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK(2012)Synaptic dysfunction in depression: potential therapeutic targets. Science 338:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH(2016)Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS(2014)Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71:681–688. [DOI] [PubMed] [Google Scholar]
- Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ(2009)What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord 116:4–11. [DOI] [PubMed] [Google Scholar]
- Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO(2004)Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol 24:123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glue P, Gulati A, Le Nedelec M, Duffull S(2011)Dose- and exposure-response to ketamine in depression. Biol Psychiatry 70:e9–e10; author reply e11. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA(2011)Distinct modes of AMPA receptor suppression at developing synapses by glun2a and glun2b: single-cell NMDA receptor subunit deletion in vivo. Neuron 71:1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC(2015)The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76:155–162. [DOI] [PubMed] [Google Scholar]
- Griffiths JJ, Zarate CA Jr, Rasimas JJ(2014)Existing and novel biological therapeutics in suicide prevention. Am J Prev Med 47:S195–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Nestler EJ(2017)Neural substrates of depression and resilience. Neurotherapeutics 14:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartberg J, Garrett-Walcott S, De Gioannis A(2018)Impact of oral ketamine augmentation on hospital admissions in treatment-resistant depression and PTSD: a retrospective study. Psychopharmacology (Berl) 235:393–398. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC(2007)Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS(1996)The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29:2–11. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, Kremer I(2006)Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord 93:239–243. [DOI] [PubMed] [Google Scholar]
- Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, Kremer I(2013)A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol 16:501–506. [DOI] [PubMed] [Google Scholar]
- Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, Tun R, Huang KH, Chang YC, Lane HY, Tsai GE(2013)Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry 74:734–741. [DOI] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CAJ(2015)Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis 6:97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA Jr(2011)Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diaz Granados N, Jolkovsky L, Brutsche N, Luckenbaugh DA, Herring WJ, Potter WZ, Zarate CA Jr (2012a) A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2b antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol 32:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr (2012b) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Fukumoto K, Chaki S(2012)Acute and sustained effects of a metabotropic glutamate 5 receptor antagonist in the novelty-suppressed feeding test. Behav Brain Res 235:287–292. [DOI] [PubMed] [Google Scholar]
- Insel TR (2014a) Director’s Blog: Ketamine. http://www.nimh.nih.gov/about/director/2014/ketamine.shtml. Accessed May 1, 2018. [Google Scholar]
- Insel TR. (2014b) The NIMH research domain criteria (RDoC) project: precision medicine for psychiatry. Am J Psychiatry 171:395–397. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, Pavone KJ, Petrie SR, Dording C, Mischoulon D, Alpert JE, Brown EN, Baer L, Nock MK, Fava M, Cusin C(2019)Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord 243:516–524. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Iglewicz A, Nelesen RA, Lo JY, Carr CH, Romero SD, Lloyd LS(2013)Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial. J Palliat Med 16:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Yuan S, Farmer C, Nugent AC, Lener MS, Niciu MJ, Park M, Yazdian A, Ballard ED, Henn FA, Henter ID, Park LT, Zarate CA Jr(2018)Clinical trial of the potassium channel activator diazoxide for major depressive disorder halted due to intolerability. J Clin Psychopharmacol 38:243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA(2015)Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Halberstam B, Gangwisch J(2015)Single-dose ketamine followed by daily D-cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry 76:737–738. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S(2005)AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042:92–98. [DOI] [PubMed] [Google Scholar]
- Kato T, Fogaca MV, Deyama S, Li XY, Fukumoto K, Duman RS(2017)BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry doi: 10.1038/mp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, et al. ; CORE/PRIDE Work Group (2016a) Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am J Psychiatry 173:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner CH, et al. ; CORE/PRIDE Work Group (2016b) A novel strategy for continuation ECT in geriatric depression: phase 2 of the PRIDE study. Am J Psychiatry 173:1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TF, Lieberman DZ(2014)The utility of the combination of dextromethorphan and quinidine in the treatment of bipolar II and bipolar NOS. J Affect Disord 167:333–335. [DOI] [PubMed] [Google Scholar]
- Kent JM, Daly E, Kezic I, Lane R, Lim P, De Smedt H, De Boer P, Van Nueten L, Drevets WC, Ceusters M(2016)Efficacy and safety of an adjunctive mglu2 receptor positive allosteric modulator to a SSRI/SNRI in anxious depression. Prog Neuropsychopharmacol Biol Psychiatry 67:66–73. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB(2011)Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol 226:2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, Correll CU(2016)Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46:1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S(2011)Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224:107–111. [DOI] [PubMed] [Google Scholar]
- Kokkinou M, Ashok AH, Howes OD(2018)The effects of ketamine on dopaminergic function: meta-analysis and review of the implications for neuropsychiatric disorders. Mol Psychiatry 23:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA(2014)Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW(2014)A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry 76:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara DR, Bisol LW, Munari LR(2013)Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol 16:2111–2117. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC.(2011)Dextromethorphan as a potential rapid-acting antidepressant. Med Hypotheses 76:717–719. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC.(2012)An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan. Med Hypotheses 78:693–702. [DOI] [PubMed] [Google Scholar]
- Lawson RP, Nord CL, Seymour B, Thomas DL, Dayan P, Pilling S, Roiser JP(2017)Disrupted habenula function in major depression. Mol Psychiatry 22:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ(2010)Ketamine modulates theta and gamma oscillations. J Cogn Neurosci 22:1452–1464. [DOI] [PubMed] [Google Scholar]
- Leal G, Comprido D, Duarte CB(2014)BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 76 Pt C:639–656. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, Tzeng NS, Wang CL, Lee IH, Yeh TL, Yang YK, Lu RB(2012)The DRD2/ANKK1 gene is associated with response to add-on dextromethorphan treatment in bipolar disorder. J Affect Disord 138:295–300. [DOI] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CAJ(2017)Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry 81:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS(2014)BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18:pii: pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R (2011a) Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)Mtor-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011b) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK(2012)Brain-derived neurotrophic factor val66met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71:996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, Burgdorf J, Moskal J, Taylor J, Aghajanian G, Duman RS(2017)GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 42:1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B.(2003)BDNF and activity-dependent synaptic modulation. Learn Mem 10:86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, Jiang Q, Hou B, Pu J, Hu H, Wang Z(2016)Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: relevance to mood disorders. Biol Psychiatry 79:765–775. [DOI] [PubMed] [Google Scholar]
- Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, Gao CG, Hashimoto K(2013)Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 8:e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA Jr(2009)Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther 123:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Byrow Y(2016)Is treatment-resistant depression a useful concept?Evid Based Ment Health 19:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallei A, Baj G, Ieraci A, Corna S, Musazzi L, Lee FS, Tongiorgi E, Popoli M(2015)Expression and dendritic trafficking of BDNF-6 splice variant are impaired in knock-in mice carrying human BDNF Val66Met polymorphism. Int J Neuropsychopharmacol 18:pii: pyv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltbie E, Gopinath K, Urushino N, Kempf D, Howell L(2016)Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology (Berl) 233:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Gueorguieva R, Brandt C, Fava M, Sanacora G(2017)A randomized, double-blind, placebo-controlled, sequential parallel comparison design trial of adjunctive riluzole for treatment-resistant major depressive disorder. Neuropsychopharmacology 42:2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH(2005)Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660. [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW(2015)A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45:693–704. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M(2014)Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 156:1–7. [DOI] [PubMed] [Google Scholar]
- Messias E, Everett B(2012)Dextromethorphan and quinidine combination in emotional lability associated with depression: a case report. Prim Care Companion CNS Disord 14:PCC.12I01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ(2014)Glun2b-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 3:e03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ(2016)Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology 100:17–26. [DOI] [PubMed] [Google Scholar]
- Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ(2017)Synaptic regulation of a thalamocortical circuit controls depression-related behavior. Cell Rep 20:1867–1880. [DOI] [PubMed] [Google Scholar]
- Mion G, Villevieille T(2013)Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 19:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Kuno S(2001)Riluzole stimulates nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis in cultured mouse astrocytes. Neurosci Lett 310:117–120. [DOI] [PubMed] [Google Scholar]
- Moaddel R, Venkata SL, Tanga MJ, Bupp JE, Green CE, Iyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW(2010)A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta 82:1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D(1997)Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sanchez E, Gutierrez-Rojas L, Meana JJ(2018)Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs doi: 10.1007/s40263-018-0519-3. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Gideons E, Kavalali ET(2013)The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Malenka RC, Deisseroth K(2014)Depression: the best way forward. Nature 515:200–201. [DOI] [PubMed] [Google Scholar]
- Morais M, Patrício P, Mateus-Pinheiro A, Alves ND, Machado-Santos AR, Correia JS, Pereira J, Pinto L, Sousa N, Bessa JM(2017)The modulation of adult neuroplasticity is involved in the mood-improving actions of atypical antipsychotics in an animal model of depression. Transl Psychiatry 7:e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD(2014)GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 23:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013a) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ (2013b) Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, Lener M, Kautz M, Kim J, Stern JB, Price RB, Perez AM, Brallier JW, Rodriguez GJ, Goodman WK, Iosifescu DV, Charney DS(2015)Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med 45:3571–3580. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Mathew SJ (2017a) Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 16:472–486. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Wade E, Sayed S, Ahle G, Kiraly DD, Welch A, Collins KA, Soleimani L, Iosifescu DV, Charney DS (2017b) Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: a proof of concept clinical trial. J Affect Disord 218:277–283. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Mallei A, Popoli M(2013)The action of antidepressants on the glutamate system: regulation of glutamate release and glutamate receptors. Biol Psychiatry 73:1180–1188. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Tornese P, Sala N, Popoli M(2017)Acute or chronic? A stressful question. Trends Neurosci 40:525–535. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments (2015)Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 172:950–966. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Matsumoto RR(2015)Involvement of AMPA receptors in the antidepressant-like effects of dextromethorphan in mice. Behav Brain Res 295:26–34. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Robson MJ, Healy JR, Scandinaro AL, Matsumoto RR(2014)Involvement of sigma-1 receptors in the antidepressant-like effects of dextromethorphan. Plos One 9:e89985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA Jr(2018)Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry doi: 10.1038/s41380-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olin B, Jayewardene AK, Bunker M, Moreno F(2012)Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. Plos One 7:e48002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA(2010)GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol 58:123–147. [DOI] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A, Szewczyk B, Pilc A(2014)Activation of the mTOR signaling pathway in the antidepressant-like activity of the mGlu5 antagonist MTEP and the mGlu7 agonist AMN082 in the FST in rats. Neuropharmacology 82:59–68. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Lason-Tyburkiewicz M, Willner P(2017)Antidepressant, anxiolytic and procognitive effects of subacute and chronic ketamine in the chronic mild stress model of depression. Behav Pharmacol 28:1–8. [DOI] [PubMed] [Google Scholar]
- Park LT, Lener MS, Hopkins M, Iadorola N, Machado-Vieira R, Ballard E, Nugent A, Zarate CA Jr(2017)A double-blind, placebo-controlled, pilot study of riluzole monotherapy for acute bipolar depression. J Clin Psychopharmacol 37:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Niciu MJ, Zarate CA Jr(2015)Novel glutamatergic treatments for severe mood disorders. Curr Behav Neurosci Rep 2:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B, Fraser H, Wang C, Marcus R(2015)A randomized, double- blind, placebo-controlled, sequential parallel study of CERC-301 in the adjunctive treatment of subjects with severe depression and recent active suicidal ideation despite antidepressant treatment. Ann Arbor, Michigan: National Network of Depression Centers Annual Conference. [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM(2018)Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol Psychiatry 84:e3–e6. [DOI] [PubMed] [Google Scholar]
- Phillips J, Norris S, Talbot J, Ortiz A, Birmingham M, Blier P(2018)Antidepressant effects of single and repeated ketamine infusions in treatment-resistant depression [abstract]. Biol Psychiatry 83:S410. [Google Scholar]
- Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM; GLYX-13 Clinical Study Group (2015)Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract 21:140–149. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW(2008)An innovative design to establish proof of concept of the antidepressant effects of the NR2b subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ(2009)Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 66:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Tamburri P, Deptula D, Banken L, Beyer U, Rabbia M, Parkar N, Fontoura P, Santarelli L(2016)Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiatry 73:675–684. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG.(2017)The PRIDE study and the conduct of electroconvulsive therapy: questions answered and unanswered. J ECT 33:225–228. [DOI] [PubMed] [Google Scholar]
- Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA(2013)Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 27:444–450. [DOI] [PubMed] [Google Scholar]
- Reese AL, Kavalali ET(2015)Spontaneous neurotransmission signals through store-driven Ca(2+) transients to maintain synaptic homeostasis. Elife 4:doi: 10.7554/eLife.09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B(2016)Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 80:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB(2013)Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 38:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo B, Choucha W, Fossati P, Rotge JY(2015)Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res 230:682–688. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M(2006)Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Sackeim HA.(2001)The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62:10–17. [PubMed] [Google Scholar]
- Sakhaee E, Ostadhadi S, Khan MI, Yousefi F, Norouzi-Javidan A, Akbarian R, Chamanara M, Zolfaghari S, Dehpour AR(2017)The role of NMDA receptor and nitric oxide/cyclic guanosine monophosphate pathway in the antidepressant-like effect of dextromethorphan in mice forced swimming test and tail suspension test. Biomed Pharmacother 85:627–634. [DOI] [PubMed] [Google Scholar]
- Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA Jr(2016)An assessment of the anti-fatigue effects of ketamine from a double-blind, placebo-controlled, crossover study in bipolar disorder. J Affect Disord 194:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Johnson M, Khan A, Atkinson S, Riesenberg R, Schronen J(2014)Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and a history of inadequate response to antidepressants: primary results from a randomized, placebo controlled study (PURSUIT). Hollywood, FL: 53rd Meeting of the ACNP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ, Pathak S(2017)Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology 42:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Schatzberg AF(2015)Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders?Neuropsychopharmacology 40:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M(2012)Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Zarate CA, Krystal JH, Manji HK(2008)Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov 7:426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ.(1965)The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 122:509–522. [DOI] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M(2001)Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 41:151–158. [DOI] [PubMed] [Google Scholar]
- Shen G, Han F, Shi WX(2018)Effects of low doses of ketamine on pyramidal neurons in rat prefrontal cortex. Neuroscience 384:178–187. [DOI] [PubMed] [Google Scholar]
- Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO(2014)Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 155:123–129. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L (2016a) Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry 80:424–431. [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L (2016b) A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 173:816–826. [DOI] [PubMed] [Google Scholar]
- Song M, Martinowich K, Lee FS(2017)BDNF at the synapse: why location matters. Mol Psychiatry 22:1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, Pough KA, Prince TA, Ramsey NS, Savsani KH, Scandlen L, Cavaretta MJ, Raffa RB(2017)What is the mechanism of ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J Clin Pharm Ther 42:147–154. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM(2017)Effects of a ketamine metabolite on synaptic NMDAR function. Nature 546:E1–E3. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X(2015)An excitatory synapse hypothesis of depression. Trends Neurosci 38:279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornese P, Musazzi L, Sala N, Seguini M, Bonini D, Milanese M, Bonifacino T, Treccani G, Racagni G, Nyengaard JR, Wegener G, Bonanno G, Barbon A, Popoli M(2017)Ketamine restores changes in glutamate release, BDNF trafficking and dendrite morphology in the hippocampus of rats vulnerable to chronic mild stress. Washington, DC: Society for Neuroscience. [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M; STAR*D Study Team (2006)Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O(2000)Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 12:3567–3574. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M(2010)The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. [DOI] [PubMed] [Google Scholar]
- Wallace M, White A, Grako KA, Lane R, Cato AJ, Snodgrass HR(2017)Randomized, double-blind, placebo-controlled, dose-escalation study: investigation of the safety, pharmacokinetics, and antihyperalgesic activity of l-4-chlorokynurenine in healthy volunteers. Scand J Pain 17:243–251. [DOI] [PubMed] [Google Scholar]
- Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW(2015)Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76:247–252. [DOI] [PubMed] [Google Scholar]
- Wang M, Arnsten AF(2015)Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull 31:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T(2013)Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet 382:1575–1586. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr, Sanacora G(2018)The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 175:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM(2016)The rapidly acting antidepressant ketamine and the mglu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. [DOI] [PubMed] [Google Scholar]
- Workman ER, Niere F, Raab-Graham KF(2013)mTORC1-dependent protein synthesis underlying rapid antidepressant effect requires GABABR signaling. Neuropharmacology 73:192–203. [DOI] [PubMed] [Google Scholar]
- Workman ER, Niere F, Raab-Graham KF(2018)Engaging homeostatic plasticity to treat depression. Mol Psychiatry 23:26–35. [DOI] [PubMed] [Google Scholar]
- Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM(2018)NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry doi: 10.1038/s41380-018-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K(2015)R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang H, Hu J, Hu H (2018a) Lateral habenula in the pathophysiology of depression. Curr Opin Neurobiol 48:90–96. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H (2018b) Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554:317–322. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP(2006)Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res 147:1–25. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD(2016)NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD(2018)Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD (2018a) Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32:197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD (2018b) Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 70:621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DS, Manji HK(2004)An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry 161:171–174. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK(2006)A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW (2012a) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012b) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA(2013)A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry 74:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K(2014)R (-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. [DOI] [PubMed] [Google Scholar]