Abstract
Background:
Patients with high-risk coronary artery disease (CAD) may be difficult to identify.
Methods/Results:
Using the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) cohort randomized to coronary computed tomographic angiography (n=4589), 2 predictive models were developed for high-risk CAD, defined as left main stenosis (≥50% stenosis) or either (a) ≥50% stenosis ‘[50]’ or (b) ≥70% stenosis ‘[70]’ of 3 vessels or 2-vessel CAD involving the proximal left anterior descending artery. Pretest predictors were examined using stepwise logistic regression and assessed for discrimination and calibration. High-risk CAD was identified in 6.6% [50] and 2.4% [70] of patients. Models developed to predict high-risk CAD discriminated well: [50], bias-corrected c-statistic=0.73 (95% CI, 0.71–0.76); [70], bias-corrected c-statistic=0.73 (95% CI, 0.68–0.77). Variables predictive of CAD in both models included family history of premature CAD, age, male sex, lower glomerular filtration rate, diabetes mellitus, elevated systolic blood pressure, and angina. Additionally, smoking history was predictive of [50] CAD and sedentary lifestyle of [70] CAD. Both models characterized high-risk CAD better than the Pooled Cohort Equation (AUC=0.70 and 0.71 for [50] and [70], respectively) and Diamond-Forrester risk scores (AUC=0.68 and 0.71, respectively). Both [50] and [70] CAD was associated with more frequent invasive interventions and adverse events than non-high-risk CAD (all p <0.0001).
Conclusions:
In contemporary practice, 2.4–6.6% of stable, symptomatic patients requiring noninvasive testing have high-risk CAD. A simple combination of pretest clinical variables improves prediction of high-risk CAD over traditional risk assessments.
Clinical Trial Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01174550.
Keywords: coronary computed tomography angiography, high-risk coronary artery disease, predictors, prediction model
Keywords: Computerized Tomography, Diagnostic Testing, Risk Factors
METHODS
Patient Population
PROMISE was a prospective randomized trial comparing health outcomes in stable symptomatic outpatients who required further evaluation and who were randomly assigned to an initial strategy of either anatomical testing or functional testing. The study design and primary results of the PROMISE study have been previously described.14 Local or central institutional review boards approved the trial, and all patients provided written informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The complete data set for the PROMISE trial has been deposited with the National Institutes of Health and is publicly available (https://biolincc.nhlbi.nih.gov/studies/promise/).
Using the PROMISE cohort who were randomized to initial anatomical testing and received an interpretable coronary computed tomographic angiography (CTA) as their first diagnostic test (n=4589), this study classified patients as high-risk or non-high-risk CAD according to two definitions based on site-read CTA results: LM coronary artery stenosis (≥50% stenosis) or either (a) ≥50% stenosis ‘[50]’ or (b) ≥70% stenosis ‘[70]’ of 3VD or 2VD involving the pLAD. Both [50] and [70] models were created to reflect previous CTA studies defining obstructive CAD as either ≥50% or ≥70% stenosis. Min et al. reported that patients with multiple (>2) vessels with ≥50% stenosis on CTA demonstrated significantly worse clinical outcomes as compared to patients with <50% stenosis.15 Indeterminate CAD was defined if there was inadequate data to determine if a specific vessel met either high- or non-high-risk criteria (i.e. uninterpretable vessel).
Cardiovascular Risk Factor Definition
Patient demographics, symptoms, and cardiovascular risk factors were collected and assessed at the time of the PROMISE study enrollment.14,16
Clinical Outcomes and Events
All patients in the anatomic testing (CTA) arm were followed for coronary artery interventions (ICA, percutaneous coronary intervention [PCI], and/or coronary artery bypass graft surgery [CABG]) within 90 days after their initial CTA. Also, the following adjudicated events were identified over a 26-month (median) follow-up period: unstable angina hospitalization (UAH), myocardial infarction (MI), cardiovascular death, and all-cause death.
Statistical Analysis
Demographics, symptoms, and risk factors were examined as potential predictors of high-risk CAD. Descriptive statistics for continuous variables are presented as means and standard deviations, and categorical variables are presented as frequencies and percentages. Characteristics in patients categorized in the high-risk CAD cohorts were compared to non-high-risk patients using a Student t test for continuous variables and a chi-square test or Fisher’s exact test in cases of low cell counts for categorical variables using SAS Version 9.4.
Baseline characteristics, risk factors, symptoms, and laboratory/electrocardiogram variables were used in a logistic multiple regression model to predict high-risk status. Models were developed using clinical guidance and stepwise selection methods. To help achieve parsimony as well as good predictive accuracy, an entry and exit criteria of p<0.05 was used, and the final models were confirmed using backward selection. The baseline factors in Table 1 represent the full set of candidate predictors considered in the model selection process. A few variables, such as high-density lipoprotein, left bundle branch block, left ventricular hypertrophy, nitroglycerin, and relationship of symptoms to stress or exertion, were excluded from prediction models due to the high number of missing or unknown values. The linearity of continuous predictors was assessed using restricted cubic splines and transformed if necessary. C-statistics were estimated to evaluate model discrimination. Model calibration was assessed via the Hosmer-Lemeshow goodness-of-fit test and graphically via calibration plots that plotted the observed proportion in the high-risk group vs. the mean predicted probability of high risk in each decile of the predicted value.17 As a method of internal validation, bootstrapping was used to estimate the optimism of the c-statistic while taking into account the uncertainty of the stepwise model selection procedure in each bootstrap sample.18,19 The final bias-corrected c-statistic was estimated by subtracting the optimism measure from the c-statistic obtained from the model build sample. Optimism quantifies the bias due to the potential overfitting of the model in the sample used to derive the model. The metric expresses the difference in model performance in the sample used to derive the model compared to model performance in the underlying population. Thus adjusting for optimism provides bias corrected c-statistics that better capture the model’s expected discriminatory capacity in the underlying population.18,19 The final models are presented with an odds ratio, 95% confidence interval (CI), and p-value for each predictor. Chi-square tests and Fisher’s exact test were used to compare 90-day interventions (ICA, PCI, and/or CABG) between the high-risk and non-high-risk groups. Log-rank tests were used to compare clinical outcomes (UAH, MI, cardiovascular death, and all-cause death). The final models were compared with existing risk scores using the method of DeLong to compare area under the ROC curve of different models in the same sample.20
Table 1:
Baseline Characteristics
| High Risk (≥50% stenosis) N=301 |
Non-High Risk (<50% stenosis) N=4207 |
p-value* | High Risk (≥70% stenosis) N=111 |
Non-High Risk (<70% stenosis) N=4409 |
p-value† | |
|---|---|---|---|---|---|---|
| Patient age (years) | 63.9 (8.33) | 60.2 (8.09) | <0.0001 | 64.2 (8.73) | 60.3 (8.12) | <0.0001 |
| Female sex | 91 (30.2%) | 2245 (53.4%) | <0.0001 | 27 (24.3%) | 2314 (52.5%) | <0.0001 |
| Racial or ethnic minority | 48 (16.1%) | 972 (23.2%) | 0.0042 | 17 (15.5%) | 1004 (22.9%) | 0.0655 |
| BMI (kg/m2) | 30.6 (5.48) | 30.4 (6.01) | 0.6455 | 30.1 (4.80) | 30.4 (6.00) | 0.5165 |
| Hypertension | 219 (72.8%) | 2685 (63.8%) | 0.0018 | 73 (65.8%) | 2841 (64.4%) | 0.7726 |
| Diabetes | 100 (33.2%) | 837 (19.9%) | <0.0001 | 33 (29.7%) | 906 (20.5%) | 0.0185 |
| Metabolic syndrome | 143 (47.5%) | 1531 (36.4%) | 0.0001 | 56 (50.5%) | 1623 (36.8%) | 0.0033 |
| Dyslipidemia | 222 (73.8%) | 2810 (66.8%) | 0.0129 | 80 (72.1%) | 2962 (67.2%) | 0.2779 |
| Any PAD | 26 (8.6%) | 205 (4.9%) | 0.0042 | 11 (9.9%) | 221 (5.0%) | 0.0210 |
| Smoker | 186 (61.8%) | 2109 (50.1%) | <0.0001 | 68 (61.3%) | 2236 (50.7%) | 0.0283 |
| Family history of premature (<55 years) CAD | 111 (37.2%) | 1349 (32.2%) | 0.0698 | 41 (36.9%) | 1426 (32.5%) | 0.3195 |
| History of depression | 61 (20.3%) | 829 (19.7%) | 0.8134 | 20 (18.0%) | 873 (19.8%) | 0.6414 |
| Sedentary lifestyle | 151 (50.3%) | 2029 (48.3%) | 0.5005 | 62 (55.9%) | 2124 (48.3%) | 0.1144 |
| Typical/atypical/non-cardiac symptoms16 | 0.0027 | 0.0001 | ||||
| Typical | 49 (16.3%) | 473 (11.2%) | 27 (24.3%) | 499 (11.3%) | ||
| Atypical | 233 (77.4%) | 3274 (77.8%) | 76 (68.5%) | 3438 (78.0%) | ||
| Non-cardiac | 19 (6.3%) | 460 (10.9%) | 8 (7.2%) | 472 (10.7%) | ||
| Chest pain/dyspnea / other symptoms | 0.4076 | 0.3791 | ||||
| Chest pain | 214 (71.1%) | 3113 (74.0%) | 77 (69.4%) | 3258 (73.9%) | ||
| Shortness of breath/dyspnea | 50 (16.6%) | 585 (13.9%) | 16 (14.4%) | 621 (14.1%) | ||
| Other | 37 (12.3%) | 506 (12.0%) | 18 (16.2%) | 527 (12.0%) | ||
| Symptoms related to physical/mental stress‡ | <0.0001 | 0.0043 | ||||
| No stress | 104 (34.6%) | 1945 (46.2%) | 40 (36.0%) | 2015 (45.7%) | ||
| Stress | 167 (55.5%) | 1809 (43.0%) | 64 (57.7%) | 1918 (43.5%) | ||
| Unknown | 30 (10.0%) | 453 (10.8%) | 7 (6.3%) | 476 (10.8%) | ||
| Primary symptoms relieved by rest or NTG within 10 min‡ | 0.0539 | 0.2090 | ||||
| Rarely/never | 54 (17.9%) | 975 (23.2%) | 18 (16.2%) | 1014 (23.0%) | ||
| Always/usually | 96 (31.9%) | 1378 (32.8%) | 37 (33.3%) | 1441 (32.7%) | ||
| Unknown | 151 (50.2%) | 1851 (44.0%) | 56 (50.5%) | 1951 (44.3%) | ||
| GFR (mL/min/1.73m2) | 76.7 (20.11) | 79.8 (18.73) | 0.0068 | 75.4 (18.90) | 79.7 (18.83) | 0.0192 |
| GFR | 0.0007 | 0.0168 | ||||
| <60 | 56 (18.9%) | 503 (12.1%) | 22 (20.0%) | 539 (12.4%) | ||
| ≥60 | 241 (81.1%) | 3661 (87.9%) | 88 (80.0%) | 3824 (87.6%) | ||
| Total cholesterol (mg/dL)‡ | 186.9 (47.06) | 197.1 (43.93) | 0.0001 | 187.0 (50.39) | 196.6 (44.00) | 0.0236 |
| HDL (mg/dL)‡ | 47.1 (13.68) | 52.2 (15.65) | <0.0001 | 45.8 (15.38) | 52.0 (15.56) | <0.0001 |
| Systolic blood pressure (mmHg) | 135.4 (17.10) | 130.8 (16.42) | <0.0001 | 135.7 (16.96) | 131.0 (16.47) | 0.0030 |
| Treated blood pressure | 93 (30.9%) | 1604 (38.1%) | 0.0124 | 41 (36.9%) | 1657 (37.6%) | 0.8897 |
| Treated SBP × SBP interaction | 41.8 (63.43) | 48.6 (62.69) | <0.0001 | 49.3 (65.61) | 48.0 (62.65) | 0.0062 |
| Q waves present | 18 (6.1%) | 178 (4.3%) | 0.1452 | 7 (6.4%) | 190 (4.3%) | 0.3088 |
| LBBB§ | 5 (1.7%) | 57 (1.4%) | 0.6048 | 1 (0.9%) | 62 (1.4%) | 1.0000 |
| ST depression§ | 4 (1.3%) | 56 (1.3%) | 1.0000 | 2 (1.8%) | 58 (1.3%) | 0.6591 |
| LVH with repolarization§ | 3 (1.0%) | 31 (0.7%) | 0.4918 | 2 (1.8%) | 33 (0.8%) | 0.2121 |
| Any of the above electrocardiographic findings | 29 (9.8%) | 315 (7.6%) | 0.1686 | 11 (10.0%) | 336 (7.7%) | 0.3721 |
Student’s t-test used for continuous variables, chi-square test for categorical variables, except where indicated. Data are presented as mean (SD) or n (%).
Comparing high risk vs. non-high risk (50% criteria).
Comparing high risk vs. non-high risk (70% criteria).
Variables excluded from prediction models due to high number of missing or unknown values: high density lipoprotein (HDL), left bundle branch block (LBBB), left ventricular hypertrophy (LVH), nitroglycerin (NTG), relationship of symptoms to stress or exertion.
Fisher’s Exact Test.
BMI = body mass index, CAD = coronary artery disease, GFR = glomerular filtration rate, PAD = peripheral arterial disease, SBP = systolic blood pressure.
RESULTS
High-Risk CAD Prevalence by CTA
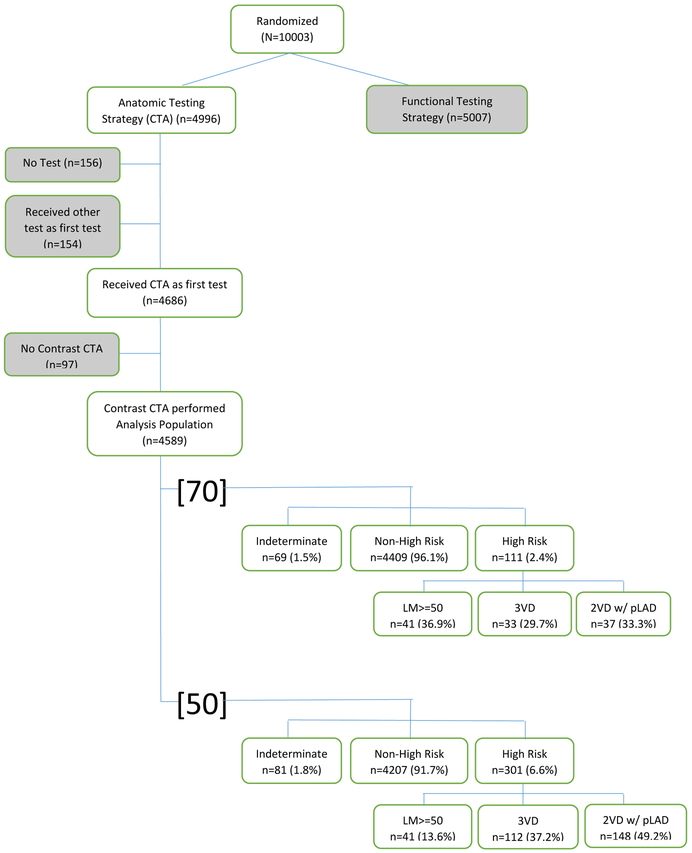
Of the 4589 included patients, 301 patients (6.6%) and 111 patients (2.4%) were identified with high-risk CAD based on the definition of ≥50% LM stenosis or ≥50% stenosis or ≥70% stenosis of 3VD or 2VD involving the pLAD, respectively (Table 1). Using the [50] and [70] high-risk criteria, there were 4207 and 4409 non-high-risk patients and 81 or 69 indeterminate patients, respectively (Figure 1). Of the 301 patients with [50] high-risk CAD, 41 (13.6%) had LM disease, 112 (37.2%) had 3VD, and 148 (49.2%) had 2VD (with pLAD). Of the 111 patients with [70] high-risk CAD, the same 41 (36.9%) had LM disease, 33 (29.7%) had 3VD, and 37 (33.3%) had 2VD (with pLAD).
Figure 1:
High-risk CAD model derivation. Of the 10,003 patients randomized in PROMISE, 4,589 patients who received CTA as an initial non-invasive test were used to derive our models. Two models for high-risk CAD were designed and defined as LM coronary artery stenosis (≥50% stenosis) or either (a) [50] (≥50% stenosis) or (b) [70] (≥70% stenosis) of 3VD or 2VD involving the pLAD. CTA indicates computed tomographic angiography; CAD, coronary artery disease; LM, left main; 3VD, three-vessel disease; 2VD w/ pLAD, two-vessel disease with proximal left anterior descending artery.
Results of Multivariable Model
Our model selection process identified eight clinical predictors of high risk using the [50] criteria: age (OR=1.06 [95% CI: 1.05–1.08] for each year increase); male sex (OR=3.52 [2.68–4.62]); diabetes mellitus (DM) (OR=2.02 [1.55–2.63]); family history of CAD (<55 years) (OR=1.65 [1.27–2.14]); anginal symptoms (typical vs. atypical, OR=1.33 [0.95–1.87]; typical vs. non-cardiac, OR=2.42 [1.35–4.34])16; smoking (OR=1.58 [1.22–2.03]); lower glomerular filtration rate (GFR) (OR=0.99 [0.98–0.99] for each mL/min/1.73 m2 increase up to 95, no additional effect for GFR>95); higher systolic blood pressure (SBP) (OR=1.01 [1.01–1.02] for each 1-mmHg increase) (Supplemental Tables 1 and 2). The [50] model demonstrated good discrimination with a c-statistic of 0.75 (95% CI: 0.72–0.78). This c-statistic was mildly optimistic (optimism of 0.018 [−0.009 to 0.045]), yielding a bias corrected c-statistic of 0.73 (95% CI: 0.71–0.76).
Our model selection process identified eight clinical predictors of high risk using the [70] criteria: age (OR=1.06 [1.04–1.09] for each year increase); male sex (OR=4.91 [3.11–7.75]); DM (OR=1.60 [1.05–2.46]); family history of CAD (<55 years) (OR=1.61 [1.07–2.43]); anginal symptoms (typical vs. atypical, OR=2.34 [1.48–3.71]; typical vs. non-cardiac, OR=2.91 [1.29– 6.54])16; sedentary lifestyle (OR=1.57 [1.06–2.33]); lower GFR (OR=0.99 [0.98–1.00] per 1 mL/min/1.73 m2 increase); higher SBP (OR=1.01 [1.00–1.02] for each 1-mmHg increase) (Supplemental Tables 3 and 4). The [70] model also demonstrated similar discrimination capacity with a c-statistic of 0.76 (95% CI: 0.71–0.81). Internal validation via bootstrapping showed that this c-statistic was mildly optimistic (optimism of 0.033 [−0.010 to 0.074], yielding a bias-corrected c-statistic of 0.73 [95% CI: 0.68–0.77]).
As a sensitivity analysis, the model development process was repeated using backward selection. This selection procedure produced the same models as stepwise selection.
Model Calibration
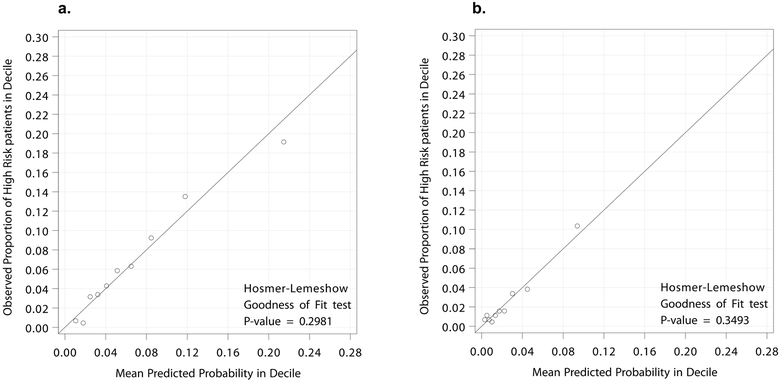
For model calibration, the observed proportions of [50] and [70] high-risk patients were compared with the mean predicted probability for high-risk CAD from each model. Using the decile plots and the Hosmer-Lemeshow goodness-of-fit test, both the [50] and [70] models demonstrated excellent calibration (p=0.298 and p=0.349, respectively) (Figure 2).
Figure 2:
Final calibration of the likelihood of high-risk patients. Actual observed proportion of high-risk CAD (LM ≥50% stenosis + (a) [50] ≥50% stenosis 3VD or 2VD involving the pLAD or (b) [70] ≥70% stenosis 3VD or 2VD involving the pLAD) compared to the mean predicted proportion of high risk. Using the decile plots and the Hosmer-Lemeshow goodness-of-fit test, both the [50] and [70] models demonstrated excellent calibration (p=0.298 and p=0.349, respectively). CAD indicates coronary artery disease; LM, left main; 3VD, three-vessel disease; 2VD w/ pLAD, two-vessel disease with proximal left anterior descending artery.
Processes of Care and Outcomes
Both patients with [50] and [70] high-risk CAD were referred for ICA significantly more than non-high-risk patients (63.1% vs. 8.0% [p<0.0001] and 73.9% vs. 10.3% [p<0.0001], respectively). Of patients with [50] high-risk CAD, 38.2% underwent PCI or CABG as compared to 3.8% of patients without high-risk CAD (p<0.001). Similarly, 52.3% of patients with [70] high-risk CAD underwent revascularization (PCI/CABG) as compared to 4.9% of patients with non-high-risk CAD (p<0.0001).
Patients with both [50] and [70] high-risk CAD experienced significantly more adverse events (composite endpoint of UAH/MI/death) over a median of 26 months of follow-up compared to patients without high-risk CAD (9.6% vs. 2.6% [p<0.0001] and 11.7% vs. 2.8% [p<0.0001], respectively). The difference between [50] and [70] high-risk CAD patients as compared to patients without high-risk CAD was greatest for UAHs (6.3% vs. 0.8% [p<0.0001] and 8.1% vs. 1.0% [p<0.0001], respectively) (Table 2).
Table 2:
Process of Care and Outcomes Measures in Patients Predicted to Have High-Risk CAD
| High Risk (≥50%) N=301 |
Non-High Risk (<50%) N=4207 |
OR [HR] (95% CI)* | p-value† | High Risk (≥70%) N=111 |
Non-High Risk (<70%) N=4409 |
OR [HR] (95% CI)* | p-value‡ | |
|---|---|---|---|---|---|---|---|---|
| Processes of Care | ||||||||
| ICA within 90 days after CTA§ | 190 (63.1%) | 338 (8.0%) | 19.6 (15.1, 25.4) |
<0.0001 | 82 (73.9%) | 452 (10.3%) | 24.8 (16.0, 38.2) |
<0.0001 |
| PCI (90 days)§ | 69 (22.9%) | 143 (3.4%) | 8.5 (6.2, 11.6) |
<0.0001 | 30 (27.0%) | 183 (4.2%) | 8.6 (5.5, 13.3) |
<0.0001 |
| CABG (90 days)‖ | 46 (15.3%) | 16 (0.4%) | 47.3 (26.4, 84.6) |
<0.0001 | 29 (26.1%) | 33 (0.7%) | 46.9 (27.2, 80.8) |
<0.0001 |
| PCI/CABG (90 days)§ | 115 (38.2%) | 158 (3.8%) | 15.8 (12.0, 21.0) |
<0.0001 | 58 (52.3%) | 216 (4.9%) | 21.2 (14.3, 31.6) |
<0.0001 |
| Outcomes | ||||||||
| CV Death/MI# | 8 (2.7%) | 52 (1.2%) | 2.11 (1.00, 4.44) |
0.0447 | 4 (3.6%) | 56 (1.3%) | 2.78 (1.01, 7.67) |
0.0391 |
| CV Death/MI/UA# | 26 (8.6%) | 84 (2.0%) | 4.47 (2.88, 6.94) |
<0.0001 | 13 (11.7%) | 97 (2.2%) | 5.74 (3.22, 10.24) |
<0.0001 |
| All Cause Death/MI# | 11 (3.7%) | 77 (1.8%) | 1.96 (1.04, 3.69) |
0.0334 | 4 (3.6%) | 84 (1.9%) | 1.86 (0.68, 5.08) |
0.2161 |
| All Cause Death/MI/UAH# | 29 (9.6%) | 109 (2.6%) | 3.85 (2.56, 5.80) |
<0.0001 | 13 (11.7%) | 125 (2.8%) | 4.48 (2.53, 7.93) |
<0.0001 |
| UAH (CEC)# | 19 (6.3%) | 34 (0.8%) | 8.05 (4.59, 14.11) |
<0.0001 | 9 (8.1%) | 44 (1.0%) | 8.52 (4.16, 17.44) |
<0.0001 |
Odds ratio (95% CI) listed for Process of Care, hazard ratios (95% CI) for Outcomes.
Comparing High Risk vs. Non-High Risk (50% criteria).
Comparing High Risk vs. Non-High Risk (70% criteria).
Chi-square test. ‖Fisher’s exact test.
Log-rank test.
CV = cardiovascular, CTA = computed tomographic angiography, CABG = coronary artery bypass graft surgery, ICA = invasive coronary angiography, MI = myocardial infarction, PCI = percutaneous coronary intervention, UAH = unstable angina hospitalization.
Model Comparison to Other Risk Scores
The [50] model (c-statistic: 0.75) was a better predictor of high-risk CAD compared to both the Pooled Cohort Equation (PCE) (c-statistic: 0.70, chi-square=18.18, p<0.0001),21 and modified Diamond-Forrester risk score (mDFS), aimed at symptomatic chest pain patients (c-statistic: 0.68, chi-square=26.90, p<0.0001). Similarly, the [70] model (c-statistic: 0.76) was a better predictive model for high-risk CAD than either the PCE (c-statistic: 0.71, chi-square=5.97, p=0.0145)21 or mDFS (c-statistic: 0.71, chi-square=9.47, p=0.0021) (Supplemental Figure 1). The [70] model performed similarly to the Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter Registry (CONFIRM) high-risk model (c-statistic: 0.73, chi-square=2.15, p=0.1422) (Supplemental Figure 2).
DISCUSSION
In the PROMISE study, high-risk CAD, defined by ≥50% LM stenosis or (a) ≥50% stenosis or (b) ≥70% stenosis of 3VD or 2VD involving the pLAD, was identified in 6.6% and 2.4% of stable symptomatic patients undergoing cardiac CTA, respectively. Two robust models, both with good calibration, were developed predicting the presence of high-risk CAD using pre-test variables, and both performed better than either the PCE or mDFS in predicting event risk or obstructive CAD, while the [70] model performed similar to the CONFIRM high-risk model. Patients identified with high-risk CAD experienced more frequent ICA, revascularization (PCI/CABG), and adverse events (UAH and UAH/MI/death) than those with non-high-risk coronary anatomy. The identification of high-risk individuals may assist in clinical decision making regarding testing, referral to catheterization, and intensity of medical treatment.
The prevalence of high-risk CAD from our contemporary cohort is remarkably less than in previous studies used to create currently recommended clinical risk scores. In the Coronary Artery Surgery Study (CASS) from 1975–1979, 20,391 symptomatic patients suspected of CAD underwent cardiac catheterizations. In this population, LM disease and/or 3VD was present in 8% of women and 27% of men.22 This prevalence is not dissimilar to the 33% of patients identified with LM disease or 3VD in the cohort used to create the Duke clinical risk score.9 Hubbard et al., who created a five-point cardiac risk score to identify high-risk CAD, reported 31% of consecutive patients referred for cardiac catheterization had LM disease or 3VD.11 These previously described studies used cardiac catheterization to identify high-risk CAD, which may have introduced a selection bias. In patients with chest pain undergoing coronary CTA, Min et al. identified 192/1127 (17%) patients with either LM disease (≥50% stenosis) or 3VD or 2VD (≥70% stenosis) with pLAD.15
CONFIRM, a similar contemporary study to PROMISE, enrolled 27,125 consecutive adults referred to coronary CTA for suspected CAD from 2005–2009.23 Using the definition of high-risk CAD as LM (≥50%) or 3VD or 2VD (≥70%) with pLAD, CONFIRM identified 3.6% of patients with high-risk CAD, a prevalence that is much closer to PROMISE’s than reported in older studies.24 However, CONFIRM reported a slightly higher prevalence of high-risk CAD compared to our PROMISE [70] high-risk cohort (2.4%), likely due to CONFIRM including a broad population with few restrictions. In fact, 24.7% of the CONFIRM high-risk patients were asymptomatic. Only symptomatic patients were enrolled in PROMISE. From the recent Scottish Computed Tomography of the HEART Trial (SCOT-HEART), 7% of the patients from the CTA arm (n=1778) were identified with three-vessel obstructive (>70% stenosis) CAD. The number of patients with obstructive LM CAD was not reported.25
We developed two models to predict [50] and [70] high-risk CAD using clinical variables easily identified in patient’s medical history. Our models had seven clinical variables in common: family history of premature CAD, older age, male sex, lower GFR, DM, elevated SBP, and angina. Smoking history was a predictor in the [50] model, while the [70] model included sedentary lifestyle. Since both models are completely derived from clinical variables, clinicians can obtain this information real-time during an outpatient visit and use it to help determine the need for testing. By enabling physicians to identify patients who may have high-risk CAD, our models can assist in recognizing patients who may potentially benefit from more aggressive medical treatment, closer follow-up, and/or consideration for early invasive catheterization.
Despite the difference in prevalence of high-risk CAD found in the population used to create the older Duke risk model compared to our study cohort, Pryor et al. identified similar predictors for high-risk CAD including age, sex, chest pain characteristics, DM, smoking, and hypertension, but also hyperlipidemia, peripheral or cerebral artery disease, carotid bruit, prior MI, and significant Q wave and ST-T wave changes.9 Similar to the Duke risk model, a risk score developed by Hubbard et al. identified five variables that were independently predictive of LM or 3VD: age, typical angina, DM, male sex, and both history and electrocardiographic evidence of a prior MI.11 In the Coronary Risk Score (CORSCORE) study, the predictive accuracy of the Duke risk model was found to be marginally higher than the Diamond–Forrester model in predicting significant 3VD.26 However, an updated Diamond–Forrester model was recently described to better predict obstructive CAD in a contemporary cohort.27 Both our [50] and [70] models were more predictive of high-risk CAD than the PCE and mDFS (Supplemental Figure 1). In addition to both of our models outperforming the PCE and Diamond and Forrester scores, our models are more clinically applicable to a contemporary population since the data set in which both the PCE and Diamond and Forrester scores were derived originated from patients in the 1960s-1990s.
Investigators from the CONFIRM study recently created a scoring system to predict high-risk CAD based on clinical risk factors and symptoms. Similar to the clinical variables from both our models, CONFIRM identified age, sex, DM, hypertension, current smoking, chest pain symptoms, family history of CAD, hyperlipidemia, and peripheral vascular disease as independent variables associated with high-risk CAD. A comparison of our [70] model with the CONFIRM model demonstrated that both performed similarly in identifying high-risk CAD (Supplemental Figure 2). Similar to both of our models, the CONFIRM clinical model demonstrated better performance than the mDFS in predicting high-risk CAD.24
Identifying patients with high-risk CAD prior to diagnostic testing can help ensure that these patients receive closer follow-up or early cardiac catheterization. As demonstrated in our study, patients with high-risk CAD have a greater risk for adverse cardiovascular events (UAH/MI/death) compared to patients with non-high-risk CAD. The difference was especially marked for UAH (Table 2). Puri et al. followed atherosclerotic progression in patients with LM CAD using serial intravascular ultrasound and reported that the patients who suffered adverse clinical events experienced more UAH as compared to MI or death.28 In our study, patients with high-risk CAD required more revascularization (PCI and/or CABG) as compared to patients without high-risk CAD. These observations were documented in previous studies in which patients with high-risk CAD on cardiac catheterization had higher cardiovascular events and derived benefit from coronary revascularization.1–2, 4–7, 9, 29–31 Min et al. described that pLAD, multivessel, and LM disease identified on CTA were significant predictors of all-cause death; LM (≥50% stenosis) on CTA had the worst survival (85%) at approximately 2 years.15
It is important to note a few limitations of our study. Although the results from our prediction models were generated from the largest contemporary evaluation of noninvasive testing among patients with stable chest pain, the prevalence of high-risk CAD was found to be small. But as previously discussed, similar contemporary studies (CONFIRM and SCOT-HEART) demonstrated a comparably low prevalence of high-risk CAD in patients suspected of CAD who were referred for coronary CTA. These recent observations of lower high-risk CAD prevalence compared to previous studies/registries and others noting a decreasing prevalence of positive noninvasive testing appear to reflect the changes in cardiovascular prevention, testing, and management practices compared to decades ago and strongly support the development of new risk scores in contemporary populations.32 Although validation of our model with a separate external cohort could potentially strengthen the model, the only large contemporary cohort similar to our symptomatic PROMISE patients would be from the SCOT-HEART study.25 However, as mentioned above, the SCOT-HEART investigators identified patients with significant 3VD but did not report obstructive LM CAD, so external validation with our model was unobtainable. High-risk CAD patients from our study were derived only from the CT cohort of the PROMISE study. Thus, the prevalence of high-risk CAD in the cohort randomized to initial functional testing in the PROMISE study is unknown, except in those referred to cardiac catheterization who are subject to bias. Since the PROMISE study randomized patients 1:1 to either the functional or anatomical arm, we would expect a similar prevalence of high-risk CAD in the functional testing. In this study, high-risk CAD was identified using CTA as opposed to the gold standard test, ICA. However, previous studies have demonstrated that CTA and ICA have similar accuracy for high-risk CAD.33 Although the PROMISE study was designed to capture only ICAs performed within 90 days after the initial CTA, 95.6% and 97.7% of the total ICAs performed in the [50] and [70] high-risk groups, respectively, were performed within those 90 days.
Conclusion
In contemporary practice, 2.4–6.6% of patients without prior CAD who have stable symptoms and an indication for noninvasive cardiovascular testing have high-risk CAD by CTA. Although the prevalence of high-risk CAD is low, a limited set of readily available pre-test clinical variables identifies these potentially high-risk patients and is easy to use at the time of initial consultation. Patients predicted to have high-risk CAD have more cardiovascular events and undergo more revascularizations than those without high-risk CAD. While requiring independent validation, our findings identify patients who may benefit from more aggressive medical treatment and consideration for early cardiac catheterization.
Supplementary Material
Clinical Perspective.
In the outpatient clinic setting it is often difficult to identify patients with high-risk CAD. In addition to a standard patient history and physical exam, physicians often order noninvasive cardiac testing, such as a coronary CTA, to assess for CAD. Patients with significant CAD often undergo invasive coronary angiography and then revascularization. Using the PROMISE study cohort randomized to CTA, we developed two predictive models to identify high-risk CAD. We provided two different criteria for high risk CAD defined as LM stenosis (≥50% stenosis), and either (a) ≥50% stenosis ‘[50]’ or (b) ≥70% stenosis ‘[70]’ of three vessels or two-vessel CAD involving the pLAD in order to provide physicians two clinical applications based on the stenosis threshold on CTA which they feel is most meaningful. Both our [50] and [70] models predicted high-risk CAD with good discrimination. A simple combination of clinical variables readily available before testing improves prediction of high-risk CAD over traditional risk assessments.
Numerous studies have demonstrated that symptomatic patients with obstructive left main (LM) coronary artery disease (CAD), three-vessel CAD (3VD), or two-vessel CAD (2VD) involving the proximal left anterior descending artery (pLAD) have significantly worse prognosis and increased cardiovascular events.1–3 In these high-risk patients, expeditious identification and revascularization have been proven to improve clinical outcomes.4–7 Decades ago, clinical predictive risk models and scores were developed to estimate pretest likelihoods for CAD, including LM CAD and 3VD.8–11 In these studies, the prevalence of LM CAD and/or 3VD ranged from 8–33% in symptomatic patients suspected of having CAD who were referred for invasive coronary angiography (ICA), far higher than is seen in current populations undergoing evaluation for suspected CAD.12 In fact, even with current risk assessment tools and noninvasive testing, more than 50% of patients are found to have non-obstructive CAD at the time of their ICA.13 Since the 1990s when many of the prediction models were developed, the presentation, treatment, and natural history of CAD have dramatically changed. This warrants the need to reevaluate these risk predictions. The aim of this study is to develop contemporary predictive models using clinical features, risk factors, and test results from the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) to identify symptomatic outpatients with high-risk CAD prior to any noninvasive testing that may help to inform care decisions.14 Secondary aims include comparing our models to older and current predictive models as well as evaluating present-day clinical outcomes in patients identified with high-risk CAD.
Acknowledgments
Sources of Funding
This project was supported by grants R01HL098237, R01HL098236, R01HL98305, and R01HL098235 from the National Heart, Lung, and Blood Institute (NHLBI). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents. This paper does not necessarily represent the official views of NHLBI.
Disclosures
S.V. reports receiving research grants from the American College of Cardiology, Abbott Vascular, Agency for Healthcare Research and Quality, and consulting fees from Novella and Premiere; and travel from Phillips Healthcare. K.L.L. reports receiving grants from the National Institutes of Health. U.H. reports receiving grants from HeartFlow and Kowa Pharmaceuticals. D.B.M. reports receiving grants from the National Institutes of Health, Eli Lilly and Company, Bristol-Myers Squibb, Gilead Sciences, AGA Medical Corporation, Merck & Company, Oxygen Therapeutics, and AstraZeneca, and personal fees from CardioDx, Medtronic, and St. Jude Medical. P.S.D. reports receiving grant support from HeartFlow and service on a data and safety monitoring board for GE HealthCare. M.R.P reports receiving grants from AstraZeneca, CSL, Heart Flow Technologies, Janssen, Johnson & Johnson, MAQUET, Medtronic, and the NHLBI; and has served on advisory boards for Astra Zeneca, Bayer, CSL, Genzyme Corp., Janssen, Medtronic, and Merck.
References
- 1.Emond Mock MB, Davis KB, Fisher LD, Holmes DR Jr, Chaitman BR, Kaiser GC, Alderman E, Killip T 3rd. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90:2645–2657. [DOI] [PubMed] [Google Scholar]
- 2.Leipsic J, Taylor CM, Grunau G, Heilbron BG, Mancini GB, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Cury R, Feuchtner G, Kim YJ, Kaufmann PA, Lin FY, Maffei E, Raff G, Shaw LJ, Villines TC, Min JK. Cardiovascular risk among stable individuals suspected of having coronary artery disease with no modifiable risk factors: results from an international multicenter study of 5262 patients. Radiology. 2013;267:718–726. [DOI] [PubMed] [Google Scholar]
- 3.Carrigan TP, Nair D, Schoenhagen P, Curtin RJ, Popovic ZB, Halliburton S, Kuzmiak S, White RD, Flamm SD, Desai MY. Prognostic utility of 64-slice computed tomography in patients with suspected but no documented coronary artery disease. Eur Heart J. 2009;30:362–371. [DOI] [PubMed] [Google Scholar]
- 4.Caracciolo EA, Davis KB, Sopko G, Kaiser GC, Corley SD, Schaff H, Taylor HA, Chaitman BR. Comparison of surgical and medical group survival in patients with left main equivalent coronary artery disease. Long-term CASS experience. Circulation. 1995;91:2335–2344. [DOI] [PubMed] [Google Scholar]
- 5.Takaro T, Hultgren HN, Lipton MJ, Detre KM. The VA cooperative randomized study of surgery for coronary arterial occlusive disease. II. Subgroup with significant left main lesions. Circulation. 1976;54(suppl III):107–117. [PubMed] [Google Scholar]
- 6.European Coronary Surgery Study Group. Coronary artery bypass surgery in stable angina pectoris: survival at two years. Lancet. 1979;1:889–893. [PubMed] [Google Scholar]
- 7.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 8.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE Jr, Muhlbaier LH, Califf RM. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. [DOI] [PubMed] [Google Scholar]
- 9.Pryor DB, Shaw L, Harrell FE Jr, Lee KL, Hlatky MA, Mark DB, Muhlbaier LH, Califf RM. Estimating the likelihood of severe coronary artery disease. Am J Med. 1991;90:553–562. [PubMed] [Google Scholar]
- 10.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard BL, Gibbons RJ, Lapeyre AC 3rd, Zinsmeister AR, Clements IP. Identification of severe coronary artery disease using simple clinical parameters. Arch Intern Med. 1992;152:309–312. [PubMed] [Google Scholar]
- 12.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–1065. [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, Khan MA, Kosinski AS, Krucoff MW, Malhotra V, Picard MH, Udelson JE, Velazquez EJ, Yow E, Cooper LS, Lee KL; PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. [DOI] [PubMed] [Google Scholar]
- 16.Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, Marco J, Morais J, Pepper J, Sechtem U, Simoons M, Thygesen K, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381 [DOI] [PubMed] [Google Scholar]
- 17.Calster BV, Niebor D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. 2016;74:167–176. [DOI] [PubMed] [Google Scholar]
- 18.Steyerberg EW. Overfitting and optimism in prediction models In: Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York, NY: Springer, 2009:83–100. [Google Scholar]
- 19.Harrell FE Jr. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag, 2001:109–115. [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 21.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 22.Chaitman BR, Bourassa MG, Davis K, Rogers WJ, Tyras DH, Berger R, Kennedy JW, Fisher L, Judkins MP, Mock MB, Killip T. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation. 1981;64:360–367. [DOI] [PubMed] [Google Scholar]
- 23.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Delago A, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Maffei E, Nasir K, Pencina MJ, Raff GL, Shaw LJ, Villines TC. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Chen L, Yam Y, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann PA, Kim YJ, Leipsic J, LaBounty T, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK, Chow BJW. A clinical model to identify patients with high-risk coronary artery disease. JACC Cardiovasc Imaging. 2015;8:427–434. [DOI] [PubMed] [Google Scholar]
- 25.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 26.Jensen JM, Voss M, Hansen VB, Andersen LK, Johansen PB, Munkholm H, Nørgaard BL. Risk stratification of patients suspected of coronary artery disease: comparison of five different models. Atherosclerosis. 2012;220:557–562. [DOI] [PubMed] [Google Scholar]
- 27.Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, Galema TW, Meijboom WB, Mollet NR, de Feyter PJ, Cademartiri F, Maffei E, Dewey M, Zimmermann E, Laule M, Pugliese F, Barbagallo R, Sinitsyn V, Bogaert J, Goetschalckx K, Schoepf UJ, Rowe GW, Schuijf JD, Bax JJ, de Graaf FR, Knuuti J, Kajander S, van Mieghem CA, Meijs MF, Cramer MJ, Gopalan D, Feuchtner G, Friedrich G, Krestin GP, Hunink MG; CAD Consortium. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J. 2011;32:1316–1330. [DOI] [PubMed] [Google Scholar]
- 28.Puri R, Wolski K, Uno K, Kataoka Y, King KL, Crowe TD, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ. Left main coronary atherosclerosis progression, constrictive remodeling, and clinical events. JACC Cardiovasc Interv. 2013;6:29–35. [DOI] [PubMed] [Google Scholar]
- 29.Myers WO, Blackstone EH, Davis K, Foster ED, Kaiser GC. CASS Registry long term surgical survival. J Am Coll Cardiol. 1999;33:488–498. [DOI] [PubMed] [Google Scholar]
- 30.VA Coronary Artery Bypass Surgery Cooperative Study Group. Eighteen-year follow-up in the Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for stable angina. Circulation. 1992;86:121–130. [DOI] [PubMed] [Google Scholar]
- 31.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, Mack MJ, Holmes DR Jr, Morel MA, Van Dyck N, Houle VM, Dawkins KD, Serruys PW. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381:629–638. [DOI] [PubMed] [Google Scholar]
- 32.Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LE, Berman DS. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol. 2013;61:1054–1065. [DOI] [PubMed] [Google Scholar]
- 33.Sheth T, Amlani S, Ellins ML, Mehta S, Velianou J, Cappelli G, Yang S, Natarajan M. Computed tomographic coronary angiographic assessment of high-risk coronary anatomy in patients with suspected coronary artery disease and intermediate pretest probability. Am Heart J. 2008;155:918–923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.