Abstract
Carotenoid supplementation can improve human visual performance, but there is still no validated rodent model to test their effects on visual function in laboratory animals. We recently showed that mice deficient in β-carotene oxygenase 2 (BCO2) and/or β-carotene oxygenase 1 (BCO1) enzymes can accumulate carotenoids in their retinas, allowing us to investigate the effects of carotenoids on the visual performance of mice. Using OptoMotry, a device to measure visual function in rodents, we examined the effect of zeaxanthin, lutein, and β-carotene on visual performance of various BCO knockout mice. We then transgenically expressed the human zeaxanthin-binding protein GSTP1 (hGSTP1) in the rods of bco2−/− mice to examine if delivering more zeaxanthin to retina will improve their visual function further. The visual performance of bco2−/− mice fed with zeaxanthin or lutein was significantly improved relative to control mice fed with placebo beadlets. β-Carotene had no significant effect in bco2−/− mice but modestly improved cone visual function of bco1−/− mice. Expression of hGSTP1 in the rods of bco2−/−mice resulted in a 40% increase of retinal zeaxanthin and further improvement of visual performance. This work demonstrates that these “macular pigment mice” may serve as animal models to study carotenoid function in the retina.
Keywords: Carotenoid, Lutein, zeaxanthin, visual performance, spatial frequency, contrast sensitivity
1. Introduction
Macular carotenoids are yellow xanthophyll pigments that accumulate in the human retina with extremely high concentration in the foveal area [1–3]. These carotenoids have been identified as lutein, zeaxanthin, and meso-zeaxanthin [4–7], of which lutein and zeaxanthin originate from the diet, whereas meso-zeaxanthin comes mainly from an isomerization reaction of lutein in the retinal pigment epithelium (RPE) [8, 9]. The uptake of macular carotenoids has been reported to be a selective and active absorption process involving many transporter proteins and enzymes [1, 10–14]. Glutathione S-transferase Pi isoform (GSTP1) and steroidogenic acute regulatory domain protein 3 (StARD3), are the two carotenoid-binding proteins responsible for the specific retinal distribution of zeaxanthin and lutein, respectively [15, 16]. Many clinical trials and studies have demonstrated that carotenoid supplementation can prevent and reduce the risk of many human eye diseases such as age-related macular degeneration (AMD) [17–19].
It has been well documented that supplementation with lutein and zeaxanthin can improve visual performance of both normal subjects and patients with eye diseases [20–26]. Loughman et al. reported that significant improvements in visual acuity were found at the sixth month in normal subjects fed with a mixture of lutein, zeaxanthin, and meso-zeaxanthin [27]. A randomized, double-blind, placebo-controlled, 1-year interventional study in 120 Chinese drivers demonstrated that lutein supplementation can increase contrast sensitivity and decrease glare disability [28]. It was also reported that visual acuity of cataract patients supplemented with lutein improved about one line on the Snellen visual acuity chart in comparison with a placebo group [29]. Stringham and Hammond found that light scattering was greatly reduced for short wavelength monochromatic light in subjects with high levels of macular carotenoids, suggesting that macular carotenoids can mitigate glare disability [20]. The visual benefits of macular carotenoids are attributed to their optical properties, antioxidant effects, and other biological mechanisms [30, 31]. Macular carotenoids are thought to be able to reduce chromatic aberration, light scatter, and glare disability by absorbing blue light [1, 32]. They also can quench free radicals and maintain retinal health [13, 33]. Until now, however, it has been difficult to test hypothesis to examine the mechanisms underlying improvement of visual performance by carotenoid supplementation due to the lack of small mammal models capable of reproducibly accumulating substantial levels of carotenoids in their retinas [34–37]. In 2014, our group discovered that zeaxanthin can be deposited in the retina of mice deficient in the β-carotene oxygenase 2 (BCO2) enzyme, generating so-called “macular pigment mice” [38]. More recently, we were also able to deliver comparable amounts of lutein and lower levels of β-carotene to the retinas of these bco2−/− mice, while bco1−/− mice were superior for delivery of β-carotene to the retina [39]. These results have been confirmed by other research groups, and no morphological difference is detected between the retinas of wild-type mice and bco2−/− mice [40–42]. All these researchers have shown that “macular pigment mice” are likely to be good laboratory animal models to study the effects of carotenoids on visual performance.
In this manuscript, we investigate the effects of zeaxanthin, lutein, and β-carotene on the spatial frequency and contrast sensitivity of rod and cone cells of the “macular pigment mice” using OptoMotry, a device to examine visual function of small animals. Furthermore, we tested if delivering more carotenoids to the retina of transgenic mice expressing the human zeaxanthin binding protein GSTP1 (hGSTP1) in their rod cells will induce further improvement of their visual function.
2. Material and methods
2.1. Animal husbandry and generation.
Bco2−/− and bco1−/− mice were bred at the University of Utah vivarium using founders from Case Western Reserve University. To express zeaxanthin-binding protein GSTP1 specifically in the mouse retina, we generated human GSTP1 transgenic (hGSTP1-tg) mice. In brief, an XhoI site was inserted immediately upstream of the translation initiation codon and a ClaI site immediately downstream of the translation stop codon of the cDNA of human GSTP1 by PCR. The XhoI/ClaI fragment was subcloned into corresponding sites of pRho 4.4 vector to place the human GSTP1 gene under the control of the mouse opsin promoter. In order to track expression of the transgene, a hemagglutinin (HA) tag was placed contiguous with the human GSTP1 cDNA sequence. After direct DNA sequencing, the 5.6-kb trangene construct containing the rhodopsin promoter, human GSTP1 cDNA, a HA-tag, and a mouse protamine polyadenylation signal was isolated from the plasmid by digestion with KpnI and XbaI, then injected into C57BL/6X129 embryos. The embryos were implanted into pseudopregnant foster female mice. Founder mice with transgene integration were identified by PCR, and mated to wildtype C57BL/6 mice to produce mice used for analysis. Subsequently, the hGSTP1-tg mice were bred with the bco2−/− mice to generate hGSTP1-tg /bco2−/− mice with the expectation that they would accumulate more zeaxanthin in their retinas relative to bco2−/− mice. All the procedures were approved by appropriate institutional animal care and use committees and were carried out according to National Institutes of Health guidelines.
2.2. Carotenoid-feeding experiments.
Bco2−/−, bco1−/−, hGSTP1-tg , and hGSTP1-tg /bco2−/− mice were employed in the carotenoid-feeding experiments in which bco2−/− mice were treated with lutein, zeaxanthin, or β-carotene, bco1−/− mice were treated with β-carotene, and hGSTP1-tg and hGSTP1-tg /bco2−/− mice were treated with zeaxanthin. In each experiment, 3-month-old mice were divided into two groups and fed with carotenoid beadlet chow (∼2.6 mg per mouse per day; DSM, Kaiseraugst, Switzerland) or placebo beadlet chow for 4 weeks after first receiving a vitamin A-deficient chow (AIN-93, (TestDiet, Richmond, IN)) for 4 weeks to help promote carotenoid uptake. Then, their visual performance and carotenoid contents were examined.
2.3. Carotenoid extraction and analysis by HPLC.
Carotenoids in liver and serum, as well as ocular tissues of the mice were extracted and analyzed as before [39]. Briefly, the ocular tissue and liver samples were extracted three times with tetrahydrofuran containing 0.1% butylated hydroxytoluene by sonication at 5°C to 10°C for 30 minutes each time. Combined extracts were evaporated to dryness under vacuum at room temperature. To extract carotenoids from serum, ethanol containing 0.1% butylated hydroxytoluene was added into the samples to precipitate the proteins, and then ethyl acetate was added to extract the carotenoids. The sample was centrifuged at 2,000 x g for 5 minutes at 4°C, and the supernatant phase was collected. Then the sample was extracted with ethyl acetate two more times and extracted with hexane once. The collected supernatants were combined and dried down under vacuum. Finally, the dried residue was re-dissolved in HPLC mobile phase and centrifuged at 2000 x g for 10 minutes, and the supernatant was injected into the HPLC system. HPLC separations were performed on a silica-based nitrile bonded column (25 cm length × 4.6 mm internal diameter; 5-μm spherical particle (Regis Chemical, Morton Grove, IL)). The eluent consisted of an isocratic mixture of hexanes (75%), dichloromethane (25%), methanol (0.3%), and N, N-diisopropylethylamine (0.1%). The column flow rate was 1 mL/min. The column temperature was maintained at 25°C, and the monitoring wavelength was 445 nm.
2.4. OptoMotry.
3- to 4-month-old mice (n=7 to 15/ group) were employed to test spatial visual acuity using the OptoMotry system (Cerebral Mechanics, Lethbridg, AB, Canada). Briefly, individual mice were placed on a platform centered in a quad-square formed by four inward facing computer screens, and their movements were monitored by an overhead video camera. Photopic measurements were conducted under illuminance of around 165 lux. Scotopic measurements were carried out in infrared light with the LCD displays masked with 5 layers of ND16 Lee299 filters. During the detection of spatial frequency threshold, the rotation speed and contrast were kept at 12 degrees/s, and 100%, respectively, while the frequency was kept at 0.19 cycle/degree in the examination of contrast sensitivity. All experiments had concurrent control mice fed with placebo chow.
2.5. RT-PCR.
Total RNA was prepared from mouse retinas. cDNA was synthesized using SuperScriptIII reverse transcriptase. PCR to detect the expression of the human GSTP1 transgene was performed with 1µl RT reaction as template. Primers were as follows: forward, 5′-TGG TGG ACA TGG TGA ATG ACG G −3′; and reverse, 5′- AGC GTA GTC TGG GAC GTC GTA TG −3′ to yield a 393 bp fragment.
2.6. Western blots and Immunohistochemistry.
Protein samples were separated on 4−15% gradient SDS−PAGE and transferred to 0.45 μm nitrocellulose membranes. After blocking with 5% nonfat dried milk, the membranes were incubated with primary and secondary antibodies. The dilution ratios were 1:1000 and 1:2000, respectively. The membranes were developed using ECL Plus Western blot detection reagents (GE Healthcare Bio-Sciences, Pittsburgh, PA). In the immunohistochemistry experiments, sections of perfusion-fixed monkey eyes were processed as described [43] with the addition of heating sections in a solution of 10 mM sodium citrate, pH 6, at 95°C ( 5 min) prior to blocking with 10% normal donkey serum in PBS-T. Antibodies used were: Anti-GSTP1 and anti-HA-tag antibodies from Alpha Diagnostic International (San Antonio, TX); anti-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO).
2.7. Statistical analysis.
Carotenoid contents of serum, liver, and the ocular tissues of the mice were analyzed using ANOVA and t-tests. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) and Stata/15.1 statistical software (StataCorp, College Station, TX, USA).
3. Results
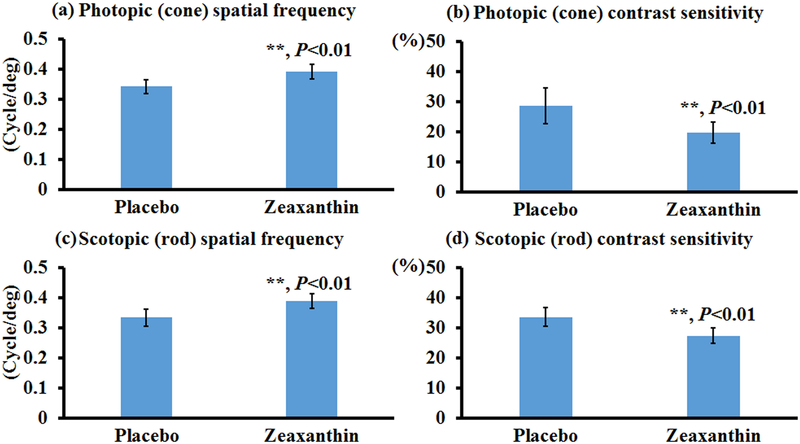
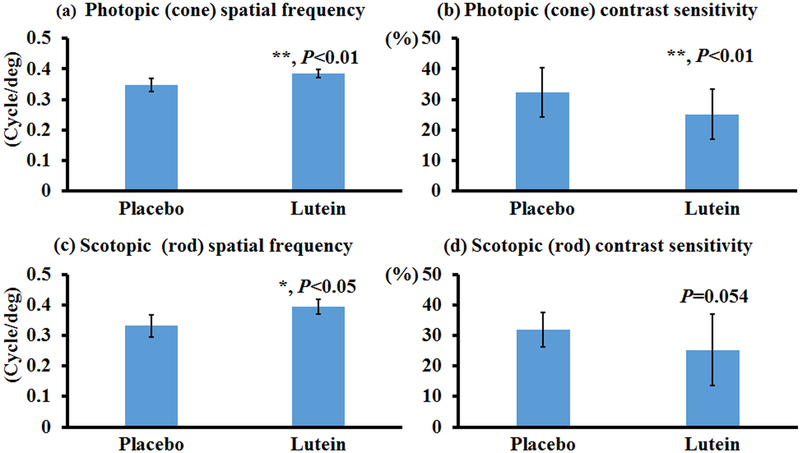
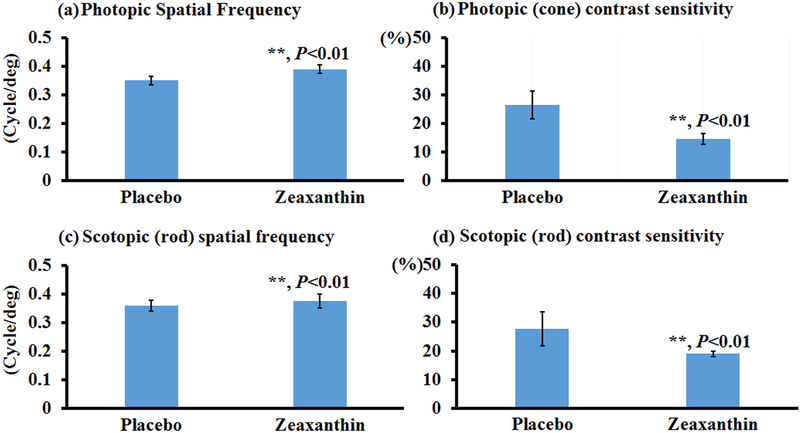
To test the effects of carotenoids on visual performance of mice, bco2−/− mice were fed with zeaxanthin, lutein, or β-carotene for one month, and then their photopic and scotopic spatial frequencies and contrast sensitivities were quantified using OptoMotry. Of note, the photopic and scotopic parameters represent visual function of the cone and rod systems, respectively. Higher values of spatial frequency correspond to better visual acuity, whereas smaller contrast sensitivities are indicative of better visual function. Figure 1 shows that zeaxanthin supplementation significantly increased the spatial frequency and contrast sensitivity of both rod and cone systems in the bco2−/− mice. In comparison with mice of the placebo group, the rod and cone spatial frequencies are increased around 15% in mice fed with zeaxanthin, while the rod and cone contrast sensitivities improved around 20% and35%, respectively. Like zeaxanthin supplementation, lutein supplementation can significantly increase the visual performance of bco2−/− mice, except for their scotopic contrast sensitivity (Figure 2). The extent of improvement by lutein supplementation is similar to zeaxanthin in regard to the rod and cone spatial frequency and rod contrast sensitivity. Lutein also was able to significantly improve the cone contrast sensitivity but was slightly less than zeaxanthin, around 20%.
Figure 1. Visual performance measured by OptoMotry in bco2−/− mice with and without zeaxanthin supplementation.
Zeaxanthin supplementation significantly improves the visual function of bco2−/− mice. (a) Photopic spatial frequency; (b) Photopic contrast sensitivity; (c) Scotopic spatial frequency; (d) Scotopic contrast sensitivity. Values indicate means ± SD; 10 mice were used in each group. **, P<0.01.
Figure 2. Visual performance measured by OptoMotry in bco2−/− mice with and without lutein supplementation.
Lutein supplementation significantly improves bco2−/− mice’s visual function except for the contrast sensitivity of the rod cells. (a) Photopic spatial frequency; (b) Photopic contrast sensitivity; (c) Scotopic spatial frequency; (d) Scotopic contrast sensitivity. Values indicate means ± SD; 15 mice were used in each group. *, P<0.05; **, P<0.01.
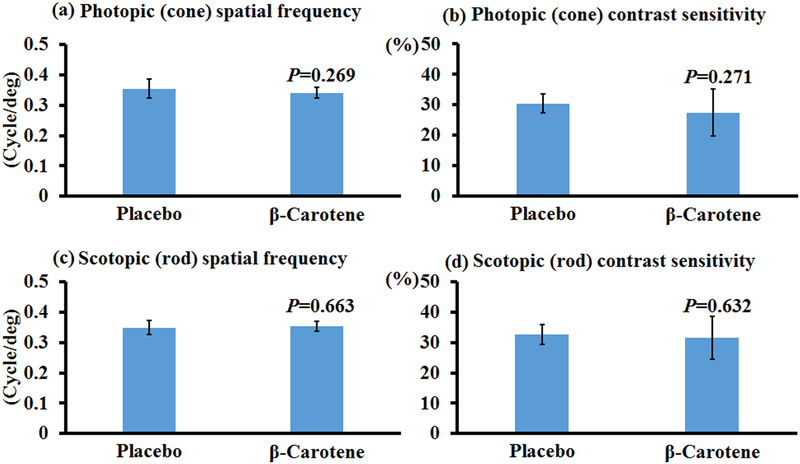
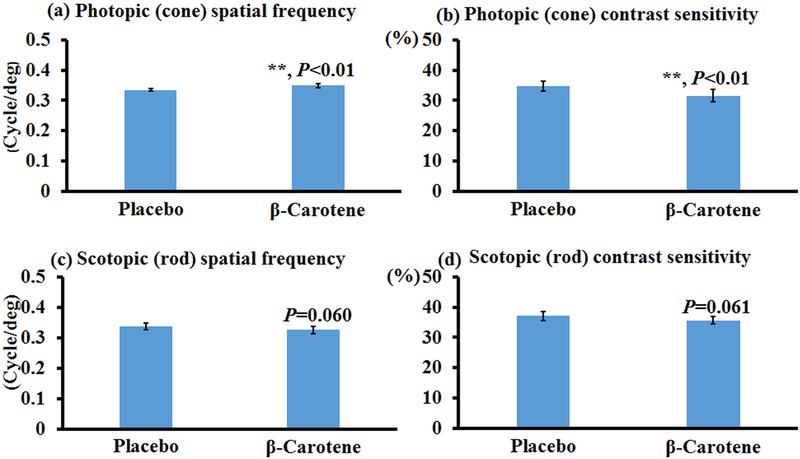
β-Carotene is not a human macular carotenoid, and only a trace amount of β-carotene is detected in the human retina overall. However, we still investigated the effects of β-carotene on visual performance as it is the precursor of retinal, a key molecule involved in vision, and it also shares the blue light filtering property of lutein and zeaxanthin. Figure 3 demonstrates that no significant improvement in visual function was detected in bco2−/− mice fed with β-carotene relative to the control mice. This is not surprising because BCO1, the critical cleavage enzyme for β-carotene, is still present in the bco2−/− mice. Our previous study revealed that the content of β-carotene is only around 5 to 10% of that of lutein and zeaxanthin in the retina of bco2−/− mice, while the content of β-carotene in the retinas of bco1−/− mice supplemented with β-carotene is comparable to the contents of lutein or zeaxanthin in the retina of supplemented bco2−/− mice [39]. Therefore, we further investigated if β-carotene can improve the visual performance of bco1−/− mice. Figure 4 shows that β-carotene supplementation can significantly improve the spatial frequency and contrast sensitivity of the cone system but not the rod system. There were a 4% and 9% improvements detected in the photopic spatial frequency and contrast sensitivity of mice fed with β-carotene relative to the control mice. All these OptoMotry data indicate that, of these three carotenoids, zeaxanthin is the best at improving the visual performance of mice, especially for cone contrast sensitivity.
Figure 3. Visual performance measured by OptoMotry in bco2−/− mice with and without β-carotene supplementation.
β-Carotene supplementation has no significant effect on the visual performance of bco2−/− mice. (a) Photopic spatial frequency; (b) Photopic contrast sensitivity; (c) Scotopic spatial frequency; (d) Scotopic contrast sensitivity. Values indicate means ± SD; 10 mice were used in each group. *, P<0.05.
Figure 4. Visual performance measured by OptoMotry in bco1−/− mice with and without β-carotene supplementation.
β-Carotene supplementation slightly improves the visual performance of bco1−/− mice. (a) Photopic spatial frequency; (b) Photopic contrast sensitivity; (c) Scotopic spatial frequency; (d) Scotopic contrast sensitivity. Values indicate means ± SD; 7 and 8 mice were used in β-carotene and placebo groups, respectively. *, P<0.05.
Next, we tested if delivering more zeaxanthin to the retina of mice will further improve their visual performance. In order to deliver more zeaxanthin to the retina of mice, we transgenically expressed the human zeaxanthin-binding protein GSTP1 (hGSTP1) in the retina of bco2−/− mice by crossing an hGSTP1 transgenic mouse line (hGSTP1-tg) with the bco2−/− mice. Figure 5 shows the transgene construct and the expression of hGSTP1. cDNA encoding hGSTP1 protein was placed under the control of the mouse rhodopsin promoter, which drives hGSTP1 protein expression specifically in rods. Confocal immunolocalization of the expressed HA-tag showed robust expression of human GSTP1 throughout rods, from the outer plexiform layers (OPL) to outer segments (OS). The hGSTP1-tg mice were mated to bco2−/− mice in order to generate hGSTP1-tg/bco2−/− mice.
Figure 5. Generation of transgenic (hGSTP1-tg) mice expressing the human zeaxanthin-binding protein GSTP1 specifically in the retina.
The transgene construct (upper panel). RT-PCR reveals presence of human GSTP1 in the transgenic mouse retina. Lane 1. Amplicon size marker; 2. Wildtype C57BL/6 mice (WT); 3. hGSTP1-tg mice. Samples are normalized by GAPDH (lower left panel). Immunoblot results of antibody directed against the HA-tag versus total protein extract from pooled mouse retinas. Lane 1. Protein size marker; 2. C57BL/6 mice (WT); 3. hGSTP1-tg mice. Samples are normalized by actin (lower middle panel). Immunolocalization with antibody to HA-tag (green) in a 1-month-old hGSTP1-tg mouse retina (far right panel). OS, outer segments; ONL, outer nuclear layers; OPL, outer plexiform layers.
We then performed a zeaxanthin-feeding experiment, in which ~ 3-month-old hGSTP1-tg/bco2−/− and bco2−/− mice were fed with DSM-beadlet diets for one month. Figure 6 shows the carotenoid contents detected by HPLC in this feeding experiment. Zeaxanthin content was ~0.84 ng/ pair of retinas in the hGSTP1-tg /bco2−/− mice, which is around 40% higher than that of bco2−/−mice. Meanwhile, there was no significant difference was found between the zeaxanthin contents of RPE/choroids, serum, or livers of hGSTP1-tg /bco2−/− mice and those of bco2−/− mice. In addition, we could not deliver zeaxanthin into the retina of the hGSTP1-tg mice. This is because the carotenoid cleavage enzyme BCO2 is still functional in these mice, so zeaxanthin molecules will be broken down before arrival at the retina.
Figure 6. Contents of zeaxanthin detected in the tissues of hGSTP1-tg, bco2−/− and hGSTP1-tg/bco2−/− mice.
The expression of zeaxanthin-binding protein GSTP1 specifically in the retina of bco2−/− mice significantly increased the retinal carotenoid contents. 8 to 10-week-old mice (n=25/ genotype) were kept on DSM zeaxanthin beadlet chow (1 g zeaxanthin/kg chow) for 4 weeks. Carotenoids were extracted from the serum and liver of each individual animal. Retina and RPE/choroid were pooled from 3 to 5 animals (5 repeats) in each mouse group. Values indicate means ± SD, N.D., not detectable. *, P<0.05; **, P<0.01.
We next examined the impact of zeaxanthin on the visual performance of hGSTP1-tg /bco2−/− mice. 3-month-old hGSTP1-tg /bco2−/− mice were divided into two groups and fed with or without zeaxanthin for 4 weeks. We then examined their visual performance using OptoMotry (Figure 7). Comparing with the control mice, the rod and cone spatial frequency and contrast sensitivity were significantly improved in the hGSTP1-tg /bco2−/− mice fed with zeaxanthin, and similar improvements in the rod and cone spatial frequency were seen when comparing the hGSTP1-tg / bco2−/− and the bco2−/− mice. An obvious improvement was found in the rod contrast sensitivity of hGSTP1-tg /bco2−/− mice in contrast to the bco2−/− mice. This increase in hGSTP1-tg /bco2−/− mice is about 35% while it is only 20% in the bco2−/− mice. This may be ascribed to the contribution of the zeaxanthin-binding protein GSTP1 expressed specifically in the rod cells. It is also shows that zeaxanthin supplementation caused a 45% increase in the cone contrast of hGSTP1-tg /bco2−/−, which is about 1.3times as high as the bco2−/− mice. No significant difference was found between the visual performance of hGSTP1-tg/bco2−/− control mice and bco2−/− control mice.
Figure 7. Visual performance measured by OptoMotry in hGSTP1-tg/bco2−/− mice with or without zeaxanthin supplementation.
Zeaxanthin supplementation significantly improves the visual function of hGSTP1-tg/bco2−/− mice, especially the rod contrast sensitivity. (a) Photopic spatial frequency; (b) Photopic contrast sensitivity; (c) Scotopic spatial frequency; (d) Scotopic contrast sensitivity. Values indicate means ± SD; 11 and 14 mice were used in the zeaxanthin and placebo groups, respectively. **, P<0.01.
4. Discussion
Besides protection against light-induced oxidative damage in the retina, improving visual performance is another primary function of the macular carotenoids. It is well known that carotenoid supplementation can improve the visual performance of both normal subjects and those with eye disease, but there is always concern that these are subjective psychophysical tests that could be influenced by subject and examiner bias. Our previous studies have established that transgenic “macular pigment mice” whose carotenoid cleavage enzymes have been selectively knocked out can serve as animal models for bioavailability and bio-efficacy of retinal carotenoids. In the present work, we demonstrate that supplementation with lutein and zeaxanthin improves the spatial frequency and contrast sensitivity of mice, especially the contrast sensitivity, mimicking the results of the recent clinical trials in humans [44, 45]. This validates that bco2−/− mice can be used to investigate the functional benefits of the macular carotenoids.
Our investigations revealed several new insights into the effects of carotenoids on visual function. We found that xanthophyll carotenoids can significantly improve the visual performance of both rod and cone cells, while β-carotene just slightly enhances the visual performance of cone cells in mice (Figures 1–4). Supplementation with lutein and zeaxanthin dramatically increased the contrast sensitivity of cone cells of bco2−/− mice, and zeaxanthin was around 1.2±0.19 time stronger than lutein. (Figures 1–2). We also examined β-carotene’s effects on visual performance in bco2−/− mice, and no improvement was detected which we ascribed to the low retinal content of β-carotene in these mice. Our previously published study has shown that only trace amounts of retinal β-carotene can be detected in the bco2−/− mice because β-carotene’s main cleavage enzyme, BCO1, is still active [39]. To raise β-carotene to a comparable level of lutein and zeaxanthin in the retina, we fed β-carotene to mice deficient in the BCO1 enzyme, and the OptoMotry data show that β-carotene can slightly increase the visual function of cone cells.
GSTP1 and StARD3 have been identified to be the zeaxanthin-binding protein and lutein-binding proteins in the human retina, respectively. In this work, we also took advantage of this property of GSTP1 and examined if delivering more zeaxanthin to the retina of mice will further improve their visual performance. The human GSTP1 protein was transgenically expressed in the retina of bco2−/− mice, causing the retinal carotenoid content to increase around 40% more than the bco2−/− mice under the same feeding conditions (Figures 5–6). Since this specific expression is driven by the mouse rhodopsin promoter, the human GSTP1 proteins have been robustly expressed in the rod cells, and correspondingly, more zeaxanthin should be deposited there. This may be responsible for the dramatic improvement in the contrast sensitivity of rod cells in hGSTP1-tg/bco2−/− mice after zeaxanthin supplementation (Figure 7), supporting the idea that increasing the retinal carotenoid levels can improve visual function. Of course, to study the role of macular carotenoids in visual performance further, we should selectively elevate the carotenoid level of various cone cells because, after all, the majority of the macular carotenoids are present in an area dominated by cone cells. We also found that no carotenoid was accumulated in the retina of hGSTP1-tg mice, indicating that BCO2 is a critical enzyme for the presence of carotenoid in the retina. All of these results are consistent with our previous findings that the relative inactivity of the human BCO2 enzyme is responsible for the accumulation of xanthophyll carotenoids in the human macula and that the high cleavage activity of mouse BCO2 is responsible for the failure of wild-type mice to accumulate any retinal carotenoids even after extreme systemic doses and overexpression of carotenoid-binding proteins in the retina.
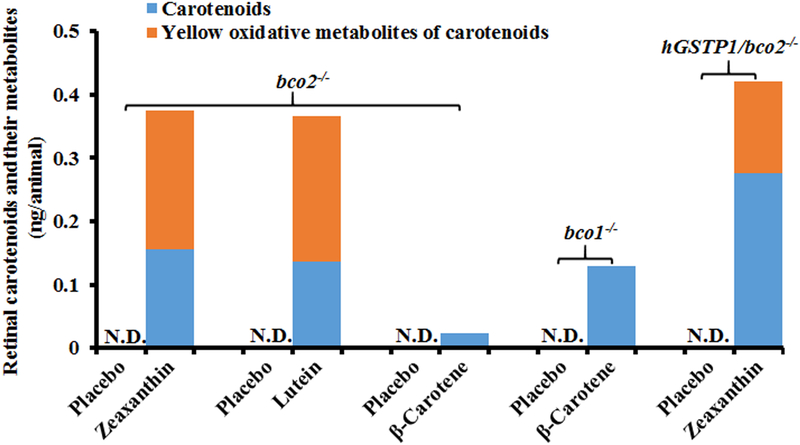
Of all of the possible mechanisms for carotenoid improvement of visual performance in humans and “macular pigment mice” (light filtering, antioxidant, and other neural and biochemical mechanisms), blue-light filtering by these yellow pigments is the most straightforward. Our OptoMotry results showed a rank order of visual performance of hGSTP1-tg/bco2−/− (zeaxanthin-fed) > bco2−/− (zeaxanthin-fed) ≈ bco2−/− (lutein-fed) > bco1−/− (β-carotene-fed) > bco2−/− (β-carotene-fed) >> any mouse (placebo-fed), yet retinal content of the intact carotenoids in our transgenic mice was hGSTP1-tg/bco2−/− (zeaxanthin-fed) > bco2−/− (zeaxanthin-fed) ≈ bco2−/− (lutein-fed) ≈ bco1−/− (β-carotene-fed) > bco2−/− (β-carotene-fed) >> any mouse (placebo-fed) (Figure 8). This disconnect between performance and carotenoid content can be explained by the fact that transgenic BCO knockout mice generate considerable amounts of yellow oxidative products when fed lutein or zeaxanthin which can also be deposited in the mouse retina, while β-carotene-fed BCO knockout mice do not generate these yellow metabolites [39]. As can be seen in Figure 8, if we sum the intact carotenoids with their yellow metabolites, the rank order of total carotenoids aligns with the visual performance results rankings. In addition, in a separate control experiment using older mice, we found that the cone visual function of bco2−/− mice was decreased 20% to 30% compared to WT (bco2+/+) mice of the same age. From the visual function data of bco2−/− mice fed with zeaxanthin (Figure 1), we can see that the visual function of bco2−/− mice fed with zeaxanthin was improved 20% to 35% relative to the control mice on placebo diet, suggesting that carotenoid supplementation may improve the impaired visual function of bco2−/− mice.
Figure 8. Contents of carotenoids and their yellow oxidative metabolites in the retinas of the mice used in the visual performance experiments.
The yellow oxidative metabolites of carotenoids were detected in the mice fed with zeaxanthin or lutein but not β-carotene, and their amounts were estimated using authentic standard of lutein or zeaxanthin as these metabolite compounds have not been identified yet. The number of mice in each feeding group varies from 7 to 15, and the retinas from 3 to 7 animals were pooled together for carotenoid analysis. N.D., not detectable.
Our results in transgenic mice are consistent with the effect of macular carotenoids on visual performance revealed in recent human clinical trials and studies. This implies that these transgenic “macular pigment mice” may be successfully employed to further dissect the molecular mechanisms underlying the beneficial effects of the macular carotenoids on visual function.
Acknowledgements:
We would like to thank Dr. Johannes von Lintig from Case Western Reserve University for generously providing the bco1−/− and bco2 −/− founder mice. We also thank Drs. Wolfgang Baehr (University of Utah, Moran Eye Center) and Ching-Kang Jason Chen (Baylor College of Medicine, Houston) for their assistance in the generation of hGSTP1-tg mice. This work was supported by NIH grants EY-11600, EY-14800, and by unrestricted departmental funds from Research to Prevent Blindness, New York City, NY.
Abbreviations
- AMD
Age-related macular degeneration
- BCO1
β-carotene oxygenase 1
- BCO2
β-carotene oxygenase 2
- GSTP1
Glutathione S-transferase Pi isoform
- HA
Hemagglutinin
- HPLC
High-performance liquid chromatography
- RPE
Retinal pigment epithelium
- StARD3
Steroidogenic acute regulatory domain protein 3
Footnotes
The authors have no conflicts of interest.
References
- [1].Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM, Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease, Progress in retinal and eye research 50 (2016) 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nussbaum JJ, Pruett RC, Delori FC, Historic perspectives. Macular yellow pigment. The first 200 years, Retina 1(4) (1981) 296–310. [PubMed] [Google Scholar]
- [3].Krinsky NI, Landrum JT, Bone RA, Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye, Annu Rev Nutr 23 (2003) 171–201. [DOI] [PubMed] [Google Scholar]
- [4].Wald G, Human Vision and the Spectrum, Science 101(2635) (1945) 653–8. [DOI] [PubMed] [Google Scholar]
- [5].Bone RA, Landrum JT, Tarsis SL, Preliminary identification of the human macular pigment, Vision research 25(11) (1985) 1531–5. [DOI] [PubMed] [Google Scholar]
- [6].Bone RA, Landrum JT, Fernandez L, Tarsis SL, Analysis of the macular pigment by HPLC: retinal distribution and age study, Investigative ophthalmology & visual science 29(6) (1988) 843–9. [PubMed] [Google Scholar]
- [7].Bone RA, Landrum JT, Hime GW, Cains A, Zamor J, Stereochemistry of the human macular carotenoids, Investigative ophthalmology & visual science 34(6) (1993) 2033–40. [PubMed] [Google Scholar]
- [8].Gorusupudi A, Shyam R, Li B, Vachali P, Subhani YK, Nelson K, Bernstein PS, Developmentally Regulated Production of meso-Zeaxanthin in Chicken Retinal Pigment Epithelium/Choroid and Retina, Investigative ophthalmology & visual science 57(4) (2016) 1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS, RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye, Proceedings of the National Academy of Sciences of the United States of America 114(41) (2017) 10882–10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li B, Vachali P, Bernstein PS, Human ocular carotenoid-binding proteins, Photochem Photobiol Sci 9(11) (2010) 1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Loane E, Nolan JM, O’Donovan O, Bhosale P, Bernstein PS, Beatty S, Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration, Survey of ophthalmology 53(1) (2008) 68–81. [DOI] [PubMed] [Google Scholar]
- [12].Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP Jr., Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA, Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study, Investigative ophthalmology & visual science 54(3) (2013) 2333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Whitehead AJ, Mares JA, Danis RP, Macular pigment: a review of current knowledge, Archives of ophthalmology 124(7) (2006) 1038–45. [DOI] [PubMed] [Google Scholar]
- [14].Shyam R, Vachali P, Gorusupudi A, Nelson K, Bernstein PS, All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids, Archives of biochemistry and biophysics 634 (2017) 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS, Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye, The Journal of biological chemistry 279(47) (2004) 49447–54. [DOI] [PubMed] [Google Scholar]
- [16].Li B, Vachali P, Frederick JM, Bernstein PS, Identification of StARD3 as a lutein-binding protein in the macula of the primate retina, Biochemistry 50(13) (2011) 2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].A.R. Group, Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL, The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1), Ophthalmology 119(11) (2012) 2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beatty S, Boulton M, Henson D, Koh HH, Murray IJ, Macular pigment and age related macular degeneration, The British journal of ophthalmology 83(7) (1999) 867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE, A one year study of the macular pigment: the effect of 140 days of a lutein supplement, Experimental eye research 65(1) (1997) 57–62. [DOI] [PubMed] [Google Scholar]
- [20].Stringham JM, Garcia PV, Smith PA, McLin LN, Foutch BK, Macular pigment and visual performance in glare: benefits for photostress recovery, disability glare, and visual discomfort, Investigative ophthalmology & visual science 52(10) (2011) 7406–15. [DOI] [PubMed] [Google Scholar]
- [21].Stringham JM, Hammond BR, Macular pigment and visual performance under glare conditions, Optom Vis Sci 85(2) (2008) 82–8. [DOI] [PubMed] [Google Scholar]
- [22].Wooten BR, Hammond BR, Macular pigment: influences on visual acuity and visibility, Progress in retinal and eye research 21(2) (2002) 225–40. [DOI] [PubMed] [Google Scholar]
- [23].Hammond BR, Fletcher LM, Roos F, Wittwer J, Schalch W, A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast, Investigative ophthalmology & visual science 55(12) (2014) 8583–9. [DOI] [PubMed] [Google Scholar]
- [24].Hammond BR Jr., Wooten BR, Snodderly DM, Preservation of visual sensitivity of older subjects: association with macular pigment density, Investigative ophthalmology & visual science 39(2) (1998) 397–406. [PubMed] [Google Scholar]
- [25].Hammond BR Jr., Wooten BR, Curran-Celentano J, Carotenoids in the retina and lens: possible acute and chronic effects on human visual performance, Archives of biochemistry and biophysics 385(1) (2001) 41–6. [DOI] [PubMed] [Google Scholar]
- [26].Hammond BR Jr., Renzi LM, Carotenoids, Advances in nutrition 4(4) (2013) 474–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Loughman J, Nolan JM, Howard AN, Connolly E, Meagher K, Beatty S, The impact of macular pigment augmentation on visual performance using different carotenoid formulations, Investigative ophthalmology & visual science 53(12) (2012) 7871–80. [DOI] [PubMed] [Google Scholar]
- [28].Yao Y, Qiu QH, Wu XW, Cai ZY, Xu S, Liang XQ, Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study, Nutrition 29(7–8) (2013) 958–64. [DOI] [PubMed] [Google Scholar]
- [29].G. Age-Related Eye Disease Study 2 Research, Huynh N, Nicholson BP, Agron E, Clemons TE, Bressler SB, Rosenfeld PJ, Chew EY, Visual acuity after cataract surgery in patients with age-related macular degeneration: age-related eye disease study 2 report number 5, Ophthalmology 121(6) (2014) 1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bone RA, Sparrock JM, Comparison of macular pigment densities in human eyes, Vision research 11(10) (1971) 1057–64. [DOI] [PubMed] [Google Scholar]
- [31].Kirschfeld K, Carotenoid pigments: their possible role in protecting against photooxidation in eyes and photoreceptor cells, Proceedings of the Royal Society of London. Series B, Biological sciences 216(1202) (1982) 71–85. [DOI] [PubMed] [Google Scholar]
- [32].Schultze M, Ueber den gelben Fleck der Retina, seinen Einfluss auf normales Sehen und auf Farbenblindheit, ohen & Sohn; 1866. [Google Scholar]
- [33].Li B, Ahmed F, Bernstein PS, Studies on the singlet oxygen scavenging mechanism of human macular pigment, Archives of biochemistry and biophysics 504(1) (2010) 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bhosale P, Bernstein PS, Vertebrate and invertebrate carotenoid-binding proteins, Archives of biochemistry and biophysics 458(2) (2007) 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fernandez-Robredo P, Sadaba LM, Salinas-Alaman A, Recalde S, Rodriguez JA, Garcia-Layana A, Effect of lutein and antioxidant supplementation on VEGF expression, MMP-2 activity, and ultrastructural alterations in apolipoprotein E-deficient mouse, Oxid Med Cell Longev 2013 (2013) 213505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shapiro SS, Mott DJ, Machlin LJ, Kinetic characteristics of beta-carotene uptake and depletion in rat tissue, The Journal of nutrition 114(10) (1984) 1924–33. [DOI] [PubMed] [Google Scholar]
- [37].Gorusupudi A, Vallikannan B, Glycolipids improve lutein bioavailability and accumulation in eyes in mice, European Journal of Lipid Science and Technology 114(7) (2012) 710–717. [Google Scholar]
- [38].Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, Bernstein PS, Inactivity of human beta,beta-carotene-9’,10’-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment, Proceedings of the National Academy of Sciences of the United States of America 111(28) (2014) 10173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li B, Vachali PP, Shen Z, Gorusupudi A, Nelson K, Besch BM, Bartschi A, Longo S, Mattinson T, Shihab S, Polyakov NE, Suntsova LP, Dushkin AV, Bernstein PS, Retinal accumulation of zeaxanthin, lutein, and beta-carotene in mice deficient in carotenoid cleavage enzymes, Experimental eye research 159 (2017) 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Widjaja-Adhi MAK, Lobo GP, Golczak M, Von Lintig J, A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption, Human Molecular Genetics 24(11) (2015) 3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Palczewski G, Widjaja-Adhi MA, Amengual J, Golczak M, von Lintig J, Genetic dissection in a mouse model reveals interactions between carotenoids and lipid metabolism, Journal of lipid research (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wu L, Guo X, Lyu Y, Clarke SL, Lucas EA, Smith BJ, Hildebrand D, Wang W, Medeiros DM, Shen X, Lin D, Targeted Metabolomics Reveals Abnormal Hepatic Energy Metabolism by Depletion of beta-Carotene Oxygenase 2 in Mice, Scientific reports 7(1) (2017) 14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bhosale P, Li B, Sharifzadeh M, Gellermann W, Frederick JM, Tsuchida K, Bernstein PS, Purification and partial characterization of a lutein-binding protein from human retina, Biochemistry 48(22) (2009) 4798–807. [DOI] [PubMed] [Google Scholar]
- [44].Akuffo KO, Beatty S, Peto T, Stack J, Stringham J, Kelly D, Leung I, Corcoran L, Nolan JM, The Impact of Supplemental Antioxidants on Visual Function in Nonadvanced Age-Related Macular Degeneration: A Head-to-Head Randomized Clinical Trial, Investigative ophthalmology & visual science 58(12) (2017) 5347–5360. [DOI] [PubMed] [Google Scholar]
- [45].Stringham JM, O’Brien KJ, Stringham NT, Contrast Sensitivity and Lateral Inhibition Are Enhanced With Macular Carotenoid Supplementation, Investigative ophthalmology & visual science 58(4) (2017) 2291–2295. [DOI] [PubMed] [Google Scholar]