Abstract
Pleasure and motivation are important factors for goal-directed behavior and well-being in both animals and humans. Intact hedonic capacity requires an undisturbed interplay between a number of different brain regions and transmitter systems. Concordantly, dysfunction of networks encoding for reward have been shown in depression and other psychiatric disorders. The development of technological possibilities to investigate connectivity on a functional level in humans and to directly influence networks in animals using optogenetics among other techniques has provided new important insights in this field of research.
In this review, we aim to provide an overview on the neurobiological substrates of anhedonia on a network level. For this purpose, definition of anhedonia and the involved reward components are described first, then current data on reward networks in healthy individuals and in depressed patients are summarized, and the roles of different neurotransmitter systems involved in reward processing are specified. Based on this information, the impact of different therapeutic approaches on reward processing is described with a particular focus on deep brain stimulation (DBS) as a possibility for a direct modulation of human brain structures in vivo.
Overall, results of current studies emphasize the importance of anhedonia in psychiatric disorders and the relevance of targeting this phenotype for a successful psychiatric treatment. However, more data incorporating these results for the refinement of methodological approaches are needed to be able to develop individually tailored therapeutic concepts based on both clinical and neurobiological profiles of patients.
Keywords: depression, reward networks, functional MRI, functional connectivity, anhedonia
Introduction
Depression is a heterogenous diagnostic concept consisting of a set of different symptoms, with decreased mood, anhedonia, and reduced energy defined as core symptomatologies in ICD-10 and depressed mood and loss of interest and pleasure in DSM-5. Current subtypes of depression are based on clinical stratification without inclusion of neurobiological information and have only limited relevance for prognosis and treatment choice.
Scientific progress with refinement and evolution of the technical possibilities to investigate brain function have led to the perception that depression is a complex network disorder rather than a dysfunction of a few specific brain regions. Thus, the description of how clinical subdomains of depressive symptomatology translate into deficits in neurocircuitry is a relevant question. This viewpoint seems to be necessary for the definition of relevant diagnostic categories, the identification of valid prognostic factors, and the development of improved treatment recommendations.
With regard to clinical subdomains, anhedonia is likely to be of particular relevance. The fact that both ICD-10 and DSM-5 determine anhedonia and loss of pleasure, respectively, as a core symptom of depression highlights its importance in this disorder. Furthermore, it was shown that anhedonia is a negative prognostic factor for treatment outcome both in adolescents (McMakin et al., 2012) and adults (Spijker et al., 2001; Vrieze et al., 2014). Also, reduction of a symptom dimension named “interest-activity dimension” in a paper by Uher et al., defined as low interest and lack of enjoyment among other symptoms, was shown to be a predictor for poor outcome in depressed patients undergoing antidepressant treatment. This result was found in a large cohort of 811 patients with major depressive disorder (MDD) and consecutively replicated in a regression analysis in a larger population of 3737 MDD patients derived from the STAR*D trial within the same publication (Uher et al., 2012). Conversely, improvement of anhedonia was shown to significantly correlate with improvement of overall functioning in depressed patients (Llorca and Gourion, 2015), and improvement of anhedonia is a strong predictor for improvement in psychosocial functioning (Vinckier et al., 2017). It was also suggested that anhedonia might be a trait marker rather than an expression of a disease state, with persistence of reduced hedonic capacity in euthymic remitted patients (Di Nicola et al., 2013). Furthermore, early increase of positive emotions after start of antidepressant treatment defined by adjectives such as “satisfied,” “enthusiastic,” and “energetic” in a paper by Geschwind et al. predicted response and remission in MDD patients after 6 weeks (Geschwind et al., 2011).
It was proposed that current standard treatment of MDD, particularly selective serotonin reuptake inhibitors (SSRIs), fail to sufficiently treat reward-related symptoms of depression (Dunlop and Nemeroff, 2007; Price et al., 2009; Di Nicola et al., 2013). Therefore, there is a need for more effective treatment of anhedonia in depression. Recently, positive results have been reported for newer antidepressants such as ketamine and agomelatine in this regard. Agomelatine, which acts via the melatonergic system (MT1 and MT2 agonist) and as a antagonist on the 5-HT2C receptor, has shown promising results in a number of studies in this context (Kasper and Hamon, 2009; Di Giannantonio et al., 2011; Martinotti et al., 2012; Gargoloff et al., 2016).
Overall, a more detailed consideration of the role of anhedonia in depression seems to be worthwhile to explore. For this purpose, we wanted to provide an overview of current state of knowledge about reward processing in depression with a particular focus on network level alterations. However, this review is a selective one and is intended as a basis for the stimulation of further research on the topic more than a systematic overview. Thus, only studies explicitly focusing on reward processing in depression at baseline and during treatment were including but not all resting-state functional connectivity studies in depression, although relevance for reward processing might be indirectly given based on the involved regions.
Definition of anhedonia and possibilities of assessment
Anhedonia is defined as the reduced ability to feel pleasure in normally pleasurable situations and is a transdiagnostic key symptom for several psychiatric disorders such as MDD and schizophrenia. The clinical phenotype of anhedonia is thought to reflect a dysfunctional processing of reward on a neurobiological level. Since it was first described in the late 19th century, the knowledge about reward processing has evolved and it has become clear that anhedonia is an umbrella term including a number of different components of reward processing that are necessary for the ability to feel pleasure in response to an event (Berridge and Kringelbach, 2008). According to current concepts, and comprehensively summarized by Kring and Barch, 2014 and Rizvi et al., 2016, reward processing is a multi-step process starting with the establishment of an association between a given stimulus and a connected reward, which is then followed by the development of interest, desire, and anticipation defined as “state of readiness for a reward” (Kring and Barch, 2014; Rizvi et al., 2016). Based on the balance between expected value of a given stimulus and the expected effort to gain this reward, a “cost-benefit calculation” is performed and motivation in favor or opposed to the initiation of goal-directed behavior is formed (Kring and Barch, 2014). If motivation is strong enough, thus the expected reward subjectively exceeds the necessary effort, an action plan is constructed, followed by the behavioral component of reward in which sustained energy is expended to gain the expected reward. If the goal is achieved, the consummatory phase of reward processing occurs, leading to hedonic response. This whole cascade of events is then subject to feedback integration and reward-based learning, influencing future reward-related processes.
The definitions of these different subdomains were shown to be relevant for the understanding of neurobiological processes underlying reward processing, because modulation of distinct circuits or local transmitters were shown to influence these in a specific manner. In remitted bipolar patients, for example, who display increased levels of anhedonia compared to healthy volunteers, only some aspects of hedonic capacity, namely interests and social interactions, seem to be impaired (Di Nicola et al., 2013). In animal models of depression, dopamine depletion in the nucleus accumbens (NAc) was shown to lead to a lack of motivation as expressed by reduction of appetitive seeking (Berridge and Robinson, 1998) but not to a reduction of liking (orofacial expression to sucrose and disgust reaction to quinine) (Wise and Raptis, 1986). Furthermore, it was suggested that treatment does not influence all aspects of reward processing to the same extent, with some studies showing stronger effects on reward anticipation than consummatory aspects of reward.
In humans, anhedonia can be assessed with questionnaires or reward-specific tasks as used in neuroimaging studies, for example. The most frequently applied anhedonia-specific rating scales include the Snaith-Hamilton Pleasure Scale (Snaith et al., 1995), the Revised Chapman Physical Anhedonia Scale (Chapman et al., 1976), and the Fawcett-Clark Pleasure Scale (Fawcett et al., 1983). These tests evaluate different domains such as social interaction and sensory experience among others and were shown to have good test validity. Other tests were designed to more specifically investigate subdomains of anhedonia such as the Temporal Experience of Pleasure Scale (Gard et al., 2007), which measures anticipatory and consummatory pleasure, the Anticipatory and Consummatory Interpersonal Pleasure Scale, which measures anticipatory pleasure and consummatory pleasure with regard to social context (Gooding and Pflum, 2014), or the Behavioral Activation for Depression Scale (Kanter et al., 2007), which measures motivated behavior (Der-Avakian et al., 2016).
Computer-based tasks were shown to selectively investigate different subdomains of anhedonia, such as reward learning, reward anticipation, and consummatory phase of reward often using monetary rewards as stimuli. These computer-based tasks were created to provide the possibility to objectively investigate reward processing; this approach was described to be particularly important in the context of translation of results (Der-Avakian et al., 2016). For a more detailed review of questionnaire-based and behavioral methods to assess anhedonia in depression, readers are referred to Rizvi et al. 2016.
Studies in humans, which are mainly based on self-report questionnaires, lack adequate comparability with results gained from behavioral tasks in animals. Furthermore, interpretation of animal data in the context of human pathophysiology is often challenging (Der-Avakian et al., 2016). For animal studies, new technological possibilities have facilitated a more detailed analysis of this processes on a causal level, using optogenetic methods or genetically modified animals. However, a major challenge exists in the translation of these results in humans. This is particularly true in females because current animal data is based nearly exclusively on male animals. This issue was recently addressed in a review and readers are referred to Heshmati and Russo (Heshmati and Russo, 2015). Also, the importance of differentiating the various aspects of reward processing has expanded in the last years, and thus more specific tasks and methods in humans have been developed to facilitate a better translation of preclinical results.
Which networks for reward have been detected?
A number of reviews focusing on reward circuits in animals and humans have been published (Haber and Knutson, 2010; Russo and Nestler, 2013; Keiflin and Janak, 2015). The most consistently described reward network is the dopaminergic mesolimbic pathways originating in the ventral tegmental area (VTA) and spreading onto the NAc located in the ventral striatum (VS), bed nucleus of the stria terminalis, amygdala, and hippocampus (Dunlop and Nemeroff, 2007). This evidence was recently further investigated in an animal study using optogenetics, which showed that activation of the VTA leads to dopamine release in the NAc (Tsai et al., 2009; for a review, see Lenz and Lobo, 2013). A total 60% of efferent projections of the VTA are dopaminergic, although it contains glutamatergic and GABAergic neurons also (Dobi et al., 2010). The NAc has also mixed dopaminergic and glutamatergic afferent connections from the VTA and the thalamus, prefrontal cortex (PFC), hippocampus and amygdala, and efferent GABAergic projections to the ventral pallidum and the VTA via its primary outputs, which run through the mediodorsal thalamus to the cortex. The complex relationship and interconnections of the involved regions outline the role of the NAc as integral hub for corticolimbic circuitry (Heshmati and Russo, 2015). In addition to this main reward axis, the medial PFC (mPFC) has been considered as core region of “reward processing,” which, together with the VTA and the NAc, forms the classical “mesocorticolimbic reward circuit”. Ferenczi et al. comprehensively investigated the modulatory relationship between the striatum and the PFC and the neurobiological correlates of reward-seeking behavior in rats using a multimodal approach including functional magnetic resonance imaging (fMRI) and optogenetics (Ferenczi et al., 2016). This study led to a number of relevant results. The optogenetic stimulation of dopaminergic neurons in the midbrain led to ipsilateral increases of the blood oxygenation level dependent (BOLD) signal in the dorsal and VS. On a behavioral level, blue light, inducing dopaminergic stimulation, was self-administered by the rodents as an expression of its rewarding effect. Furthermore, increased excitability of the mPFC, as shown in depression, led to reductions of BOLD signal in response to stimulation of midbrain dopaminergic neurons in the striatum and of reward-seeking behavior as measured with the sucrose preference test and social interaction test (Ferenczi et al., 2016). Thus, the results of this study implicate that the undisturbed interaction between the striatum and mPFC via the dopaminergic transmitter system is essential for the functioning of reward-related behavior. Disruption of this circuit as demonstrated by increased mPFC excitability might be a correlate for dysfunctional reward processing and anhedonia. Besides the mPFC, the orbitofrontal cortex (OFC) was shown to be implicated in reward processing. In a review by Berridge et al., it was stated that the OFC is “best thought as an important nexus for sensory integration, emotion processing and hedonic experience” (Berridge and Kringelbach, 2008). However, the importance of the OFC has been challenged in this and other work (Berridge and Kringelbach, 2008; Stalnaker et al., 2015). Importantly, it has to be highlighted that the ventromedial PFC (vmPFC) and the OFC, although they differ in terms of function, are two highly interconnected, overlapping regions with limited possibilities to clearly distinguish these two areas in lesioning and other studies depending on the methodology (Pujara and Koenigs, 2014).
More recently, it was shown that additional regions are involved in reward-related processes in a differentiated manner; thus, the hypothalamus, lateral habenula (LHb), and dorsal striatum have been detected as major players in this regard.
The LHb has dopaminergic projections to the substantia nigra pars compacta and the VTA as well as serotonergic projections to the median and dorsal raphe nuclei (Herkenham and Nauta, 1979). It was shown that the LHb has an inhibitory influence on dopaminergic neurons (Matsumoto and Hikosaka, 2007) via GABAergic interneurons in the rostromedial tegmental nucleus (Ji and Shepard, 2007; Jhou et al., 2009), which is reflected by the fact that lesioning of the habenula leads to an increase of cortical and striatal dopamine levels (Nishikawa et al., 1986). On a behavioral level, it was shown that the habenula has a inhibitory effect on dopaminergic neurons in reinforcement learning. Thus, using a reward prediction error paradigm in monkeys with a smaller than expected reward led to activation of LHb neurons, whereas a larger than expected reward led to their inhibition (Matsumoto and Hikosaka, 2007). For a review on habenula function, see (Hikosaka, 2010).
In terms of fibers that mediate reward, the medial forebrain bundle (MFB) has gained increased attention, as it has become a potential target for DBS treatment (see below). This structure connects the VTA with the NAc via the mesolimbic dopaminergic connection and the VTA with the PFC via the mesocortical dopaminergic connection (Döbrössy et al., 2015).
Which reward networks are disturbed in major depression?
In 2006 a review by Nestler and Carlezon focusing on the mesolimbic reward circuit in depression was published. In this article, the authors stressed the need to “better define the detailed circuitry of the numerous and diverse molecular pathways” of the VTA-NAc pathways partly “because the depression field has focused largely on serotonergic and noradrenergic mechanisms in other brain circuits (e.g., hippocampus and cortex)” (Nestler and Carlezon, 2006). Since then, a number of studies have been published linking the dopaminergic reward circuit and depression (Table 1).
Table 1.
Overview of studies evaluating reward processing in MDD using fMRI and functional connectivity analysis.
| Authors | Year | n (total) | n (patients) | n (HC) | Task/Stimulus | ROI/Seeds | Observed Effects (HCs vs MDDs) Decreased (↓)/ Increased (↑) Functional Connectivity or Activiton in fMRI |
|---|---|---|---|---|---|---|---|
| Johnston | 2015 | 40 | 20 MDD | 20 | Modified Pessiglione task | Dorsal raphe nuclei | ↑: Significant hippocampal overactivity during loss events in MDD compared to HC |
| Cheng | 2016 | 909 | 421 MDD | 488 | None | Voxel-level BWAS |
↓: Altered functional connectivity in MDD in circuit involving the medial OFC (BA 13), the parahippocampal gyrus, and medial temporal lobe (perirhinal cortex BA 36 and entorhinal cortex BA 28) |
| Dombrovski | 2015 | 78 | 47 MDD | 31 | Probabilistic learning | Striatum, left operculoinsular cortex, right vlPFC, bilateral STG, bilateral thalamus, PCC | ↓: Disrupted differential connectivity between striatum and operculo insular cortex in response to unpredicted rewards vs. unpredicted punishments in MD |
| Gong | 2017 | 123 | 80 MDD | 43 | None | NAc | ↓: Decreased FUCO between NAc and caudate, mid OFC and mOFC, rACC, insular lobe, superior temporal gyrus |
| Greenberg | 2015 | 148 | 148 MDD | 0 | Monetary reward | VS | ↓: Greater anhedonia severity was associated with a reduced reward expectancy-prediction error inverse relationship in the VS |
| Pizzagalli | 2009 | 61 | 30 MDD | 31 | Monetary incentive delay | - | ↓: MDD participants showed significantly weaker responses to gain in the left NAc and bilateral caudate |
| Robinson | 2012 | 27 | 13 MDD | 14 | Mixed reward and punishment learning | Bilateral caudate and putamen | ↓: Impaired reward (but not punishment) reversal accuracy was found alongside attenuated anteroventral striatal response to unexpected reward in MDD |
| Young | 2016 | 43 | 21 MDD | 22 | Pleasant/unpleasant sound stimuli | pVMPFC | ↓: Negative correlation between anhedonia and FUCO between pVMPFC and NAc, left VTA/SN, left OFC and right mid- insula and pVMPFC to middle temporal gyrus/superior temporal sulcus, right inferior frontal gyrus pars opercularis |
All studies investigating reward processing in depression in MDD vs healthy volunteers are included, if functional connectivity was also analyzed these results are also specified.
Abbreviations: BA, Broca area; BWAS, brain-wide association study; FUCO, functional connectivity; HC, healthy controls; MDD, major depressive disorder; mOFC, medial orbitofrontal cortex; midOFC, middle orbitofrontal cortex; NAc, Ncl. accumbens (ventral striatum); OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; pVMPFC, posterior ventromedial prefrontal cortex; rACC, rostral ACC; STG, superior temporal gyrus; vlPFC, ventrolateral prefrontal cortex; VS, ventral striatum; VTA, ventral tegmental area.
On a neural activation level, studies have evaluated reward processing often using fMRI with regions-of-interest (ROIs) in the “classical” dopaminergic reward-associated regions such as the VS. As hypothesized, these studies revealed significantly altered activation of reward-related regions such as the VS, thalamus, insula, and prefrontal regions during reward anticipation and consummatory reward phase. A meta-analysis of 22 fMRI studies including a total of 341 patients and 367 controls found decreased activation mainly in subcortical and limbic areas (caudate, thalamus and anterior cingulate cortex [ACC], putamen and insula) during reward processing in patients with MDD. An increased neural activation was found in cortical regions (cuneus, middle, and superior frontal gyrus, fusiform gyrus, and lingual gyrus). When distinguishing between different aspects of reward processing, namely reward anticipation and consummatory pleasure, reduction of caudate response was reported for both conditions along with an increased activation in the middle frontal gyrus and dorsal ACC during reward anticipation in patients vs healthy controls (HCs) (Zhang et al., 2013).
Several studies showed a disruption of striatal activity during reward learning, prediction-error encoding, and rewarding outcomes (Table 1) (Kumar et al., 2008; Gradin et al., 2011; Robinson et al., 2012). In line with these results, an fMRI study in a large sample of unmedicated MDD patients showed that depressed patients show a disruption of the inverse correlation between reward expectancy and prediction error-associated reactivity in the VS, which is found in healthy volunteers (Greenberg et al., 2015). This disruption was associated with anhedonia severity both in HCs and MDD patients. The association between decreased activation of the striatum during prediction-error processing and anhedonia severity was also found in other studies in MDD patients (Gradin et al., 2011). It was also proposed that the capacity to modulate the striatal activity and neural activation of the putamen in response to different sizes of reward is disrupted in MDD patients compared with HCs. Importantly, this difference in the VS was reduced by SSRI treatment and correlated with the extent of symptom reduction (Takamura et al., 2017). Furthermore, reduced activation during the consummatory phase of reward was shown for the NAc and caudate in MDD patients compared with HCs (Pizzagalli et al., 2009; Redlich et al., 2015).
It was suggested that depression is associated with deficits in reward motivation to a greater extent than consummatory aspects of reward (Chentsova-Dutton and Hanley, 2010; Lally et al., 2014). However, this fact is not supported by other studies that showed significantly reduced response to rewarding monetary outcomes in the NAc and the caudate (Pizzagalli et al., 2009) and equally disrupted anticipatory and consummatory pleasure in daily activities in depressed patients (Wu et al., 2017).
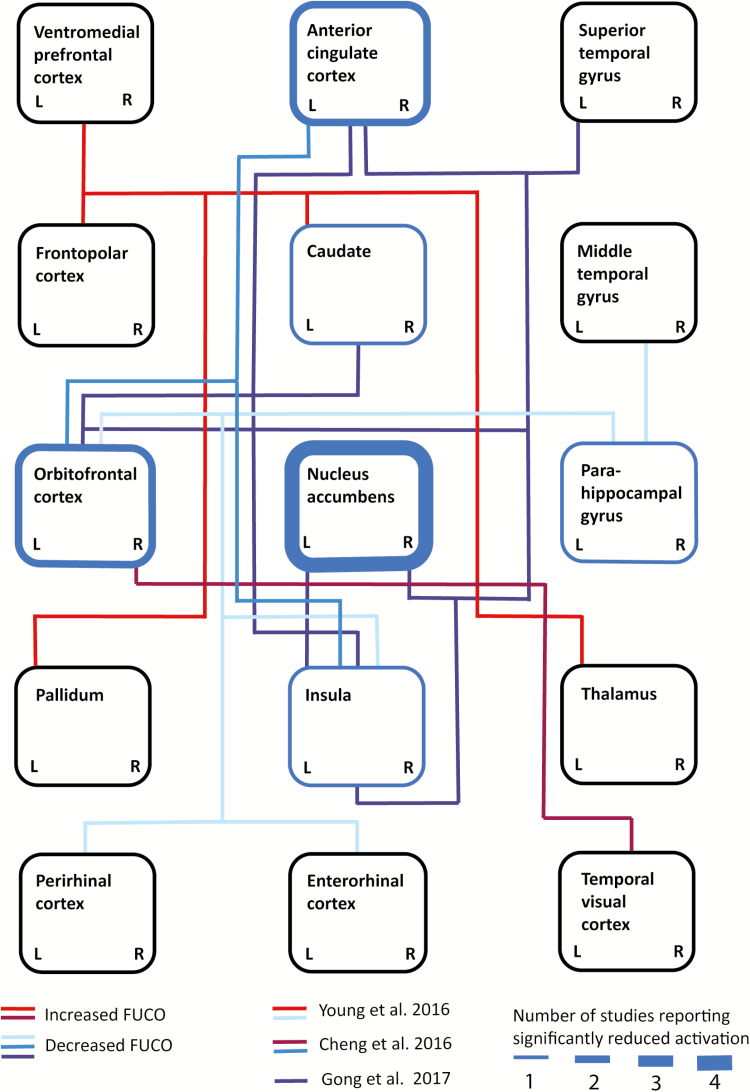
Compared with fMRI studies investigating neural activation, less data are available on the disruption of reward processing in MDD on a network level (Kraus et al., 2017b; Spies et al., 2017; Kraus et al., 2018). These studies paint the picture of significantly disturbed interaction between cortical and subcortical brain regions in depression as a correlate of dysfunctional reward processing. In a resting-state fMRI study in 75 unmedicated MDD patients and 42 HCs, disrupted functional connectivity was reported for the NAc with the caudate, medial OFC, rostral ACC, superior temporal gyrus (left), insular lobe, dorsomedial PFC (dmPFC), and dorsal ACC (Gong et al., 2017). A recent investigation in a large cohort of 421 MDD patients and 488 controls using a “brain-wide” voxel level analysis found a significant reduction in functional connectivity of the medial OFC, BA 13, which is known to be linked to reward processing (Cheng et al., 2016). Overall, studies report a significant reduction in neural activation in the caudate, NAc, OFC, and ACC. From a network perspective, the NAc has been described as a central hub, with reduced functional connections to cortical regions such as the OFC and ACC as well as reduced connectivity between cortical regions such as the insula, ACC, and OFC (Figure 1). However, results are too heterogenous to make a definite statement about a pattern of MDD-associated alterations of reward processing on both a neural activation and a network level.
Figure 1.
Schematic depiction of altered functional connectivity and neural activation during reward processing in patients with major depressive disorder (MDD) at baseline. Blue borders of rectangles indicate brain regions with significantly reduced activation during reward processing in depressed patients vs healthy controls (HCs; line thickness indicates the number of replications, as reported by Kumar et al. 2008; Pizzagalli et al. 2009; Redlich et al. 2015; Robinson et al. 2012). Blue-colored connections indicate decreased functional connectivity and red-colored connections indicate increased functional connectivity between areas as measured by functional magnetic resonance imaging (fMRI). Different connectivity studies are distinguishable by different colors. L and R serve to indicate hemispheric differences: changes represented by lines connecting laterally in the cartoon were reported only for the represented side (Cheng et al. 2016: blue and burgundy; Gong 2017: dark red; Young 2016: light blue and vermilion).
During reward processing, more specifically loss vs reward conditions, higher coupling between the VS and prefrontal areas, including the ACC, as well as connectivity of the VS and the left caudate and mid-cingulate was shown, which was mainly driven by higher connectivity during loss/disappointment vs relief/win (Quevedo et al., 2017). Furthermore, increased connectivity between the NAc and the VTA in MDD patients vs HCs was described during reward processing in an fMRI task focusing on reward feedback (Redlich et al., 2015). In late-life depression, MDD patients showed significantly reduced increase of functional connectivity between the striatum and operculoinsular cortex (left), the ventrolateral PFC (right), temporal gyrus, thalamus, and posterior cingulate cortex (PCC) in response to unpredicted reward vs unpredicted punishment compared with age-matched nondepressed controls (Dombrovski et al., 2015). A study using music listening vs scrambled stimuli as reward paradigm found a negative correlation between anhedonia and functional connectivity of the posterior vmPFC (seed region) and the NAc, VTA/substantia nigra, OFC (left), and insula (right) (Young et al., 2016). Thus, results point toward baseline disruption of subcortical connectivity and functional coupling between striatal and frontocortical regions. However, the direction of these changes are not consistent, which might be related to differences in used stimuli and investigated reward subdomains (Table 1).
Some studies have evaluated differences in functional connectivity between treatment responders and nonresponders with regard to reward networks. Applying resting-state fMRI and network-based statistics, it was proposed that treatment-resistant depression (TRD) patients and treatment-sensitive depression (TSD) patients differ in terms of disrupted subnetworks. In a study by He et al., TRD vs TSD patients showed more extensive hypoconnectivity of OFC, limbic regions, lateral parietal cortex, lateral temporal cortex, and medial/inferior occipital cortex (He et al., 2016). In another study, TRD patients showed disruption of functional connectivity of the OFC and reduced volume of the caudate compared with TSD patients. When using the caudate as seed region, significantly decreased connectivity to frontal regions, more precisely the middle and inferior frontal gyrus, were found (Ma et al., 2012). Disturbed function of the caudate in TRD patients was also found in a single-photon emission computed tomography study comparing cerebral perfusion between TRD patients and healthy individuals along with fronto-temporal hypoperfusion, including the ACC and the insula (Richieri et al., 2015). With relevance to reward processing, a translational study in an animal model of TRD (congenital learned helplessness [cLH]) showed significant differences in relative cerebral blood flow within the LHb in rats suffering from cLH compared with non-cLH rats. In the same study, significant differences in functional connectivity were found within regions with major relevance to serotonergic transmission such as between the dorsal raphe nucleus (DRN) and the OFC (Gass et al., 2014). Overall, studies propose that TRD is associated with more pronounced hypoconnectivity between cortical regions, mainly in frontal areas and subcortical areas such as the striatum, compared with TSD. However, differences in reward tasks used, analysis approaches, and presentation in published material hamper the comparability of results and their summarized presentation (Figure 1). Furthermore, the analysis of functional connectivity allows for only the indirect measurements of connections between areas because it represents the statistical analysis of synchronous fluctuations in neural activity of two brain regions, without statement about anatomical tracts and fibers as well as uncertainty if this connectivity might be based on the shared modulation by a third region or if other regions are interposed in this connection, for example.
Only recently have efforts been made to allocate neurobiological changes to distinct clinical phenotypes. Using a machine-learning approach, different “biotypes” have been defined based on fMRI resting-state connectivity patterns and clinical data (Drysdale et al., 2017). This method led to a number of relevant results in the context of this review. First, a “neuroanatomical core pathology” was proposed including reward-related areas such as the insula, OFC, vmPFC, and VS among other subcortical areas. It was further described that 3 “core” symptoms were present in >90% of included patients, one of which was anhedonia (96.7%). In addition to this shared pattern of dysfunctional connectivity, 4 distinct biotypes were found, each associated with a specific symptom profile. Particularly, 2 biotypes should be highlighted that showed hyperconnectivity in thalamic and frontostriatal networks and were related to increased anhedonia and psychomotor retardation. These biotypes showed different response rates for repeated transcranial magnetic stimulation (rTMS) treatment of the dmPFC and the combination of biotype classification and connectivity features had a high predictive accuracy of 89.6% (Drysdale et al., 2017).
Which neurotransmitters are involved?
The dopaminergic system is traditionally associated with reward processing, which has been confirmed in a number of preclinical and clinical studies. More recently, interactions with other transmitter systems such as the serotonergic, opioid, and glutamatergic systems have broadened the picture of mediation of reward on a transmitter level. It has been described that a number of brain regions, such as the frontal cortex and the hippocampus, provide glutamatergic input to the VTA and NAc (Nestler, 2015). In fact, the VTA contains not only dopaminergic neurons, which constitute about 60% of all VTA neurons, but also GABAergic (25%) and glutamatergic (15%) neurons (Volman et al., 2013). It has been suggested that different transmitter systems might mediate different domains of reward, with consummatory pleasure being linked to the opioid and serotonergic system and anticipatory pleasure mainly to dopaminergic mechanisms (Barbano and Cador, 2007; Der-Avakian et al., 2016). In the following section, results regarding the implicated transmitter systems will be summarized.
Dopaminergic System
It is largely known that the dopaminergic transmitter system is strongly linked to reward processes. It was shown in positron emission tomography (PET) studies that reward leads to dopamine release in the striatum and more specifically in the NAc as indicated by reduction of the binding potential of the dopamine receptor subtype 2 using the radioligand [11C] raclopride (Koepp et al., 1998; Zald et al., 2004; Jonasson et al., 2014). A multimodal study using the same radioligand and fMRI during a monetary incentive delay (MID) task could show a correlation between dopamine release, again measured using [11C] raclopride, and hemodynamic response in the substantia nigra/VTA and the VS during reward anticipation in fMRI (Schott et al., 2008). Importantly, the concept of reward prediction-error has provided an elegant first explanation on how dopamine may drive reward learning and reward choice. It was thus shown that dopaminergic neurons increase the firing rate for unpredicted reward outcomes only, whereas expected rewards provoke no change in dopamine firing rate. The increase of firing rate is affected in a size-dependent manner (Schultz, 2016). This is also true for aversive stimuli, with decreased firing rates of dopaminergic neurons below baseline.
Serotonergic and Glutamatergic System
It was shown that reward-related areas, both cortical and subcortical, show extensive innervation by serotonergic neurons, which originate in the raphe nuclei. It was further reported that the serotonergic transmitter system has a modulatory impact on the reward network via different receptors such as the 5-HT1A, 5-HT2A receptors, and the serotonin transporter (5-HTT) (Lanzenberger et al., 2012; Hahn et al., 2014; Kraus et al., 2014; Spies et al., 2015). A recent meta-analysis reported that MDD is associated with a significant reduction of the 5-HTT in the midbrain and striatum (Gryglewski et al., 2014). Although the serotonergic system has been mainly associated with consummatory pleasure, some evidence suggests an involvement of serotonin in reward anticipation (Marutani et al., 2011). Thus, animal data show tonically increased firing of serotonergic neurons in the DRN during reward anticipation (Li et al., 2016). For a review of the role of the serotonergic system in reward processing, see also Kranz et al. (Kranz et al., 2010). Importantly, an animal study showed that the DRN increases firing rates during reward-associated tasks and that effects are mediated via the serotonergic and glutamatergic system on a postsynaptic level. Optogenetic stimulation of DRN neurons led to exploration of stimulation-coupled spatial regions, shift in sucrose preference, optical self-stimulation, and guidance of sensory discrimination learning on a behavioral level. Blockage of either serotonin synthesis or glutamate release resulted in partial impairments in reward experiments (Liu et al., 2014). It is believed that the interaction between glutamate and striatal activity serves as an important modulator of synaptic plasticity and neural excitability (Surmeier et al., 2007; Kreitzer et al., 2009; Lovinger et al., 2010; Gleich et al., 2015). Especially glutamatergic innervation originating in the frontal cortex has been highlighted in this regard, with studies reporting an influence of glutamatergic frontal neurons on dopaminergic neurotransmission in the VS via GABAergic neurons (Carlsson et al. 1999) and associations between glutamate concentration and fronto-limbic functional connectivity (Duncan et al. 2013).
Opioid System
The opioid system in the NAc shell and the ventral pallidum has been shown to mediate consummatory pleasure, with 2 to 3 times increases of hedonic experience to sucrose taste when a microinjection of neuronal opioid was performed in these areas (Berridge and Kringelbach, 2008). However, in the recent years, a more complex role of the opioid system has been proposed, including its involvement in the mediation of incentive motivation and learning via μ- and δ- opioid receptors in the NAc; for a detailed review, see also Laurent et al. 2015.
Treatment studies: effects on a network level?
Based on the described baseline differences between MDD patients and HCs, some studies have investigated how different treatment approaches affect reward processing (Table 2). These studies involved classical antidepressant drugs such as SSRIs but also “newer” antidepressant drugs such as ketamine (Kraus et al., 2017a, 2017c) and agomelatine (Kasper and Hamon, 2009; Di Giannantonio et al., 2011; Martinotti et al., 2012; Gargoloff et al., 2016) as well as nonpharmacological neuromodulatory approaches (TMS, DBS, ECT) (Lanzenberger et al., 2013) and psychotherapy (Dichter et al., 2009; Walsh et al., 2017).
Table 2.
Overview of studies investigating treatment effects of different therapeutic approaches to determine effects on reward processing in MDD.
| Authors | Year | n (total) | n (patient) | n (HC) | Task/Stimulus | Therapeutic Intervention | ROI/Seeds | Observed Effects Decreased (↓)/Increased (↑) FUCO or Activation in fMRI |
|---|---|---|---|---|---|---|---|---|
| Admon | 2017 | 43 | 46 MDD | 43 | Monetary incentive delay | Amisulprid (50mg); MDD: (23*, 23 Pl); HC: (23*, 20Pl) | Caudate, NAc, putamen | ↑: Increased FUCO between striatum and dorsal ACC and midcingulate cortex in response to reward outcomes in MDD treated with Amisulprid vs. placebo |
| Dichter | 2009 | 27 | 12 MDD | 15 | Wheel of fortune | Behavioral Activation Treatment for Depression | - | ↑: Increased dorsal striatal activation during reward anticipation after BATD in MDD |
| Downar | 2014 | 47 | 38 TRD, 9 TRBPD | 0 | None | rTMS (DMPFC), 20 sessions | 516 | ↑: Responders: increased VMPFC connectivity to VTA, left caudate nucleus, left DMPFC, left DLPFC, left Occipital cortex, right anterior insula, and the rigth Precuneus; Non-responders: increased connectivity to the right fusiform gyrus, right DLPFC, right PCC, right Middle temporal gyrus, right frontopolar cortex, left MCC and left Middle paracingulate cortex |
| Quevedo | 2017 | 68 | 38 MDD | 30 | Monetary reward | Various antidepressants in MDD groups | Bilateral VS | ↑: Increased FUCO between VS and midline cortical areas during loss versus reward trials.in MDD; depression severity linked to higher VS to caudate and precuneus connectivity |
| Carl | 2016 | 53 | 33 MDD | 20 | Monetary incentive delay | Brief Behavioral Activation Treatment for Depression | NAc, caudate nucleus, putamen, frontal medial cortex, OFC, and ACC | ↓: Decreased activity in MDD group when comparing first and second run of the task reflecting decreased capacity for sustained activation of the right NAc |
| Redlich | 2015 | 100 | 33 UD, 33 BD | 34 | Car-guessing paradigm | Various medications in all but 2 patients | NAc | ↓: Decreased activity in UD and BD patients compared to HC in the NAc during reward processing |
| Takamura | 2017 | 24 | 12 MDD | 12 | Variable sized monetary reward | Medication for less than 7 days | Left VS. bilateral putamen | ↓: In HC, striatal activity increased in proportion to the size of monetary reward during reward anticipation. This was altered in MDD patients, and significant group-by-reward size interaction effects detected in ROIs |
| Duprat | 2017 | 50 | 50 MDD | 0 | Probabilistic learning | Accelerated intermittent TBS | - | ↑↓: No significant group differences found in treatment vs sham condition. Patients with low anhedonia displayed higher activity in caudate and putamen at baseline and reduced activity in the reward system after one week of treatment. High anhedonia was linked to increased activity in the reward system after one week of treatment. |
Studies including functional MRI and/or functional connectivity analysis in MDD patients receiving pharmacological or non-pharmacological treatment are included.
Abbreviations: ACC, anterior cingulate cortex; BA, broca area; BATD, behavioral activation treatment for depression; BD, bipolar depression; DLPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; FUCO, functional connectivity; HC, healthy controls; MDD, major depressive diorder; MCC, midcingulate cortex; NAc, nucleus accumbens; OFC, orbitoftrontal cortex; PCC, posterior cingulate cortex; PFC, prefrontal cortex; Pl, placebo; pVMPFC, posterior ventromedial prefrontal cortex; rTMS, repeated transcranial magnetic stimulation; STG, superior temporal gyrus; TRD, treatment-resistant depression; TBS, theta burst stimulation; UD, unipolar depression; VMPFC, ventromedial prefrontal cortex; VS, ventral striatum; VTA, ventral tegmental area.
Serotonergic Drugs
Studies examining the effect of serotonergic drugs on reward processing used fMRI and different reward-related tasks, including financial, sensory, and erotic stimuli in HCs. These studies revealed significant effects both of acute and prolonged treatment with drugs enhancing serotonergic transmission such as SSRIs and SNRIs and suppression of serotonergic transmission using acute tryptophan depletion in HCs (for a review, see (Macoveanu, 2014). Only limited data are available for patients suffering from major depression. Stoy et al. showed that MDD patients exhibit reduced activation of the VS during reward anticipation compared with HCs in an MID task. After 6-week treatment with escitalopram, this difference was no longer measurable (Stoy et al., 2012). However, another study using citalopram found a reduction of the activation within the VS as well as the vmPFC/OFC to positive stimuli and of the OFC in response to aversive stimuli (McCabe et al., 2010). These results could reflect the fact that anhedonia has a rather low response to SSRI treatment compared with other symptoms (Opbroek et al., 2002; Price et al., 2009). It was, for example, shown that approximately 40% of the patients achieving remission after 8 weeks of fluoxetine treatment still exhibited residual diminished pleasure or interest (Nierenberg and Wright, 1999). With relevance to reward processing, it was shown that SSRI treatment leads to changes in interregional relation of 5-HTT availability, for example, the ACC, insula, and putamen, as measured with the radioligand [11C]DASB and PET (James et al., 2017).
Dopaminergic Drugs
Drugs with assumed modulatory impact on the dopaminergic system, such as bupropion, either did not have reliable effect on the dopaminergic neurotransmitter system in therapeutic dosages or failed to have enough efficacy on overall depressive symptoms. PET and single-photon emission computed tomography (SPECT) studies using different tracers in individuals treated with bupropion revealed rather low occupancy rates of 14% to 26% (Meyer et al., 2002; Learned-Coughlin et al., 2003; Argyelán et al., 2005). However, a study about residual symptoms in depressed patients treated with a variety of different antidepressants (SSRIs, SNRIs, tricyclic antidepressants, NaSSa, bupropion) reported a lower rate of anhedonia in patients treated with bupropion (33%) compared with an overall rate of 46% (Goodwin et al., 2017). Using nonantidepressant drugs with a mainly dopaminergic mechanism of action, Admon et al. investigated if amisulpride, which is thought to provoke a dose-dependent increase of dopaminergic neurotransmission via autoreceptor blockage, might lead to changes of reward processing on a behavioral and neurobiological levels as measured with fMRI and an MID task (Admon et al., 2017). Results of this study showed that patients receiving amisulpride showed increased neural activation in the striatum in response to reward cues and increased functional connectivity between the NAc and the midcingulate cortex in response to reward outcomes compared with patients receiving placebo. This effect was not mirrored by significant normalization of reward processing on a behavioral level; the authors therefore concluded that longer term treatment might be necessary for behavioral effects (Admon et al., 2017).
Glutamatergic Drugs
Lally et al. proposed a favorable effect of ketamine on anhedonia in bipolar patients currently experiencing a depressive episode (Lally et al., 2014). Their study investigated whether a single infusion of ketamine might lead to a significant reduction of anhedonia as measured with the Snaith-Hamilton Pleasure Scale questionnaire. Results showed that ketamine had significant effects on anhedonia, which lasted for up to 2 weeks and were accompanied by a significant increase of metabolism in the VS (Lally et al., 2014). The functional circuit between the hippocampus and the mPFC was shown to be of particular importance for the antidepressant response of ketamine in rats; thus, recent investigations aimed to specifically target the hippocampus to extract ketamine-like antidepressant effects without side effects (Carreno et al., 2016).
Psychotherapy
With regard to psychotherapy (more specifically, behavioral activation therapy for depression [BATD]), it was proposed that MDD patients show a lack of capacity of sustained activation during reward outcomes in the NAc compared with HCs at baseline. BATD, which uses a reward-oriented approach, led to functional changes in prefrontal regions during reward selection and the dorsal striatum during reward anticipation using a wheel-of-fortune task (Dichter et al., 2009). Anhedonia was again a predictive marker for response, possibly because of the specificity of this treatment approach for symptoms of anhedonia (Walsh et al., 2017).
With regard to TMS, nonresponders showed significant reduction of functional connectivity of the vmPFC to the caudate and VTA. Importantly, at baseline, nonresponders scored significantly higher in reward-related items of the Becks Depression Inventory and Quick Inventory of Depressive Symptomatology compared with responders to rTMS (Downar et al., 2014). Furthermore, different aspects of reward (consummatory, appetitive), which were assessed clinically, were found significantly predictive of nonresponse in this study. However, the predictive effect was not replicated in another study using theta burst stimulation (TBS) in depressive patients. In this study, effects of stimulation in low- vs high-anhedonia MDD patients on striatal activity were described using a probabilistic learning task in fMRI after completed treatment. In patients with high anhedonia load, neural activity increased in the caudate and putamen after TBS, whereas patients with lower anhedonia displayed decreased activity in the right striatum after treatment (Duprat et al., 2017).
Deep Brain Stimulation
Deep brain stimulation offers the opportunity to investigate the influence of direct modulation of neural circuits on neurobiological and clinical parameters (Höflich et al., 2013). Recently, several studies have been published, which include DBS treatment for TRD in areas with relevance for reward processing, such as the NAc, VTA, and MFB. Schlaepfer et al. could show that stimulation of the NAc as a core region of reward processing leads to a significant increase of metabolism in the NAc, amygdala, and the dorsolateral PFC and dmPFC and decreased metabolism in the ventral and ventrolateral PFC 1 week after starting DBS as measured by FDG-PET (Schlaepfer et al., 2008). In a following study in 10 patients with a longer observation period, FDG-PET scans 1 year after implantations partly confirmed these results with significant decreases of metabolism within prefrontal subregions, including the OFC and subgenual ACC, thalamus, caudate, and PCC and an increase of metabolism in the precentral gyrus (Bewernick et al., 2010). In animal studies, NAc stimulation specifically led to a reversal of decreased sucrose preference as an indicator of normalization of hedonic capacity (Gersner et al., 2010). However, not all studies could show significant effects of DBS on the dopaminergic system in the NAc with animal studies failing to show effects of DBS in the NAc core on dopamine release and metabolites (Sesia et al., 2010; van Dijk et al., 2011). VTA stimulation in animals was shown to significantly alter BOLD signal in several subcortical and cortical areas, with BOLD increases in the dorsolateral PFC, ACC, and PCC, insula, premotor cortex, primary somatosensory cortex, and striatum and decreases of the BOLD signal in the parahippocampal cortex, anterior PFC, insula, inferior temporal gyrus, and primary somatosensory cortex. Furthermore, VTA stimulation led to dopamine release in the NAc as measured with fast-scan cyclic voltammetry in this study (Settell et al., 2017). Overall, these data could reflect a restoration of the balance between subcortical and cortical areas via the dopaminergic system. As described above, the disturbance of functional interaction between subcortical and cortical regions has been proposed as one of the neurobiological correlates of MDD on a network level.
With regard to the MFB, studies showed that DBS in this area leads to dopamine release in the striatum (Schlaepfer et al., 2008) and to increase of dopamine receptor subtype 2 receptor and dopamine transporter expression within the PFC and hippocampus (Dandekar et al., 2017). Furthermore, bilateral stimulation within the MFB in rats led to increases of intracranial selfstimulation paradigm as an indicator of an effect on the reward system (Edemann-Callesen et al., 2015). Another target, which has been proposed for DBS in TRD patients, is the LHb, with a case report showing significant efficacy. However, no specific effect on reward processing has been reported in this patient (Sartorius et al., 2010).
Conclusion
In conclusion, current studies paint the picture of a complex interaction of different functional networks and transmitter systems to permit hedonic capacity. The failure of one link of this complex functional circuitry leads to deficits of reward domains as seen in psychiatric disorders such as depression. As current efforts in medical science aim for the precision of treatment approaches and new strategies for the development of more adequate biopsychological subdomains, the consideration of anhedonia as a relevant phenotype is important. Current studies emphasize the consideration of subdomains of reward processing involved in anhedonia in future studies, both in animals and humans. This is important because it has been shown that both disorder and treatment do not always affect all reward processes to the same extent. First, direct application of the results gained on dysfunctional networks was performed in neuromodulatory treatment approaches such as DBS and TMS. Furthermore, refined analysis of symptom-specific effects of different drugs and treatment options is an important starting point for the establishment of precision treatment approaches in anhedonia.
Statement of Interest
Siegfried Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Rupert Lanzenberger received travel grants and/or conference speaker honoraria from Shire, AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. The other authors declared no conflicts of interest.
References
- Admon R, Kaiser RH, Dillon DG, Beltzer M, Goer F, Olson DP, Vitaliano G, Pizzagalli DA(2017)Dopaminergic enhancement of striatal response to reward in major depression. Am J Psychiatry 174:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelán M, Szabó Z, Kanyó B, Tanács A, Kovács Z, Janka Z, Pávics L(2005)Dopamine transporter availability in medication free and in bupropion treated depression: a 99mTc-TRODAT-1 SPECT study. J Affect Disord 89:115–123. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M(2007)Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 191:497–506. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML(2008)Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 199:457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE(1998)What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?Brain Res Brain Res Rev 28:309–369. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, Brockmann H, Lenartz D, Sturm V, Schlaepfer TE(2010)Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry 67:110–116. [DOI] [PubMed] [Google Scholar]
- Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Dichter GS, Smoski MJ (2016) Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord 203:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Carlsson ML(1999)Neurotransmitter interactions in schizophrenia–therapeutic implications. Biol Psychiatry 46:1388–1395. [DOI] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ(2016)Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML(1976)Scales for physical and social anhedonia. J Abnorm Psychol 85:374–382. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, Wang X, Zhang J, Lin W, Zheng L, Pu J, Tsai SJ, Yang AC, Lin CP, Wang F, Xie P, Feng J(2016)Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 139:3296–3309. [DOI] [PubMed] [Google Scholar]
- Chentsova-Dutton Y, Hanley K(2010)The effects of anhedonia and depression on hedonic responses. Psychiatry Res 179:176–180. [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Luse D, Hoffmann C, Cotton P, Peery T, Ruiz C, Hussey C, Giridharan VV, Soares JC, Quevedo J, Fenoy AJ(2017)Increased dopamine receptor expression and anti-depressant response following deep brain stimulation of the medial forebrain bundle. J Affect Disord 217:80–88. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Barnes SA, Markou A, Pizzagalli DA(2016)Translational assessment of reward and motivational deficits in psychiatric disorders. Curr Top Behav Neurosci 28:231–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ(2009)The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry 66:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannantonio M, Di Iorio G, Guglielmo R, De Berardis D, Conti CM, Acciavatti T, Cornelio M, Martinotti G(2011)Major depressive disorder, anhedonia and agomelatine: an open-label study. J Biol Regul Homeost Agents 25:109–114. [PubMed] [Google Scholar]
- Di Nicola M, De Risio L, Battaglia C, Camardese G, Tedeschi D, Mazza M, Martinotti G, Pozzi G, Niolu C, Di Giannantonio M, Siracusano A, Janiri L(2013)Reduced hedonic capacity in euthymic bipolar subjects: a trait-like feature?J Affect Disord 147:446–450. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M(2010)Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci 30:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döbrössy MD, Furlanetti LL, Coenen VA(2015)Electrical stimulation of the medial forebrain bundle in pre-clinical studies of psychiatric disorders. Neurosci Biobehav Rev 49:32–42. [DOI] [PubMed] [Google Scholar]
- Dombrovski AY, Szanto K, Clark L, Aizenstein HJ, Chase HW, Reynolds CF 3rd, Siegle GJ(2015)Corticostriatothalamic reward prediction error signals and executive control in late-life depression. Psychol Med 45:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, Bakker N, Blumberger DM, Daskalakis ZJ, Kennedy SH, Flint AJ, Giacobbe P(2014)Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 76:176–185. [DOI] [PubMed] [Google Scholar]
- Drysdale AT, et al. (2017)Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Tiret B, Marjańska M, Marjańska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G(2013)Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. Plos One 8:e60312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB(2007)The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. [DOI] [PubMed] [Google Scholar]
- Duprat R, Wu GR, De Raedt R, Baeken C(2017)Accelerated iTBS treatment in depressed patients differentially modulates reward system activity based on anhedonia. World J Biol Psychiatry 9:1–12. [DOI] [PubMed] [Google Scholar]
- Edemann-Callesen H, Voget M, Empl L, Vogel M, Wieske F, Rummel J, Heinz A, Mathé AA, Hadar R, Winter C(2015)Medial forebrain bundle deep brain stimulation has symptom-specific anti-depressant effects in rats and as opposed to ventromedial prefrontal cortex stimulation interacts with the reward system. Brain Stimul 8:714–723. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD(1983)Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry 40:79–84. [DOI] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K(2016)Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351:aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF(2007)Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargoloff PD, Corral R, Herbst L, Marquez M, Martinotti G, Gargoloff PR(2016)Effectiveness of agomelatine on anhedonia in depressed patients: an outpatient, open-label, real-world study. Hum Psychopharmacol 31:412–418. [DOI] [PubMed] [Google Scholar]
- Gass N, Cleppien D, Zheng L, Schwarz AJ, Meyer-Lindenberg A, Vollmayr B, Weber-Fahr W, Sartorius A(2014)Functionally altered neurocircuits in a rat model of treatment-resistant depression show prominent role of the habenula. Eur Neuropsychopharmacol 24:381–390. [DOI] [PubMed] [Google Scholar]
- Gersner R, Toth E, Isserles M, Zangen A(2010)Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol Psychiatry 67:125–132. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Nicolson NA, Peeters F, van Os J, Barge-Schaapveld D, Wichers M(2011)Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. Eur Neuropsychopharmacol 21:241–247. [DOI] [PubMed] [Google Scholar]
- Gleich T, Lorenz RC, Pöhland L, Raufelder D, Deserno L, Beck A, Heinz A, Kühn S, Gallinat J(2015)Frontal glutamate and reward processing in adolescence and adulthood. Brain Struct Funct 220:3087–3099. [DOI] [PubMed] [Google Scholar]
- Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, Zhang H, Lv L, Zhang H, Xie C, Zhang Z(2017)Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res 84:9–17. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ (2014). The assessment of interpersonal pleasure: introduction of the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS) and preliminary findings. Psychiatry Res 215:237–243. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Price J, De Bodinat C, Laredo J(2017)Emotional blunting with antidepressant treatments: A survey among depressed patients. J Affect Disord 221:31–35. [DOI] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Reid I, Hall J, Steele JD(2011)Expected value and prediction error abnormalities in depression and schizophrenia. Brain 134:1751–1764. [DOI] [PubMed] [Google Scholar]
- Greenberg T, et al. (2015)Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: findings from the EMBARC study. Am J Psychiatry 172:881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski G, Lanzenberger R, Kranz GS, Cumming P(2014)Meta-analysis of molecular imaging of serotonin transporters in major depression. J Cereb Blood Flow Metab 34:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B(2010)The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Haeusler D, Kraus C, Höflich AS, Kranz GS, Baldinger P, Savli M, Mitterhauser M, Wadsak W, Karanikas G, Kasper S, Lanzenberger R(2014)Attenuated serotonin transporter association between dorsal raphe and ventral striatum in major depression. Hum Brain Mapp 35:3857–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Cui Q, Zheng J, Duan X, Pang Y, Gao Q, Han S, Long Z, Wang Y, Li J, Wang X, Zhao J, Chen H(2016)Frequency-specific alterations in functional connectivity in treatment-resistant and -sensitive major depressive disorder. J Psychiatr Res 82:30–39. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ(1979)Efferent connections of the habenular nuclei in the rat. J Comp Neurol 187:19–47. [DOI] [PubMed] [Google Scholar]
- Heshmati M, Russo SJ(2015)Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep 2:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O.(2010)The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci 11:503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höflich A, Savli M, Comasco E, Moser U, Novak K, Kasper S, Lanzenberger R(2013)Neuropsychiatric deep brain stimulation for translational neuroimaging. Neuroimage 79:30–41. [DOI] [PubMed] [Google Scholar]
- James GM, Baldinger-Melich P, Philippe C, Kranz GS, Vanicek T, Hahn A, Gryglewski G, Hienert M, Spies M, Traub-Weidinger T, Mitterhauser M, Wadsak W, Hacker M, Kasper S, Lanzenberger R(2017)Effects of selective serotonin reuptake inhibitors on interregional relation of serotonin transporter availability in major depression. Front Hum Neurosci 11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS(2009)The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of tsai and substantia nigra compacta. J Comp Neurol 513:566–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD(2007)Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci 27:6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston BA, Tolomeo S, Gradin V, Christmas D, Matthews K, Douglas Steele J(2015)Failure of hippocampal deactivation during loss events in treatment-resistant depression. Brain 138:2766–2776. [DOI] [PubMed] [Google Scholar]
- Jonasson LS, Axelsson J, Riklund K, Braver TS, Ögren M, Bäckman L, Nyberg L(2014)Dopamine release in nucleus accumbens during rewarded task switching measured by [¹¹C]raclopride. Neuroimage 99:357–364. [DOI] [PubMed] [Google Scholar]
- Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR(2007)The Behavioral activation for depression scale (BADS): Psychometric properties and factor structure. Journal of Psychopathology and Behavioral Assessment, 29, 191–202. [Google Scholar]
- Kasper S, Hamon M(2009)Beyond the monoaminergic hypothesis: agomelatine, a new antidepressant with an innovative mechanism of action. World J Biol Psychiatry 10:117–126. [DOI] [PubMed] [Google Scholar]
- Keiflin R, Janak PH(2015)Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron 88:247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM(1998)Evidence for striatal dopamine release during a video game. Nature 393:266–268. [DOI] [PubMed] [Google Scholar]
- Kranz GS, Kasper S, Lanzenberger R(2010)Reward and the serotonergic system. Neuroscience 166:1023–1035. [DOI] [PubMed] [Google Scholar]
- Kraus C, Ganger S, Losak J, Hahn A, Savli M, Kranz GS, Baldinger P, Windischberger C, Kasper S, Lanzenberger R(2014)Gray matter and intrinsic network changes in the posterior cingulate cortex after selective serotonin reuptake inhibitor intake. Neuroimage 84:236–244. [DOI] [PubMed] [Google Scholar]
- Kraus C, Lanzenberger R, Kasper S (2017a) Ketamine for the treatment of depression. JAMA Psychiatry 74:970. [DOI] [PubMed] [Google Scholar]
- Kraus C, Castrén E, Kasper S, Lanzenberger R (2017b) Serotonin and neuroplasticity - links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev 77:317–326. [DOI] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S (2017c) Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract 21:2–12. [DOI] [PubMed] [Google Scholar]
- Kraus C, Klobl M, Tik M, Auer B, Vanicek T, Geissberger N, Pfabigan DM, Hahn A, Woletz M, Paul K, Komorowski A, Kasper S, Windischberger C, Lamm C, Lanzenberger R(2018)The pulvinar nucleus and antidepressant treatment: dynamic modeling of antidepressant response and remission with ultra-high field functional MRI. Mol Psychiatry doi: 10.1038/s41380-018-0032-6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC.(2009)Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32:127–147. [DOI] [PubMed] [Google Scholar]
- Kring AM, Barch DM(2014)The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol 24:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD(2008)Abnormal temporal difference reward-learning signals in major depression. Brain 131:2084–2093. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA(2014)Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzenberger R, Kranz GS, Haeusler D, Akimova E, Savli M, Hahn A, Mitterhauser M, Spindelegger C, Philippe C, Fink M, Wadsak W, Karanikas G, Kasper S(2012)Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage 63:874–881. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Baldinger P, Hahn A, Ungersboeck J, Mitterhauser M, Winkler D, Micskei Z, Stein P, Karanikas G, Wadsak W, Kasper S, Frey R(2013)Impact of electroconvulsive therapy on 5-HT1a receptor binding in major depression. Mol Psychiatry 18:1. [DOI] [PubMed] [Google Scholar]
- Laurent V, Morse AK, Balleine BW(2015)The role of opioid processes in reward and decision-making. Br J Pharmacol 172:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B(2003)In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry 54:800–805. [DOI] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK(2013)Optogenetic insights into striatal function and behavior. Behav Brain Res 255:44–54. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, Jia C, Hu F, Zeng J, Guo Q, Fu L, Luo M(2016)Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7:10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, Bao J, Kim JY, Chen ZF, El Mestikawy S, Luo M(2014)Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81:1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca P, Gourion D (2015) Management of anhedonia and depressive symptoms in depressed outpatients: benefit for Functioning. European Psychiatry 30:364.
- Lovinger DM.(2010)Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Ding J, Li J, Guo W, Long Z, Liu F, Gao Q, Zeng L, Zhao J, Chen H(2012)Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. Plos One 7:e45263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoveanu J.(2014)Serotonergic modulation of reward and punishment: evidence from pharmacological fMRI studies. Brain Res 1556:19–27. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Sepede G, Gambi F, Di Iorio G, De Berardis D, Di Nicola M, Onofrj M, Janiri L, Di Giannantonio M(2012)Agomelatine versus venlafaxine XR in the treatment of anhedonia in major depressive disorder: a pilot study. J Clin Psychopharmacol 32:487–491. [DOI] [PubMed] [Google Scholar]
- Marutani T, Yahata N, Ikeda Y, Ito T, Yamamoto M, Matsuura M, Matsushima E, Okubo Y, Suzuki H, Matsuda T(2011)Functional magnetic resonance imaging study on the effects of acute single administration of paroxetine on motivation-related brain activity. Psychiatry Clin Neurosci 65:191–198. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O(2007)Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ(2010)Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR, Ryan ND, Birmaher B, Shamseddeen W, Mayes T, Kennard B, Spirito A, Keller M, Lynch FL, Dickerson JF, Brent DA(2012)Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 51:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S(2002)Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 163:102–105. [DOI] [PubMed] [Google Scholar]
- Nestler EJ.(2015)Role of the brain’s reward circuitry in depression: transcriptional mechanisms. Int Rev Neurobiol 124:151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr(2006)The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Wright EC(1999)Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry 60:7–11. [PubMed] [Google Scholar]
- Nishikawa T, Fage D, Scatton B(1986)Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res 373:324–336. [DOI] [PubMed] [Google Scholar]
- Opbroek A, Delgado PL, Laukes C, McGahuey C, Katsanis J, Moreno FA, Manber R(2002)Emotional blunting associated with SSRI-induced sexual dysfunction. Do SSRIs inhibit emotional responses?Int J Neuropsychopharmacol 5:147–151. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M(2009)Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 166:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM(2009)Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 195:211–217. [DOI] [PubMed] [Google Scholar]
- Pujara M, Koenigs M(2014)Mechanisms of reward circuit dysfunction in psychiatric illness: prefrontal-striatal interactions. Neuroscientist 20:82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Ng R, Scott H, Kodavaganti S, Smyda G, Diwadkar V, Phillips M(2017)Ventral striatum functional connectivity during rewards and losses and symptomatology in depressed patients. Biol Psychol 123:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R, Dohm K, Grotegerd D, Opel N, Zwitserlood P, Heindel W, Arolt V, Kugel H, Dannlowski U(2015)Reward processing in unipolar and bipolar depression: A functional MRI study. Neuropsychopharmacology 40:2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richieri R, Boyer L, Faget-Agius C, Farisse J, Mundler O, Lançon C, Guedj E(2015)Determinants of brain SPECT perfusion and connectivity in treatment-resistant depression. Psychiatry Res 231:134–140. [DOI] [PubMed] [Google Scholar]
- Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH(2016)Assessing anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev 65:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC(2012)Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry 169:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ(2013)The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A(2010)Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry 67:e9–e11. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V(2008)Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 33:368–377. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Düzel E, Bauer A(2008)Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci 28:14311–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W.(2016)Dopamine reward prediction error coding. Dialogues Clin Neurosci 18:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesia T, Bulthuis V, Tan S, Lim LW, Vlamings R, Blokland A, Steinbusch HW, Sharp T, Visser-Vandewalle V, Temel Y(2010)Deep brain stimulation of the nucleus accumbens shell increases impulsive behavior and tissue levels of dopamine and serotonin. Exp Neurol 225:302–309. [DOI] [PubMed] [Google Scholar]
- Settell ML, Testini P, Cho S, Lee JH, Blaha CD, Jo HJ, Lee KH, Min HK(2017)Functional circuitry effect of ventral tegmental area deep brain stimulation: imaging and neurochemical evidence of mesocortical and mesolimbic pathway modulation. Front Neurosci 11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P(1995)A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br J Psychiatry 167:99–103. [DOI] [PubMed] [Google Scholar]
- Spies M, Knudsen GM, Lanzenberger R, Kasper S(2015)The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2:743–755. [DOI] [PubMed] [Google Scholar]
- Spies M, Kraus C, Geissberger N, Auer B, Klöbl M, Tik M, Stürkat IL, Hahn A, Woletz M, Pfabigan DM, Kasper S, Lamm C, Windischberger C, Lanzenberger R(2017)Default mode network deactivation during emotion processing predicts early antidepressant response. Transl Psychiatry 7:e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, Nolen WA(2001)Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands mental health survey and incidence study (NEMESIS). Acta Psychiatr Scand 103:122–130. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Cooch NK, Schoenbaum G(2015)What the orbitofrontal cortex does not do. Nat Neurosci 18:620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Adli M, Bauer M, Heinz A, Ströhle A(2012)Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol 26:677–688. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W(2007)D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30:228–235. [DOI] [PubMed] [Google Scholar]
- Takamura M, Okamoto Y, Okada G, Toki S, Yamamoto T, Ichikawa N, Mori A, Minagawa H, Takaishi Y, Fujii Y, Kaichi Y, Akiyama Y, Awai K, Yamawaki S(2017)Patients with major depressive disorder exhibit reduced reward size coding in the striatum. Prog Neuropsychopharmacol Biol Psychiatry 79:317–323. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K(2009)Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, Hauser J, Dernovsek MZ, Souery D, Bajs M, Maier W, Aitchison KJ, Farmer A, McGuffin P(2012)Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med 42:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk A, Mason O, Klompmakers AA, Feenstra MG, Denys D(2011)Unilateral deep brain stimulation in the nucleus accumbens core does not affect local monoamine release. J Neurosci Methods 202:113–118. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Gourion D, Mouchabac S(2017)Anhedonia predicts poor psychosocial functioning: results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry 44:1–8. [DOI] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK(2013)New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci 33:17569–17576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, Hompes T, de Boer P, Schmidt M, Claes S(2014)Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord 155:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, Gibbs D, Petty C, Bizzell J, Smoski MJ, Dichter GS(2017)Attenuation of frontostriatal connectivity during reward processing predicts response to psychotherapy in major depressive disorder. Neuropsychopharmacology 42:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Raptis L(1986)Effects of naloxone and pimozide on initiation and maintenance measures of free feeding. Brain Res 368:62–68. [DOI] [PubMed] [Google Scholar]
- Wu H, Mata J, Furman DJ, Whitmer AJ, Gotlib IH, Thompson RJ(2017)Anticipatory and consummatory pleasure and displeasure in major depressive disorder: an experience sampling study. J Abnorm Psychol 126:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CB, Chen T, Nusslock R, Keller J, Schatzberg AF, Menon V(2016)Anhedonia and general distress show dissociable ventromedial prefrontal cortex connectivity in major depressive disorder. Transl Psychiatry 6:e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, Dagher A(2004)Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci 24:4105–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J(2013)The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 151:531–539. [DOI] [PubMed] [Google Scholar]