Abstract
Cell free protein synthesis has become a powerful method for the high-throughput production of proteins that are difficult to express in living cells. The protein SAP2 of Fasciola hepatica (FhSAP2), which has demonstrated to be both, an excellent vaccine candidate against experimental fascioliasis and a good antigen for serodiagnosis of human chronic fascioliasis, is a typical example of a molecule that is difficult to produce. This is mainly due to its tendency to get over-expressed in inclusion bodies by prokaryotes. FhSAP2 expressed in an Escherichia coli-based expression system is poorly glycosylated, insoluble and often undergoes improper folding leading it to reduced immunogenicity. In this work, FhSAP2 was expressed in vitro using the eukaryote cell free system, TNT T7 Quick coupled transcription / translation, that has been designed for the expression of PCR-generated DNA templates. FhSAP2 was expressed in micro-volumes and purified by an affinity chromatography method, which gave a protein yield of 500μg/ml as determined by Bicinchoninic Acid Assay (BCA) method. Circular dichroism, Western blotting and enzyme-linked immunosorbent assay (ELISA) analysis were used to confirm the secondary structure, purity and integrity of protein. Results demonstrate that FhSAP2 can be expressed in a cell-free system retaining its main conformational and antigenic properties. The protein purified could be used in immunization experiments and immunodiagnostic techniques.
Keywords: Fasciola hepatica, saposin-like protein-2, cell-free expression system
Introduction
Over the last half century there has been a continuous interest in the development of cell-free expression systems based on eukaryotic organisms, which have been attributed a wide spectrum of applications that range from bioengineering [1], identification of novel drugs [2], nano-biotechnology [3], evolutionary biology [4] and high-throughput proteomics [5]. Compared to the cell-based expression systems, cell free expression systems offer great advantages, such as a shorter production process and the possibility to express multiple proteins simultaneously using PCR products without the tedious, complex and laborious cloning and transformation steps [6]. Moreover, eukaryotic cell free expression systems have the additional advantage of providing post-translational modifications, such as glycosylations; an option that is not possible in any prokaryotic expression system [7]. In the current study, we explored the use of cell-free synthesis for the rapid expression of an antigenic protein belonging to the saposin-like protein family of Fasciola hepatica (FhSAP2), which is the parasite responsible for acute and chronic fascioliasis disease. Although not as deadly as malaria or schistosmiasis, fascioliasis is a disease that substantially burdens the human population in two main endemic hubs: Peru and Bolivian Altiplano [8]. Moreover, according to the world health organization, not only there are about 180 million persons at risk of infection worldwide, but fascioliasis is also responsible for more than US$2 billion in economic losses per year worldwide [9].
FhSAP2 was primarily identified by immuno-screening an adult fluke cDNA library, and was further cloned in an E. coli expression vector and characterized immunologically and biochemically [10]. FhSAP2 has shown to be a highly specific antigen capable of detecting both, acute and chronic infection in experimental animals [10,11], as well as chronic human fascioliasis cases using ELISA or Luminex assays [4,12]. Additionally, FhSAP2 has shown to be an excellent immunogen capable of inducing partial protective immune responses in mice and rabbits challenged with F. hepatica [11,13]. As do all members of the saposin-like protein family (SAPLIP) family, FhSAP2 possesses in its protein moiety 6-conserved cysteines and 7 hydrophobic residues arranged within 5 amphipathic α-helical domains at positions that are strictly conserved [10]. FhSAP2 also possesses lineal and conformational B-cell epitopes throughout its whole molecule [14]. Therefore, the antigenicity of this molecule greatly depends on the disulfide bond formations and proper folding, which are both very difficult to achieve because FhSAP2 is usually expressed as insoluble inclusion bodies in E. coli. Although several strategies to solubilize inclusion bodies and achieve the protein refolding have been successfully assayed [10,15,16], the whole process to express and purify FhSAP2 is tedious, time-consuming and often inefficient when relative large protein batches are needed. In the present study, we accomplished the expression of FhSAP2 in vitro using a TNT T7 Quick Coupled Transcription/Translation system. The protein was expressed fused with a His-tag at the carboxyl end and was purified by immobilized metal affinity chromatography (IMAC). Circular dichroism, western blotting and ELISA analysis demonstrated that the purified protein retains its main structural and antigenic properties. Compared to the conventional expression in E. coli, this approach allows a relatively higher protein yield with minimal processing time. It can thus be used for the large-scale production of FhSAP2 for subsequent immunization protocols or immunodiagnostic use.
Material and methods
Rabbit sera
For this study, we produced two rabbit polyclonal antibodies against recombinant FhSAP2 expressed in bacteria [10] and against F. hepatica excretory-secretory products (ESPs) obtained by in vitro culture of adult flukes for 24-h in RPMI- media [17]. Rabbits were immunized with 250μg FhSAP2 or ESPs mixed with an equal amount of complete Freund’s adjuvant by subcutaneous injection. The rabbits were boosted twice with equal amounts of protein mixed with in- complete Freund’s adjuvant at 2-week intervals. Blood was collected 2 weeks after the last immunization and evaluated by ELISA for specific antibody production. Four New Zealand White (NZW) rabbits were infected orally with 60 F. hepatica metacercariae each. Blood was obtained for the collection of serum before infection and then at 4th and 12th weeks after infection. Antisera preparation and animal infections were performed after obtaining the authorization of the Institutional Animal Care and Use Committee of University of Puerto Rico (IACUC # 7870110).
Preparation of enzyme conjugate
Serum IgG was precipitated from the rabbit anti-FhSAP2 serum by adjusting the injection solution to 40% saturation with saturated ammonium sulfate. The precipitated IgG fraction was suspended in a small volume of 0.01M phosphate buffered saline (PBS), pH7.2, dialyzed against an excess of PBS, and passed through a prepacked Protein A-Horseradish peroxidase (HRP) 5/5 column (GE Healthcare). IgG was concentrated to 10 mg/ml by using a high-flow membrane filter (YM-100; Amicon Corp.) and conjugated to horseradish peroxidase (type VI; Sigma) using the Nakane and Kawaoi method [18].
PCR- template preparation
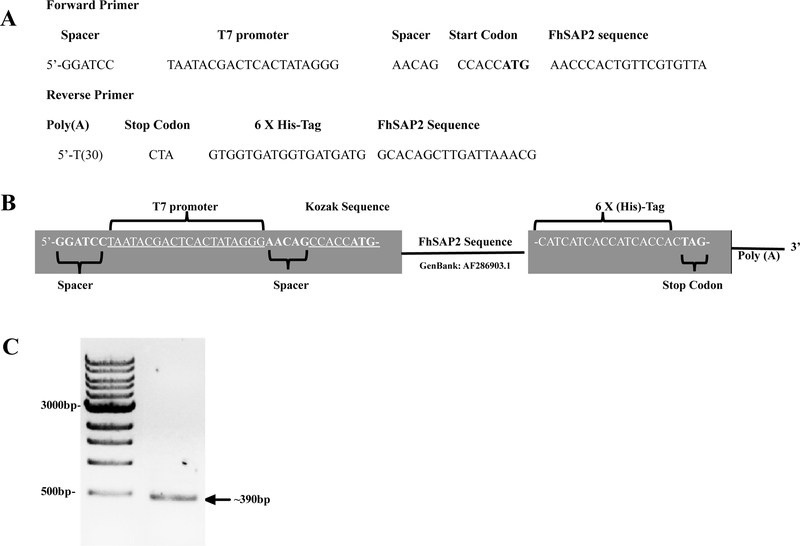
FhSAP2 protein is encoded by 300-bp complementary DNA (cDNA) (GenBank # AF286903), which is cloned in frame into pBAB-His-B expression vector [10]. cDNA-FhSAP2 was used to amplify a PCR product using primers of 50-mers length each specifically designed for this study. The forward primer containing the T7 promoter matches 21-bases of the encoding region of the FhSAP2 gene, including the ATG start codon. A Kozak consensus sequence and two short spacer sequences were also added at the 5’ forward primer to ensure the efficiency of the translation initiation. To enhance the translation of the PCR-product, a Poly (T)30 tail was added to the 5’-end reverse primer, which matches 21-bases of the carboxyl terminus of the FhSAP2 gene including the stop codon (TAG). Reverse primers also contained a poly-histidine region (6xHis) to facilitate the purification of recombinant protein by immobilized metal affinity chromatography (IMAC) (Fig. 1A–B). PCR was performed using a KOD Extreme Hot Start DNA polymerase (EMD Millipore 71975–3) in a thermocycler Model Veriti (Thermofisher, USA) and 200-pMol primers. The cycling parameters were: 94°C for 2 min as an initial denaturing step, followed by 98°C for 10 sec, annealing at 58°C for 30 sec, 1 min extension at 68°C for 30 cycles, and a final extension at 68°C for 5 min. After, the PCR product was analyzed by 1.5% agarose gel electrophoresis, as expected we obtained a unique band of ~390-bp (Fig. 1C).
Figure-1. Primers designed and genetic construct assembly.
(A-B) Forward primer-containing T7 polymerase promoter, spacer sequences and the Kozak sequence (CCACC) was synthesized. In the reverse primer a tag of 6-Histidine and a poly Adenine tail sequence for RNA product stability were added. (C) PCR product amplified with these primers was analyzed by 1.5% agarose gel electrophoresis resulting in a ~390-bp product, which include ~300-bp FhSAP2 sequence + 39-bp T7 forward + 51-bp T7 reverse.
Cell-free protein expression
To achieve the synthesis of FhSAP2 in vitro we used the TNT (Rabbit Reticulocyte Lysate) T7 Quick Coupled Transcription/Translation system (Promega Catalog # L1170) designated for expression of PCR-generated DNA templates. Eight micro-tubes containing each the following reaction mixture were prepared: 40μl TNT T7 Quick Master Mix + 1μl 1mM methionine, 5μl PCR-generated DNA template (829ng/μl) +1μl of TNT T7 PCR enhancer + 3μl nuclease free water (final volume 50μl). After a gentle agitation, the tubes were incubated at 300C for 90 min. The protein synthesized in each micro-tube was analyzed by 12.5% sodium dodecyl sulfate gel electrophoresis (SDS-PAGE) under no reduction conditions using the Laemmli method [19]. The unstained gel was transferred onto a nitrocellulose membrane and incubated overnight at 4°C with specific anti-FhSAP2-HRP conjugate diluted 1:2,500 revealing the presence of monomeric and oligomeric forms of FhSAP2. Tubes were pooled and loaded onto a 5ml HisTrap HP column (GE Healthcare, USA). His-tagged protein was eluted by washing the column with 20mM sodium phosphate containing 0.5 M NaCl + 500mM imidazole, pH 7.4. Fractions collected were pooled, desalted against PBS using a PD-10 column (sephadex G-25), and concentrated by an AMICON ultrafiltration system using an YM-3 membrane (>3kDa). Purified protein, designated as S-FhSAP2, was analyzed by SDS-PAGE, as described above, in the presence or absence of 2-mercaptoethanol and coomassie blue was used for gel staining. Protein concentration was measured using the BCA method.
Secondary structure analysis and circular dichroism (CD)
The absorption spectra of S-FhSAP2 was recorded spectrophotometrically at 20°C with a scan speed of 20 nm/ min (200–320 nm). CD measurements in the far-UV region (190–350 nm) were performed with a Jasco J-1500 CD spectrometer and protein concentrations of 0.1 mg mL−1 in 50 mM phosphate buffer, pH 8.0 in the 190–250 nm ranges at 20 °C and 95°C using 0.1-cm pathlength cell.
Determination of the antigenicity of the cell-free synthesized protein
To determine the antigenicity of S-FhSAP2, the protein was tested against the anti-ESP polyclonal antibody and against sera from rabbits with 4 weeks of F. hepatica infection. For this purpose, an ELISA protocol was developed, which was optimized via checkerboard titration. Antigen, serum, and conjugate were assayed at different concentrations to maximize the sensitivity of the assay. The optimal concentration of S-FhSAP2 was determined to be 2.5μg/ml, and the optimal dilution of serum and conjugate was determined to be 1:200 and 1:5,000, respectively (data not shown). The ELISA was performed following a basic protocol as previously reported [12].
Statistical analysis
All ELISA determinations were performed in duplicate and the experiment was replicated twice. Absorbance values were expressed as mean absorbance value for each determination. The Student’s t-test to determine differences between serum samples was performed using Graphpad Prism software (Prism-6). For all tests, a p value of < 0.05 was deemed significant.
Results and discussion
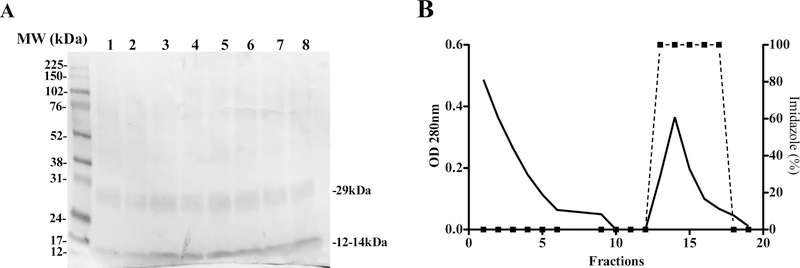
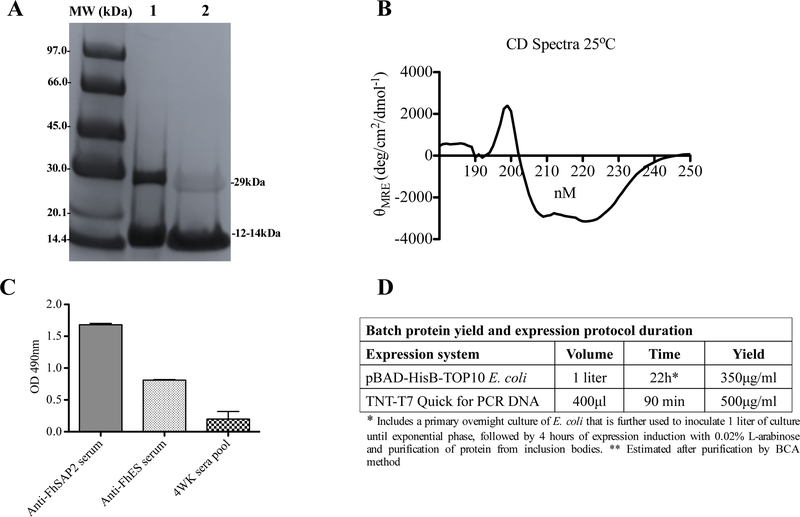
When the micro-reactions containing the FhSAP2-PCR template were incubated at 30°C for 90 min, the protein obtained from the PCR product demonstrated no variation within the expression tubes in terms of total yield and soluble fractions, which was determined by testing each reaction mix via western blotting against the anti-FhSAP2 antibody-HRP conjugates. As expected, the antibody recognized the typical 12–14kDa polypeptide of recombinant FhSAP2, which includes 11.5kDa of the amino sequence plus 2.5kD corresponding to the His-tag located at the carboxyl terminus of the protein moiety (Fig. 2A). In addition, the antibody reacted with a polypeptide of ~29kDa that presumably corresponds to the oligomeric forms of FhSAP2, which are formed due to the presence of several intrachain or interchain disulfide bonds [20]. Once we confirmed that all tubes contained FhSAP2, they were pooled and the protein was purified by IMAC using a HisTrap HP 5/5 column (Fig. 2B) and again analyzed by SDS-PAGE in the presence of 2-mercaptoethanol, to achieve disulfide bonds reduction. Results demonstrated that after 2-mercaptoethanol treatments, the polypeptide band of 29kDa reduced in intensity (Fig. 3A), confirming that these oligomers are formed due to the presence of several intrachain or interchain disulfide bonds. Both, monomeric and oligomeric forms of FhSAP2 have been detected using monoclonal antibodies in soluble crude extracts of F. hepatica adult flukes [20]. Circular dichroism analysis also revealed that the protein synthesized in vitro exhibits a high content of alpha helixes with a maximum and minimum in spectra between 197 to 201nm and 210 to 225nm respectively, which is the typical secondary structure all members in the saposin-like protein family exhibit [21–23]. The maximum molar ellipticity values (θ197-200) ranged between 1517 and 2124 and the minimum values (θ210-225) ranged between −2879 and −3149, respectively (Fig. 3B). The presence of six alpha helical motif and six cysteine residues at positions strictly conserved is one of the most notable structural characteristic of FhSAP2 [10].
Figure-2. Cell free expression of F. hepatica saposin-like protein-2.
Target gene was PCR amplified from the cloned vector and used as the template for the cell-free protein synthesis. (A) Two polypeptides of ~12–14kDa and ~29kDa corresponding to monomeric and oligomeric forms of FhSAP2, respectively were revealed in all tubes. (B) The content of all tubes was pooled. The His-tagged protein was purified by Ni-affinity chromatography by elution with a buffer containing 20mM sodium phosphate containing 0.5 M NaCl + 500mM imidazole, pH 7.4 (dashed line).
Figure-3. Synthetic FhSAP2 retains its main structural and antigenic properties.
(A) 12.5% SDS-PAGE analysis of S-FhSAP2 was performed under reduction conditions. Lane-1 represents untreated S-FhSAP2 and lane-2 represents S-FhSAP2 treated with 2-mercaptoethanol. (B) Circular dichroism (CD) spectra of S-FhSAP2 were performed by measurements in the far-UV region (180–250 nm). A Jasco J-1500 CD spectrometer was used with protein concentration of 0.1 mg mL−1 in 50mM phosphate buffer, pH 8.0 in the 190–250 nm ranges at 25°C using 0.1-cm path-length cell. (C) ELISA was used to test the ability of S-FhSAP2 to react with specific anti-FhSAP2, anti-ESP sera or a pool of sera from animals with 4 weeks of F. hepatica infection. (D) Protein yield and expression protocol duration when FhSAP2 is expressed in E. coli vs. a cell-free expression system. *Includes a primary overnight culture of E. coli that is further used to inoculate 1 liter of culture until exponential phase, followed by 4 hours of expression induction with 0.02% L-arabinose, and purification of protein from inclusion bodies. **Estimated after purification by BCA method.
A previously optimized ELISA [11] was used to test the protein’s capacity to react with a pool of sera raised against F. hepatica excretory-secretory products, a pool of serum from animals with four weeks of F. hepatica infection and the anti-FhSAP2 antibody. We had previously demonstrated that FhSAP2 is a component of the F. hepatica ES products [10], is expressed at early stages of parasite development [20] and is reactive with early sera infection [10,11]. Therefore, the finding that our cell-free expressed protein is reactive with all these sera (Fig. 3C) indicates that FhSAP2 is not only structurally similar to the native protein, but that it also retained its antigenic properties. According to the relative concentration of FhSAP2 determined by the bicinchoninic acid method [24], the current cell free expression approach had the ability to produce a batch yield of up to 500μg/ml active FhSAP2 in 90 minutes, which is a yield higher than the conventional E. coli expression system obtained after purifying protein in soluble form from inclusion bodies with a significantly lower processing time (Fig. 3D).
Conclusions
In summary, we have successfully applied an existing commercial cell-free expression system as an approach to produce recombinant FhSAP2 in vitro. Our results show that this F. hepatica protein can be efficiently expressed with relatively high yields and displayed the same conformational antigenicity without the tedious, and time-consuming process of expression in E. coli. With this approach, the issue of soluble expression was fully addressed. Because the presented methodology could be easily scaled up for production, we consider it a viable option when large batches of protein are required. Based on this study, we conclude that the protein produced by this approach could be used in immunization experiments and immunodiagnostic techniques.
Acknowledgement
This research was supported by Grants number G12MD007600, R25GM061838 from the National Institute on Minority Health and Health Disparities and from Puerto Rico Louis Stokes Alliance for Minority Participation-Bridge to the Doctorate Program Fellowship; Funded by the National Sciences Foundation, NSF Grant Award HRD-1400870 and Support Competitive Research (SCORE) Research Advancement (SC1) Grant award 1SC1AI096108-01A2. The content is solely responsibility of the authors and does not necessary represent the official views of NSF.
Footnotes
Compliance Ethical Statement
The authors of the current manuscript declare that there is no potential conflict of interest.
Rabbits used in the study were maintained in the facilities of the Animal Resources Center of Medical Sciences Campus, University of Puerto Rico, and all experiments were performed under an MSC-IACUC-approved animal study protocol (No. 7870110), which follows AAALAC guidelines.
References
- 1.Rivera F, Espino AM (2016) Adjuvant-enhanced antibody and cellular responses to inclusion bodies expressing FhSAP2 correlates with protection of mice to Fasciola hepatica. Exp Parasitol 160: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swartz J (2006) Developing cell-free biology for industrial applications. J Ind Microbiol Biotechnol 33: 476–485. [DOI] [PubMed] [Google Scholar]
- 3.Caban-Hernandez K, Gaudier JF, Ruiz-Jimenez C, Espino AM (2014) Development of two antibody detection enzyme-linked immunosorbent assays for serodiagnosis of human chronic fascioliasis. J Clin Microbiol 52: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin SH, Hsu A, Chastain HM, Cruz LA, Elder ES, Sapp SG, McAuliffe I, Espino AM, Handali S (2016) Development of Two FhSAP2 Recombinant-Based Assays for Immunodiagnosis of Human Chronic Fascioliasis. Am J Trop Med Hyg 95: 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spirin AS (2004) High-throughput cell-free systems for synthesis of functionally active proteins. Trends Biotechnol 22: 538–545. [DOI] [PubMed] [Google Scholar]
- 6.Espino AM, Rivera F (2010) Quantitation of cytokine mRNA by real-time RT-PCR during a vaccination trial in a rabbit model of fascioliasis. Vet Parasitol 169: 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landis JR, Koch GG (1977) An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics 33: 363–374. [PubMed] [Google Scholar]
- 8.Solano-Parada J, Gonzalez-Gonzalez G, Torro LM, dos Santos MF, Espino AM, Burgos M, Osuna A (2010) Effectiveness of intranasal vaccination against Angiostrongylus costaricensis using a serine/threonine phosphatase 2 A synthetic peptide and recombinant antigens. Vaccine 28: 5185–5196. [DOI] [PubMed] [Google Scholar]
- 9.Mas-Coma S (2005) Epidemiology of fascioliasis in human endemic areas. J Helminthol 79: 207–216. [DOI] [PubMed] [Google Scholar]
- 10.Espino AM, Hillyer GV (2003) Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J Parasitol 89: 545–552. [DOI] [PubMed] [Google Scholar]
- 11.Espino AM, Morales A, Delgado B, Rivera FM, Figueroa O, Suarez E (2010) Partial immunity to Fasciola hepatica in mice after vaccination with FhSAP2 delivered as recombinant protein or DNA construct. Ethn Dis 20: S1–17-23. [PMC free article] [PubMed] [Google Scholar]
- 12.Figueroa-Santiago O, Delgado B, Espino AM (2011) Fasciola hepatica saposin-like protein-2-based ELISA for the serodiagnosis of chronic human fascioliasis. Diagn Microbiol Infect Dis 70: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espino AM, Hillyer GV (2004) A novel Fasciola hepatica saposinlike recombinant protein with immunoprophylactic potential. J Parasitol 90: 876–879. [DOI] [PubMed] [Google Scholar]
- 14.Torres D, Espino AM (2006) Mapping of B-cell epitopes on a novel 11.5-kilodalton Fasciola hepatica-Schistosoma mansoni cross-reactive antigen belonging to a member of the F. hepatica saposin-like protein family. Infect Immun 74: 4932–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kesik M, Jedlina-Panasiuk L, Kozak-Cieszczyk M, Plucienniczak A, Wedrychowicz H (2007) Enteral vaccination of rats against Fasciola hepatica using recombinant cysteine proteinase (cathepsin L1). Vaccine 25: 3619–3628. [DOI] [PubMed] [Google Scholar]
- 16.Wedrychowicz H, Kesik M, Kaliniak M, Kozak-Cieszczyk M, Jedlina-Panasiuk L, Jaros S, Plucienniczak A (2007) Vaccine potential of inclusion bodies containing cysteine proteinase of Fasciola hepatica in calves and lambs experimentally challenged with metacercariae of the fluke. Vet Parasitol 147: 77–88. [DOI] [PubMed] [Google Scholar]
- 17.Espino AM, Dumenigo BE, Fernandez R, Finlay CM (1987) Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am J Trop Med Hyg 37: 605–608. [DOI] [PubMed] [Google Scholar]
- 18.Nakane PK, Kawaoi A (1974) Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem 22: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 20.Caban-Hernandez K, Espino AM (2013) Differential expression and localization of saposin-like protein 2 of Fasciola hepatica. Acta Trop 128: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grams R, Adisakwattana P, Ritthisunthorn N, Eursitthichai V, Vichasri-Grams S, Viyanant V (2006) The saposin-like proteins 1, 2, and 3 of Fasciola gigantica. Mol Biochem Parasitol 148: 133–143. [DOI] [PubMed] [Google Scholar]
- 22.Reed MB, Strugnell RA, Panaccio M, Spithill TW (2000) A novel member of the NK-lysin protein family is developmentally regulated and secreted by Fasciola hepatica. Mol Biochem Parasitol 105: 297–303. [DOI] [PubMed] [Google Scholar]
- 23.Sano A, Mizuno T, Kondoh K, Hineno T, Ueno S, Kakimoto Y, Morita N (1992) Saposin-C from bovine spleen; complete amino acid sequence and relation between the structure and its biological activity. Biochim Biophys Acta 1120: 75–80. [DOI] [PubMed] [Google Scholar]
- 24.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85. [DOI] [PubMed] [Google Scholar]