Abstract
Liposomes are versatile and well-proven as a means to deliver nucleic acids into cells. Most of the formulation procedures used are labour intensive and result in unstable end products. We have previously reported on the development of a simple, yet efficient, hydration-of-freeze-dried-matrix (HFDM) method to entrap siRNA within lipid particles. Here we show that the particles are stable up to 12 months after storage at room temperature (RT), 4°C or -20°C. While RT storage results in changes in particle size and polydispersity, gene silencing of all particles was similar to freshly prepared particles following storage for 3, 6, 9 or 12 months at all temperatures. This is the first report of such long-term stability in siRNA-loaded liposomes.
Introduction
Various methods for formulating polynucleotide-loaded PEGylated particles have been reported to date, including post-insertion [1], reverse-phase evaporation [2], detergent dialysis [3] and ethanol dialysis [4–6]. However, most of these methods, though effective, require relatively complicated and lengthy formulation procedures with the resulting particles suspended in an aqueous state. This has led to long term storage issues including aggregation and/or fusion of the particles [7, 8], hydrolysis of the lipids [7], and instability of siRNA nucleotides in an aqueous environment. Moreover, these formulations are also prone to be affected by stresses occurred during transport, such as agitation or temperature fluctuation [8, 9]. These problems, along with the significantly increased effort required for large-scale production of these particles using the existing formulation procedures has held back clinical development and adoption.
To address these issues, we developed a novel method to formulate stable, siRNA-loaded, PEGylated lipid particles using the hydration of freeze-dried matrix (HFDM) method[10]. This simple, yet-efficient, procedure results in lipid particles that are have highly favourable characteristics for in vivo delivery. Indeed, these particles have been used for in vivo systemic delivery in animal models to target a range of cancer-related genes, which have resulted in significant tumour elimination and increased survival [11, 12]. HDFM lipoplexes have also been used to reduce gene expression in lung tissues [13], the peritoneal cavity via intraperitoneal delivery [14] and in the vaginal epithelia with the aid of a novel aliginate matrix to increase retention time [15].
The simple mixing of lipids (DOTAP, cholesterol and PEG2000-C16Ceramide–molar ratio of 45:45:10) dissolved in tert-butanol with nucleic acids dissolved in sucrose followed by freeze-drying and rehydration results in lipid particles that are isotonic and ready for direct in vivo injection. Particles are ~190nm in size with a polydispersity index of 0.32 and an overall charge of 45mV. The encapsulated siRNA was protected from serum [10] and show excellent pharmacokinetics with T1/2 z>40h compared to other systems such as galactosylated cationic liposomes (T1/2 z>1h)[16], PEGylated polyplexes (T1/2 z>1.5h)[17], or SNALPS (T1/2 z>6.5h)[18]. This represented an advance in lipid formulation of siRNA for in vivo use.
As mentioned above, liposomes are prepared fresh for in vivo use as they aggregate, fuse or hydrolyse in aqueous solutions over time. As the HFDM method results in a dried matrix we postulated that these resulting lipoplexes would be highly stable over time. Indeed, our initial studies examined the longevity of the freeze-dried siRNA/lipid matrix stored at 4°C, or room temperature (RT), for 4 weeks and showed that silencing was still significant at this time [10]. Here, we have extended these studies out to 12 months and looked at a range of different storage conditions. We examined the physical characteristics of the particles and their ability to silence target genes in vitro at various time points. Our data show that HFDM lipoplexes are highly stable and still active for in vitro silencing even 12 months post-production. Such post-production longevity has never previously been reported and represents a significant advance in the field.
Materials and methods
Cells and siRNA
HeLa cells were originally obtained from the American Type Culture Collection (ATCC) and were cultured as described previously [19]. The siRNA used in this study was Lamin A/C siRNA [20] obtained from Genesearch (Shanghai, China). siGlo red (Dharmacon, Lafayette, CO) was used as a qualitative transfection indicator as transfection efficiency.
Liposome formulations
Lipoplexes were prepared by Hydration of Freeze-Dried Matrix (HFDM) method as described previously [10]. Required amounts of DOTAP, cholesterol and PEG2000- C16Ceramide were dissolved in 1 mL of tert-butanol at a molar ratio of 45:45:10. 40μg of siRNA was added to 1 mL of filtered sucrose solution before mixing with the lipid solution. The resultant formulation was then snap-frozen and freeze-dried overnight (ALPHA 1–2 LDplus, Martin Christ, Germany) at a condensing temperature of −80°C and pressure of less than 0.1 mbar. Distilled H2O was then added to the lyophilised product with gentle shaking. A Nitrogen/Phosphate (N/P) ratio of 4:1 was used for all formulations and three separate batches were made for each formulation condition (n = 3). The final product contained 40μg siRNA in 300μL isotonic sucrose solution.
Particle characterisation
Size, polydispersity and zeta potential of the resultant lipoplexes were measured using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) following appropriate dilution in distilled water. Measurements were carried out at room temperature (RT), 4°C and -20°C with 10 runs per measurement undertaken.
siRNA entrapment efficiency
siRNA entrapment efficiencies were determined using the Quant-iT PicoGreen reagent (Invitrogen, California USA) as previously described [10].
Lipoplex cell uptake analysis
Cells were forward-transfected with 40μM of siGlo red. Cell uptake efficiency was assessed by flow cytometry 24h post-transfection on a BD LSR FORTESSA cell analyser (BD bioscience, San Jose, CA).
In vitro siRNA transfection
In vitro siRNA transfection of liposome-complexed siRNA was performed as previously described [10]. HeLa cells were seeded the day before the transfection experiment at a density of 75,000 cells per well in a 6 well plate. A final concentration of 40 nM liposome-entrapped siRNA suspended in primocin-free complete media was then added to each well. siRNAs were left on cells for 8 h, and cells were then incubated in primocin-containing media for 3 days at 37°C. The level of gene knockdown was determined by qPCR analysis and percentage of knockdown efficiency calculated as previously described [21].
qPCR
Extract of RNA, cDNA generation, and qPCR were carried out as described previously [19]. Real time primers used: Lamin A/C forward 5’-TGGAGATGATCCCTTGCTGA-3’, Lamin A/C reverse 5’-GCATGGCCACTTCTTCCCA-3’, β-actin forward 5’-AGCCTCGCCTTTGCCGA-3’and β-actin reverse 5’-CTGGTGCCTGGGGCG-3’.
Statistical analysis
All data were subjected to two-way ANOVAs with Dunnett's post-hoc analyses using GraphPad Prism 7.
Results and discussion
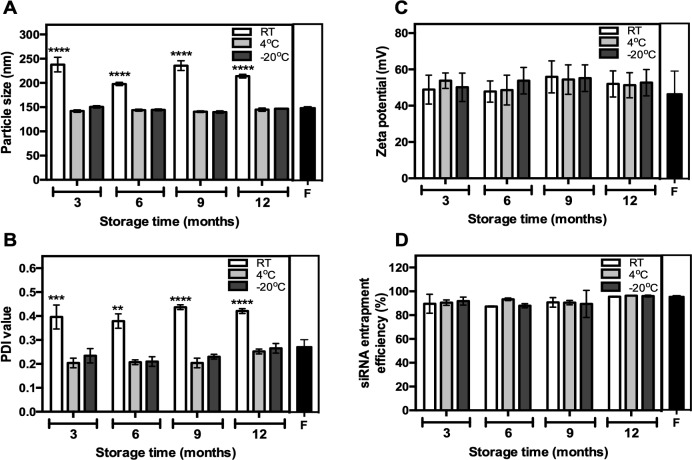
We have previously showed that storage of our formulated lipoplexes for 1 month at RT and 4°C did not affect its physiochemical properties [10]. However, the physiochemical stability of the lipoplexes have never been tested outside these storage conditions. The stability of dried-state lipoplexes was investigated up to 12 months post-manufacture. Following formulation, the particles were stored at either RT, 4°C or -20°C. When compared to freshly-prepared lipoplexes, lipoplex powder stored at RT displayed greater variation in both size and PDI upon rehydration, likely due to varying humidity levels during storage (Fig 1A and 1B). More importantly, no variation in the surface charge (or zeta potential) of particles was observed when stored at any temperature over a 12-month period (Fig 1C), which is important for the stability of the particles and remaining free from agglutination when delivered into the bloodstream. In contrast to what we previously observed [10], the siRNA entrapment efficiency did not fluctuate over time at different temperatures when compared to freshly-prepared lipoplexes, indicating the siRNA encapsulated in the lipoplexes were stable (Fig 1D). Overall, there was minimal effect of the storage time and temperature on the lipoplex physiochemical properties and the stability of the siRNA-loaded lipoplexes, with 4°Cand -20°C showing superior stability in terms of size, charge and size variation.
Fig 1. Lipoplex size, polydispersity, zeta potential and siRNA entrapment efficiency remains unchanged with long term storage at various temperatures except the lipoplex size and the polydispersity at room temperature.
(A) Size, (B) polydispersity and (C) zeta potential of the resultant lipoplex were measured using a Zetasizer Nano ZS following appropriate dilution in distilled water. Formulations were stored at either -20°C, 4°C or room temperature (RT) and 2 separate batches were made for each condition. (n = 2). (D) siRNA entrapment efficiencies were determined using the Quant-iT PicoGreen reagent. Tests were performed on freshly prepared lipoplex (F–Black shaded bars), and lipoplex stored at various temperatures for different lengths of time post manufacture. Bar graphs represent the mean and the error bars represent the standard deviation. **p value < 0.005, ***p value < 0.0005, ****p value <0.0001, two-way ANOVA using a Dunnett's post-hoc analysis against freshly prepared lipoplexes.
To assess whether storage time and temperature have any effect on the functional capability of the lipoplexes, the gene knockdown ability of siRNA-loaded lipoplexes was tested in vitro. In vitro cell uptake efficiency of lipoplexes stored over time at varying temperatures were comparable to that of freshly prepared lipoplexes (Fig 2A). Liposome powders were rehydrated at each time point, complexed with siRNAs and applied to HeLa cells to determine knockdown of the non-essential structural protein, lamin A/C (LMNA). qPCR analysis revealed notable decrease of LMNA mRNA expression in HeLa cells treated with LMNA-specific siRNA compared to untransfected cells, irrespective of the storage conditions (Fig 2B). Furthermore, the knockdown efficiency (Fig 2C) at the mRNA level was comparable to that of freshly prepared liposomes suggesting that storage time and temperature have minimal effect on the gene-silencing efficiency of the siRNAs-complexed with lipoplexes. Although some liposome preparation groups had significantly different LMNA mRNA knockdown levels when compared to freshly prepared liposomes (Fig 2B and 2C), overall knockdown efficiencies for all preparations were well above 50% (Fig 2C).
Fig 2. siRNA-mediated target gene knockdown capability was maintained using lipoplexes stored at different temperatures over time in vitro.
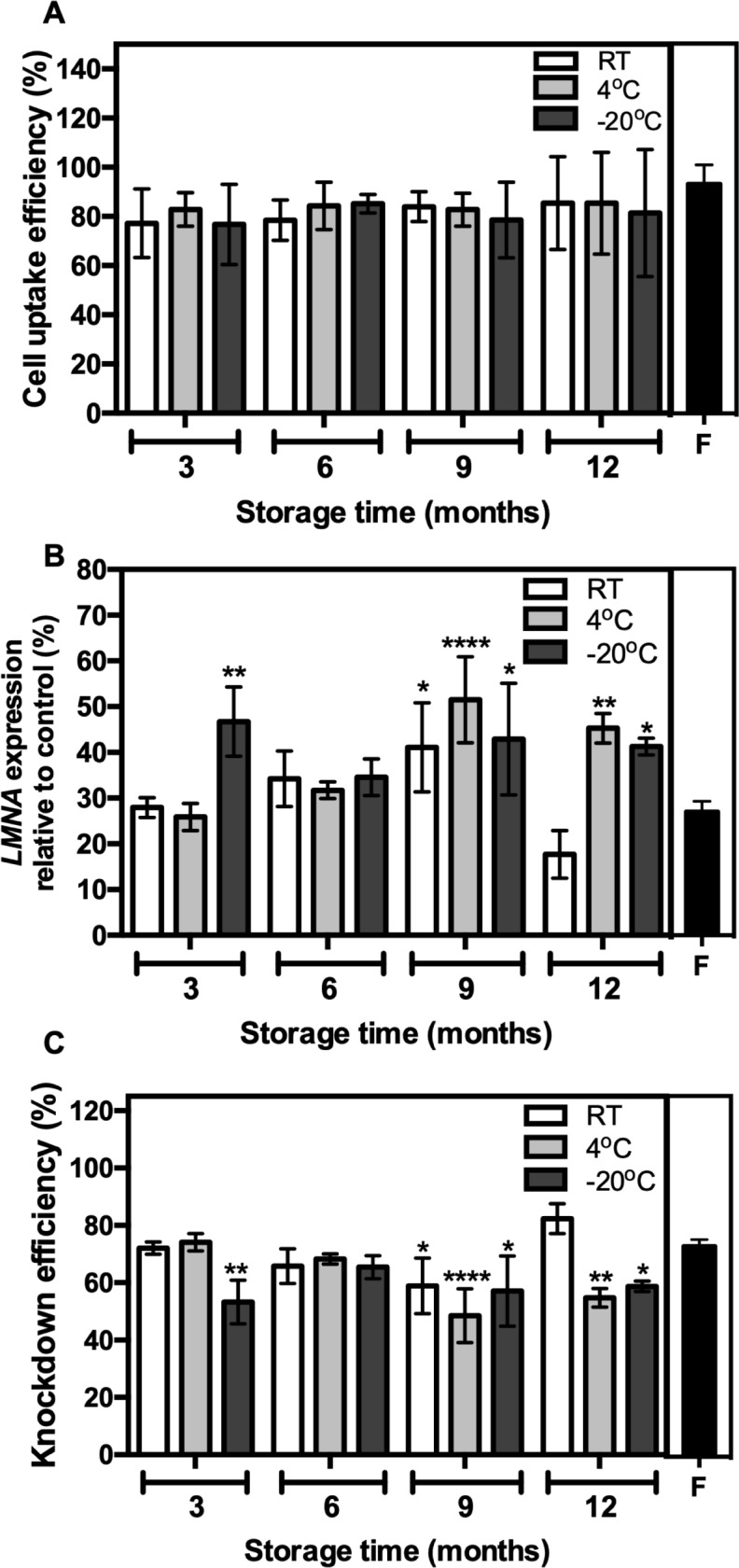
(A) HeLa cells were treated with a final concentration of 40 nM of siGlo complexed into lipoplexes and incubated at 37°C for 24h before assessing siGlo uptake (%) by flow cytometry. Tests were performed on freshly prepared lipoplexes (F–Black shaded bars), and lipoplexes stored at various temperatures for different lengths of time post manufacture. Data is presented as percentage sum of fluorescence intensity. Bar graphs represent the mean and the error bars represent the standard deviation from three independent experiments. (B) HeLa cells were treated with a final concentration of 40 nM of Lamin A/C (LMNA) siRNA complexed into lipoplexes and incubated at 37°C for 3 days. Expression levels were calculated relative to non-transfected cells (control) by qPCR analysis and data presented as percentage of LMNA expression compared to control. β-actin was used as housekeeping control gene. (C) Percentage of knockdown efficiency was also determined as previously described [21]. Tests were performed on freshly prepared lipoplexes (F–Black shaded bars), and lipoplexes stored at various temperatures for different lengths of time post manufacture. Bar graphs represent the mean and the error bars represent the standard deviation from three independent experiments. **p value < 0.005, ***p value < 0.0005, ****p value <0.0001, two-way ANOVA using a Dunnett's post-hoc analysis against freshly prepared lipoplexes.
Previous studies have investigated the stability of dried-state liposomal preparations at different temperatures (i.e. 37°C to 60°C) over a 1-month period [22], this is the first study to investigate the physiochemical and functional effects of the prolonged storage of lipoplexes over a longer period of time (i.e. 12 months) and at lower temperatures. We developed this lyophilization procedure to ensure effective long-term storage of our lipoplexes, even at RT, as lipoplex suspensions are known to be unstable in aqueous suspension for long-term storage, especially with respect to hydrolysis and size stability. To date no data on the long term (up to 12 months) viability of siRNA-loaded liposomes have, to our knowledge, been published. There are longevity studies investigating traditional liposomes loaded with plasmid DNA. For example, an early study of unextruded liposomes made from DMRIE:DOPE complexed with plasmid DNA showed that when frozen as a aqueous solution they retained activity when thawed 12 months later [23]. More recently it was showed that DC- Cholesterol: DOPE liposomes loaded with plasmid DNA and freeze-dried were stable following 3 months storage at RT and 40°C, although storage at 60°C resulted in lost DNA structure [24]. Others have investigated the stability of DNA oligonucleotide-loaded liposomes for antisense therapies, which changed size and lost ODN content over 12 months (RT) or 90 days (frozen)[25]. However, the functionality of these stored lipoplexes was not examined. Overall, we show that RT was inferior for storage, resulting in an increased particle size and polydispersity from 3 months onwards. Storage at 4°C and -20°C resulted in no changes in lipoplexes and silencing ability. Overall, HFDM liposomes exhibit excellent long-term storage characteristics.
Data Availability
Minimal underlying data set is contained within the manuscript itself.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Li SD, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharm. 2006;3(5):579–88. 10.1021/mp060039w [DOI] [PubMed] [Google Scholar]
- 2.Stuart D, Allen T. A new liposomal formulation for antisense oligodeoxynucleotides with small size, high incorporation efficiency and good stability. Biochimica et Biophysica Acta. 2000;1463:219–29. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler JJ, Palmer L, Ossanlou M, MacLachlan I, Graham RW, Zhang YP, et al. Stabilized plasmid-lipid particles: construction and characterization. Gene Ther. 1999;6(2):271–81. 10.1038/sj.gt.3300821 [DOI] [PubMed] [Google Scholar]
- 4.Maurer N, Wong KF, Stark H, Louie L, McIntosh D, Wong T, et al. Spontaneous entrapment of polynucleotides upon electrostatic interaction with ethanol-destabilized cationic liposomes. Biophys J. 2001;80:2310–26. 10.1016/S0006-3495(01)76202-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharmaceutical research. 2005;22(3):362–72. [DOI] [PubMed] [Google Scholar]
- 6.Morrissey D, Lockridge J, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–7. 10.1038/nbt1122 [DOI] [PubMed] [Google Scholar]
- 7.Li C, Deng Y. A novel method for the preparation of liposomes: Freeze drying of monophase solutions. Journal of Pharmaceutical Sciences. 2004;93(6):1403–14. 10.1002/jps.20055 [DOI] [PubMed] [Google Scholar]
- 8.Anchordoquy TJ, Carpenter JF, Kroll DJ. Maintenance of transfection rates and physical characterization of lipid/DNA complexes after freeze-drying and rehydration. Arch Biochem Biophys. 1997;348(1):199–206. 10.1006/abbi.1997.0385 [DOI] [PubMed] [Google Scholar]
- 9.Clement J, Kiefer K, Kimpfler A, Garidel P, Peschka-Suss R. Large-scale production of lipoplexes with long shelf-life. Eur J Pharm Biopharm. 2005;59(1):35–43. 10.1016/j.ejpb.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Wu SY, Putral LN, Liang M, Chang HI, Davies NM, McMillan NA. Development of a novel method for formulating stable siRNA-loaded lipid particles for in vivo use. Pharmaceutical research. 2009;26(3):512–22. 10.1007/s11095-008-9766-1 [DOI] [PubMed] [Google Scholar]
- 11.Wu SY, Singhania A, Burgess M, Putral LN, Kirkpatrick C, Davies NM, et al. Systemic delivery of E6/7 siRNA using novel lipidic particles and its application with cisplatin in cervical cancer mouse models. Gene Ther. 2011;18(1):14–22. 10.1038/gt.2010.113 [DOI] [PubMed] [Google Scholar]
- 12.Sheng YH, He Y, Hasnain SZ, Wang R, Tong H, Clarke DT, et al. MUC13 protects colorectal cancer cells from death by activating the NF-kappaB pathway and is a potential therapeutic target. Oncogene. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCaskill J, Singhania R, Burgess M, Allavena R, Wu S, Blumenthal A, et al. Efficient Biodistribution and Gene Silencing in the Lung epithelium via Intravenous Liposomal Delivery of siRNA. Mol Ther Nucleic Acids. 2013;2:e96 10.1038/mtna.2013.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singhania A, Wu SY, McMillan NA. Effective Delivery of PEGylated siRNA-Containing Lipoplexes to Extraperitoneal Tumours following Intraperitoneal Administration. J Drug Deliv. 2011;2011:192562 10.1155/2011/192562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu SY, Chang HI, Burgess M, McMillan NA. Vaginal delivery of siRNA using a novel PEGylated lipoplex-entrapped alginate scaffold system. Journal of controlled release: official journal of the Controlled Release Society. 2011;155(3):418–26. [DOI] [PubMed] [Google Scholar]
- 16.Sato A, Takagi M, Shimamoto A, Kawakami S, Hashida M. Small interfering RNA delivery to the liver by intravenous administration of galactosylated cationic liposomes in mice. Biomaterials. 2007;28(7):1434–42. 10.1016/j.biomaterials.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 17.Merkel OM, Librizzi D, Pfestroff A, Schurrat T, Buyens K, Sanders NN, et al. Stability of siRNA polyplexes from poly(ethylenimine) and poly(ethylenimine)-g-poly(ethylene glycol) under in vivo conditions: effects on pharmacokinetics and biodistribution measured by Fluorescence Fluctuation Spectroscopy and Single Photon Emission Computed Tomography (SPECT) imaging. Journal of controlled release: official journal of the Controlled Release Society. 2009;138(2):148–59. [DOI] [PubMed] [Google Scholar]
- 18.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–7. 10.1038/nbt1122 [DOI] [PubMed] [Google Scholar]
- 19.Gabrielli B, Bokhari F, Ranall MV, Oo ZY, Stevenson AJ, Wang W, et al. Aurora A Is Critical for Survival in HPV-Transformed Cervical Cancer. Mol Cancer Ther. 2015;14(12):2753–61. 10.1158/1535-7163.MCT-15-0506 [DOI] [PubMed] [Google Scholar]
- 20.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–8. 10.1038/35078107 [DOI] [PubMed] [Google Scholar]
- 21.Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015;11:393–403. 10.1016/j.actbio.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payton NM, Wempe MF, Xu Y, Anchordoquy TJ. Long-term storage of lyophilized liposomal formulations. Journal of Pharmaceutical Sciences. 2014;103(12):3869–78. 10.1002/jps.24171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zelphati O, Nguyen C, Ferrari M, Felgner J, Tsai Y, Felgner PL. Stable and monodisperse lipoplex formulations for gene delivery. Gene Ther. 1998;5(9):1272–82. 10.1038/sj.gt.3300707 [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Anchordoquy TJ. Effects of moisture content on the storage stability of dried lipoplex formulations. Journal of Pharmaceutical Sciences. 2009;98(9):3278–89. 10.1002/jps.21846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyrozumska P, Meissner J, Toporkiewicz M, Szarawarska M, Kuliczkowski K, Ugorski M, et al. Liposome-coated lipoplex-based carrier for antisense oligonucleotides. Cancer Biol Ther. 2015;16(1):66–76. 10.4161/15384047.2014.987009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Minimal underlying data set is contained within the manuscript itself.