Abstract
We hypothesized that weight loss would ameliorate dyspnea on exertion (DOE) and feelings of unpleasantness related to the DOE in obese men.
Eighteen men (34±7yr, 35±4kg/m2 BMI, mean±SD) participated in a 12-week weight loss program. Body composition, pulmonary function, cardiorespiratory measures, DOE, and unpleasantness (visual analog scale) were assessed before and after weight loss. Subjects were grouped by Ratings of Perceived Breathlessness (RPB, Borg 0–10 scale) during submaximal cycling: Ten men rated RPB≥4 (+DOE), eight rated RPB≤2 (–DOE).
Subjects lost 10.3±5.6kg (9.2±4.5%) of body weight (n=18). RPB during submaximal cycling was significantly improved in both groups (+DOE: 4.1±0.3 to 2.8±1.1; –DOE: 1.3±0.7 to 0.8±0.6, p<0.001). Several submaximal exercise variables (e.g., , ) were decreased similarly in both groups (p<0.01). Unpleasantness associated with the DOE was reduced (p<0.05). The improved RPB was not significantly correlated with changes in body weight or cardiopulmonary exercise responses (p>0.05).
Moderate weight loss appears to be an effective option to ameliorate DOE and unpleasantness related to DOE in obese men.
Keywords: Breathlessness, Sensory Perception, Exercise, Obesity, Shortness of breath, Negative Emotions, Respiratory Perception
1. INTRODUCTION
Obesity has reached epidemic levels; in 2015–16, an estimated 39.8% of U.S. adults were considered obese (Hales et al., 2017) (body mass index ≥ 30 kg/m2). Commonly, self-care treatments for obesity include a calorie restriction diet and exercise. Unfortunately, both strategies can be difficult to follow and maintain in isolation and a combined weight loss and exercise regimen is rarely successful. In addition, a major barrier to starting an exercise program is breathlessness or dyspnea on exertion (DOE) (Dalle Grave et al., 2010); a condition experienced by many otherwise healthy, obese individuals. For example, we previously reported that 37% of obese men had a higher than expected DOE (compared with nonobese men) after six minutes of cycling at 105 W (Bernhardt et al., 2013). A similarly high prevalence of 44% was found in obese women at 60 W cycling (Bernhardt and Babb, 2014a). Thus, in the present study, we focused on the effects of weight loss without aerobic exercise training on DOE.
Obesity, in the absence of other health issues, affects several physiological variables that could cause dyspnea, especially during exercise. For example, lung volume subdivisions and chest wall compliance are reduced due to the excess accumulation of adipose tissue in the chest and abdomen (Luce, 1980). Consequently, a low end-expiratory lung volume potentially predisposes obese individuals to expiratory flow limitation during exercise (Babb et al., 1989; Babb et al., 2002; Babb et al., 2008b; DeLorey et al., 2005; Ofir et al., 2007). Furthermore, the work of breathing is increased as the respiratory muscles must work against the excess chest wall fat; this heightened respiratory neural drive to breathe puts the obese individual at risk for exercise-related dyspnea (Babb, 2013; Lin and Lin, 2012; Schaeffer et al., 2014). In contrast, sedentary behavior, rather than obesity, reduces cardiorespiratory fitness, which could also cause dyspnea on exertion.
In our previous study (Bernhardt and Babb, 2014b), we found that moderate weight loss alone (i.e., without aerobic exercise training) significantly improved DOE in otherwise healthy obese women who experienced strong DOE at baseline. In continuation of that study, here we investigated whether weight loss in obese men would similarly reduce DOE. There are several reasons why outcomes could be different (or similar) in men than in women. In general, men have larger lung volumes and stronger respiratory muscles than women, which allows for a greater maximal ventilatory capacity and less expiratory flow limitation during exercise (i.e., less obesity-related restrictions) (Carey et al., 2007; Crapo et al., 1982; Mead, 1980; Schaeffer et al., 2014; Sheel and Guenette, 2008; Thurlbeck, 1982). On the other hand, men carry more weight in the visceral cavity than women (Babb et al., 2008b), which could limit diaphragm movement, lower end-expiratory lung volumes during exercise, and therefore cause greater DOE. Thus, weight loss could have no influence on DOE, or a greater effect on DOE than in obese women.
Dyspnea is a multidimensional symptom comprised of three distinct domains: sensory-perceptual (i.e., dyspnea intensity and quality), affective distress (i.e., unpleasantness and emotional responses), and symptom impact/burden (i.e., quality of life) (Parshall et al., 2012). We recently reported that unpleasantness, anxiety, and fear are higher in obese women with DOE (Marines-Price et al., 2018). It was unknown if the same was true in obese men and if so, if weight loss could reduce both dyspnea intensity as well as unpleasantness/negative emotions.
The objectives of the present study were to investigate (1) whether a 12-week weight loss program could reduce DOE and unpleasantness/negative emotions in otherwise healthy obese men, and (2) whether changes in body composition, pulmonary function, oxygen cost of breathing, and/or cardiorespiratory measures during submaximal and maximal exercise were associated with the potential reduction in DOE. Based on previous studies, it was hypothesized that DOE and unpleasantness/negative emotions would be reduced following weight loss, but without clear physiological mechanisms explaining the reduce DOE.
2. METHODS
2.1. Subjects
Eighteen obese men, aged between 20–45 years, came to the laboratory for testing visits before and after a 12-week weight loss program. Testing procedures were the same as in our previous study in obese women (Bernhardt and Babb, 2014b).
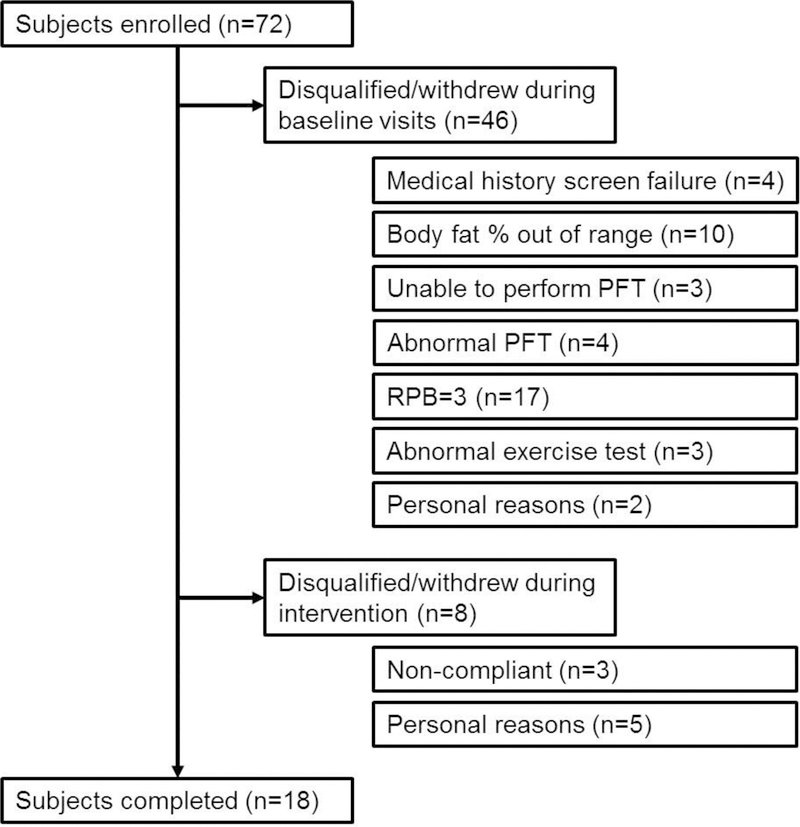
Subjects were recruited based on body mass index (≥ 30 and ≤ 50 kg/m2) and obesity was confirmed by hydrostatic weighing during their first visit (≥ 30.3 and ≤ 50.8% body fat). Exclusion criteria included current or past history of smoking, asthma, cardiovascular disease, sleep disorders, and musculoskeletal abnormalities. Regularly physically active (i.e., purposeful exercise more than 2 times per week during the last 6 months) subjects were also excluded. The study was approved by the UT Southwestern IRB (STU122010–108). Initially, seventy-two men were consented. The number of subjects who were disqualified, or who withdrew during the initial testing visits or during the weight loss program are shown in Figure 1.
Figure 1:
Subject recruitment/retention.
2.2. Body composition and pulmonary function
Standard measures of height, weight, and body circumferences (neck, chest, waist, hips) were taken. Hydrostatic weighing was performed to determine total body fat percentage, total body fat mass, and lean body mass. Standard pulmonary function testing was performed according to ATS/ERS guidelines (Society, 1995) and included spirometry, lung volumes, and diffusing capacity for carbon monoxide (model V62W body plethysmograph, SensorMedics). To determine airway hyperresponsiveness, subjects then inhaled four actuations of 90μg of albuterol sulfate (Ventolin HFA) and repeated the spirometric tests at least 15 minutes later. If FEV1 increased by ≥12% and ≥150 mL from baseline value, the subject was excluded from further study.
2.3. Submaximal constant load cycling exercise
Ratings of Perceived Breathlessness (RPB) were obtained several times using the modified Borg scale (Borg, 1982) and the value recorded during the last minute of the constant load exercise test was used for analysis. Before beginning the test, subjects read the following instructions for accurately describing their RPB: “The number 0 represents no breathlessness. The number 10 represents the strongest or greatest breathlessness that you have ever experienced. During the exercise test you will be asked to point to a number, which represents your perceived level of breathlessness at the time.” Borg RPB results have been demonstrated to be reliable and valid (Bernhardt et al., 2014; Meek et al., 1999).
Resting baseline measurements were collected with the subject seated on the cycle ergometer (Lode Corival) with hands on the handlebar. Then, subjects cycled at a constant load of 105 W with approximately 60–70 rpm for 6 minutes. Cardiorespiratory responses, including heart rate (HR), blood pressure (Tango, SunTech Medical, Inc, Morrisville, NC), ventilation (), and gas exchange ( and ) were measured at rest and throughout exercise (custom gas exchange system). Blood lactate concentration via finger prick was measured at the end of the test while the subject was still cycling (i.e., beginning of minute 7).
Following the test, subjects who rated RPB ≥ 4 completed two dyspnea questionnaires to examine the quality of their respiratory sensations they felt during the exercise. The first questionnaire consisted of 15 descriptors relating to dyspnea adapted from Mahler et al (Mahler et al., 1996); the second included six visual analog scales (10 cm length, ranging from “none” to “maximal imaginable”) on which subjects were asked to mark how unpleasant the breathlessness was and how much of the negative emotions - depression, anxiety, frustration, anger, and fear - were associated with the breathlessness (adapted from (Wade et al., 1996)).
2.4. Peak cardiovascular exercise capacity
After 10–15 minutes of rest following the submaximal cycling exercise test, the subject performed a maximal incremental cycling test to volitional exhaustion. Starting with an initial work load of 30 W, the load was increased by 30 W each minute until the termination of the test. Cardiorespiratory measures were collected throughout the test. Blood lactate concentration was measured 2–3 minutes after the test with the subject recovering, seated in a chair. Maximal effort was confirmed by predicted peak heart rate > 90%, lactate concentration > 7 mmol/L, and respiratory exchange rate > 1.10. Predicted peak was calculated using the equation by Wasserman et al. (Wasserman et al., 2005).
2.5. Oxygen cost of breathing
The oxygen cost of breathing was determined from 6-min measurements of and at rest and 4-min measurements of and during eucapnic voluntary hyperpnea at 60 L/min and 90 L/min as previously described (Babb et al., 2008a). Oxygen cost of breathing was determined by calculating the slope of the versus relationship at rest and during the two levels of hyperpnea. The oxygen cost of breathing regression for each subject was used with averaged during the constant load exercise to calculate the oxygen consumption by respiratory muscles ().
2.6. Weight loss program
Subjects completed a 12-week weight loss program (Biometrics Nutrition & Fitness ®). Each participant received dietary counseling from a registered dietician and an individualized meal plan (3 meals and 3 snacks per day) based on personal food preferences, including weekly shopping lists, recipes, and amount of calories per meal. Subjects also performed specific resistance exercises under the guidance of a personal trainer three times per week. The focus of the resistance training was to minimize the loss of lean body mass. No aerobic endurance training was performed so that changes after the program could be attributed to weight loss only, not improvements in cardiorespiratory fitness. Subjects weighed in once per week with a weight loss goal of 1–2 lb per week.
2.7. Data analysis
Subjects were grouped based on their RPB during the constant load 105 W exercise test. Those with an RPB ≤ 2 were classified as having no or mild dyspnea on exertion (–DOE) and those with an RPB ≥ 4 were classified as having strong dyspnea on exertion (+DOE). Those men who rated RPB of 3 were excluded from further study to better delineate any differences between the +DOE and –DOE groups.
Differences between +DOE and –DOE groups before and after the weight loss program were analyzed using two-way ANOVA (i.e., group and weight loss) with repeated measures on one factor (weight loss). Dyspnea questionnaire data from the +DOE group before and after weight loss were analyzed using t-test. Relationships among variables were determined using Pearson correlation for continuous variables or Spearman rank correlation for ordinal variables. Values are presented as mean ± SD. A p value of 0.05 was considered significant.
3. RESULTS
Ten men were categorized as +DOE and eight men as –DOE. Table 1 shows pre- and post-weight loss values of body composition, fat distribution, pulmonary function, oxygen cost of breathing, and cardiorespiratory responses during submaximal and maximal exercise.
Table 1:
Pre- and post-weight loss values of body composition, fat distribution, pulmonary function, oxygen cost of breathing, and cardiorespiratory responses during submaximal and peak exercise (mean ± SD).
| +DOE | -DOE | p-value Weight Loss | p-value Group Differences | p-value interact ion | |||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | ||||
| Body composition | |||||||
| Age (yr) | 37.5 ±6.3 | 29.8 ± 5.2 | 0.013 | ||||
| Height (cm) | 174.9 ± 9.1 | 182.8 ± 9.4 | NS | ||||
| Weight (kg) | 108.2 ± 19.0 | 99.2 ± 17.9 | 116.8 ± 21.0 | 104.9 ± 19.0 | <0.0001 | NS | NS |
| Body mass index (kg/m2) | 35.2 ± 4.3 | 32.3 ± 4.6 | 34.7 ± 3.4 | 31.2 ± 3.5 | <0.0001 | NS | NS |
| Body fat (%) | 38.6 ± 5.2 | 35.8 ± 6.9 | 36.3 ± 6.1 | 32.2 ± 7.6 | <0.0001 | NS | NS |
| Fat mass (kg) | 42.2 ± 11.0 | 36.2 ± 11.7 | 42.7 ± 12.4 | 34.2 ± 12.5 | <0.0001 | NS | NS |
| Lean body mass (kg) | 65.9 ± 10.0 | 63.0 ± 8.8 | 73.8 ± 13.2 | 70.3 ±13.0 | 0.0001 | NS | NS |
| Neck Circumference (cm) | 40.3 ± 2.5 | 39.0 ± 2.7 | 41.0 ± 2.0 | 39.1 ± 1.7 | <0.0001 | NS | NS |
| Chest Circumference (cm) | 113.1 ± 9.4 | 111.0 ± 10.0 | 114.4 ± 10.3 | 109.9 ± 9.7 | 0.0382 | NS | NS |
| Waist Circumference (cm) | 112.6 ± 9.6 | 101.4 ± 10.9 | 112.4 ± 10.1 | 103.1 ± 11.2 | <0.0001 | NS | NS |
| Hip Circumference (cm) | 115.4 ± 10.4 | 108.6 ± 10.6 | 118.8 ± 12.5 | 112.4 ± 10.4 | <0.0001 | NS | NS |
| Waist-hip ratio | 0.98 ± 0.05 | 0.94 ± 0.08 | 0.95 ± 0.05 | 0.92 ± 0.05 | 0.0060 | NS | NS |
| Pulmonary function | |||||||
| TLC (%pred) | 91 ± 7 | 94 ± 9 | 98 ± 12 | 102 ± 13 | 0.000 | NS | NS |
| FRC (%pred) | 78 ± 18 | 90 ± 22 | 86 ± 16 | 86 ± 25 | 0.030 | ||
| FRC (%TLC) | 38 ± 8 | 45 ± 10 | 40 ± 8 | 42 ± 11 | 0.002 | NS | NS |
| FVC (%pred) | 96 ± 11 | 97 ± 11 | 103 ± 14 | 106 ± 16 | NS | NS | NS |
| FEV1 (%pred) | 98 ± 11 | 100 ± 11 | 100 ± 13 | 105 ± 15 | 0.029 | NS | NS |
| PEF (%pred) | 98 ± 13 | 97 ± 11 | 102 ± 14 | 106 ±18 | NS | NS | NS |
| ERV (%TLC) | 13 ± 7 | 19 ± 10 | 22 ± 6 | 22 ± 7 | 0.0530 | NS | NS |
| RV (%pred) | 76 ± 11 | 77 ± 16 | 69 ± 12 | 80 ± 11 | 0.057 | NS | NS |
| DLCO (%pred) | 91 ± 10 | 96 ± 14 | 92 ± 14 | 92 ± 11 | NS | NS | NS |
| MVV (%pred) | 96 ± 17 | 103 ± 10 | 95 ± 15 | 102 ± 14 | 0.056 | NS | NS |
| Oxygen cost of breathing | |||||||
| O2 cost slope (mL of O2/L of ) | 2.07 ± 0.73 | 1.91 ± 0.63 | 2.01 ± 0.93 | 1.36 ± 0.34 | 0.014 | NS | NS |
| Constant load exercise @ 105W | |||||||
| RPB | 4.1 ± 0.3 | 2.8 ± 1.1 | 1.3 ± 0.7 | 0.8 ± 0.6 | 0.0009 | <0.0001 | NS |
| RPE | 13.0 ± 0.7 | 11.0 ± 1.9 | 9.4 ± 1.8 | 8.4 ± 1.4 | 0.0004 | 0.0001 | NS |
| (L/min) | 1.90 ± 0.11 | 1.83 ± 0.10 | 1.97 ± 0.14 | 1.78 ± 0.10 | 0.0157 | ||
| (%peak) | 74 ± 11 | 69 ± 9 | 62 ± 9 | 55 ± 10 | 0.0038 | 0.0082 | NS |
| (L/min) | 60.3 ± 10.7 | 55.4 ± 6.5 | 53.7 ± 3.6 | 49.2 ± 4.9 | 0.0045 | 0.0585 | NS |
| (%MVV) | 40 ± 12 | 33 ± 6 | 32 ± 8 | 27 ± 4 | 0.0036 | 0.0540 | NS |
| RER | 1.07 ± 0.06 | 1.03 ± 0.07 | 1.01 ± 0.06 | 1.00 ± 0.07 | NS | NS | NS |
| HR (bpm) | 140 ± 22 | 140 ± 17 | 134 ± 16 | 130 ± 16 | NS | NS | NS |
| HR (%peak) | 76 ± 13 | 78 ± 5 | 70 ± 8 | 68 ± 7 | NS | NS | NS |
| [Lactate] (mmol/L) | 5.8 ± 1.9 | 5.6 ± 1.9 | 4.3 ± 1.7 | 3.9 ± 1.5 | NS | NS | NS |
| (ml/min) | 125 ± 52 | 107 ± 43 | 113 ± 52 | 66 ± 19 | 0.0049 | NS | NS |
| Peak exercise | |||||||
| Work rate (W) | 216 ± 34 | 222 ± 25 | 255 ± 45 | 266 ± 52 | NS | 0.0328 | NS |
| Time to exhaustion (min) | 7.2 ± 1.3 | 7.3 ± 1.0 | 8.5 ± 1.4 | 9.0 ± 1.7 | 0.0391 | 0.0380 | NS |
| (L/min) | 2.60 ± 0.39 | 2.67 ± 0.39 | 3.19 ± 0.57 | 3.32 ± 0.66 | NS | 0.0120 | NS |
| (%pred) | 99 ± 16 | 101 ± 15 | 100 ± 14 | 100 ± 16 | NS | NS | NS |
| (L/min) | 104 ± 29 | 111 ± 18 | 140 ± 31 | 146 ±28 | NS | 0.0059 | NS |
| RER | 1.25 ± 0.08 | 1.28 ± 0.09 | 1.25 ± 0.05 | 1.26 ± 0.08 | NS | NS | NS |
| HR (bpm) | 184 ± 17 | 178 ± 17 | 187 ± 10 | 187 ± 11 | NS | NS | NS |
| [Lactate] (mmol/L) | 11.1 ± 1.5 | 10.4 ± 2.0 | 11.5 ± 2.4 | 11.6 ± 2.4 | NS | NS | NS |
TLC, total lung capacity; FRC, functional residual capacity; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow; ERV, expiratory reserve volume; RV, residual volume; DLCO, diffusing capacity for carbon monoxide; MVV, maximal voluntary ventilation; RPB, rating of perceived breathlessness; RPE, rating of perceived exertion;, oxygen uptake;, minute ventilation; RER, respiratory exchange rate; HR, heart rate; resp, work of breathing; %pred, percent of predicted values; NS, non-significant.
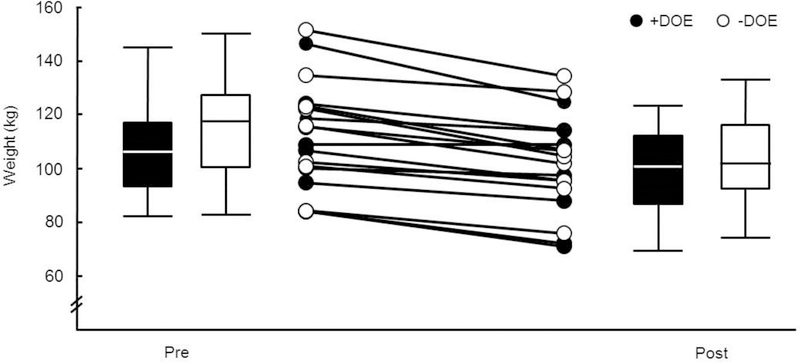
The +DOE group was on average older than the –DOE group (37.5 ± 6.3 years, range 23 – 44 vs 29.8 ± 5.2, range 24–40, p < 0.05). Otherwise, there were no significant differences between the +DOE and –DOE groups in body composition, pulmonary function, or the oxygen cost of breathing. There were no significant differences between groups following weight loss regarding body composition and pulmonary function (p > 0.05). All participants lost weight, on average −10.3 ± 5.6 kg or −9.2 ± 4.5% (Figure 2). Virtually all body composition measurements, including BMI (9%), circumferences, and body fat percentage (10%) were decreased following weight loss. On average (n=18), subjects lost 7.1 ± 3.9 kg of fat mass and 3.1 ± 2.6 kg of lean body mass. Several pulmonary function variables increased significantly following weight loss in both groups. In terms of absolute values (i.e., in L), there was a 3% increase in FVC, 3% increase in TLC, 16% increase in FRC, and 3% increase in DLCO (all p < 0.05), as well as trends towards increased ERV and RV (p < 0.06). The work of breathing as measured during eucapnic voluntary hyperpnea (O2cost slope) as well as during submaximal exercise () was reduced significantly in both groups (p < 0.05) after weight loss.
Figure 2:
Weight loss before and after intervention. Filled bars, +DOE; open bars, -DOE.
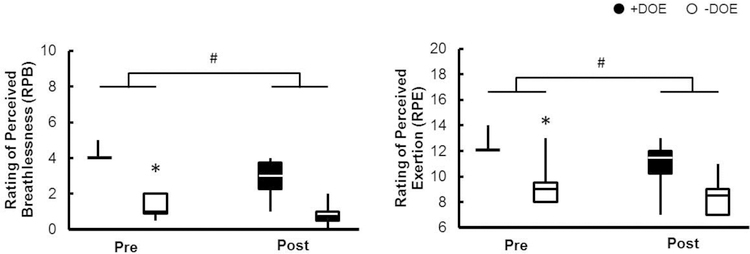
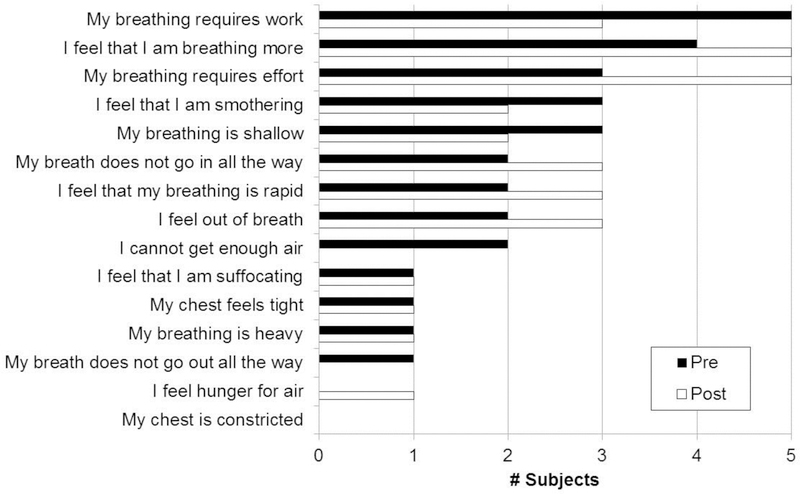
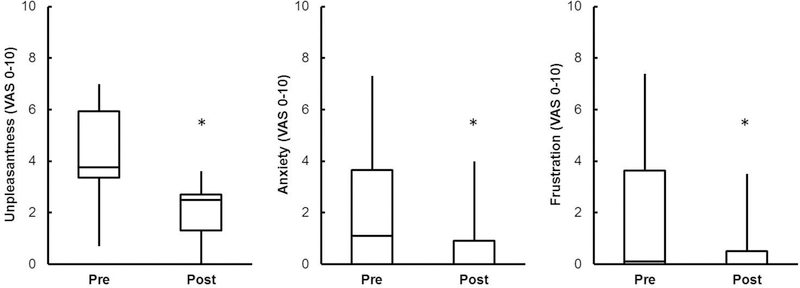
During submaximal cycling at 105 W, no significant differences were observed between groups in absolute (L/min), RER, HR, or lactate (p > 0.05). RPB, RPE, and relative exercise intensity ( as % of peak) were significantly higher in the +DOE group (p < 0.001), while trended in the same direction but failed to reach significance. There was a significant correlation between age and submaximal exercise intensity (Pearson r = 0.50, p < 0.05), and between RPB and submaximal exercise intensity (Spearman ρ = 0.54, p < 0.05). RPB during submaximal constant load cycling was significantly improved in both groups following weight loss (p < 0.001, Figure 3). Also, and were lower in both groups during submaximal exercise following weight loss (p < 0.01). Figure 4 shows the dyspnea descriptors and clusters that the men +DOE used to describe the respiratory sensations they felt during the submaximal exercise. Half of the men chose “my breathing requires work”, “my breathing requires effort”, or “I feel that I am breathing more”. There were no marked changes in descriptors after weight loss. Unpleasantness (4.2 ± 2.0 to 2.1 ± 1.1), anxiety (2.1 ± 2.6 to 0.8 ± 1.3), and frustration (2.0 ± 3.0 to 0.7 ± 1.3) related to the breathlessness during submaximal exercise significantly decreased after weight loss (Figure 5); no changes were observed in depression or anger.
Figure 3:
Weight loss decreased RPB and RPE. Filled bars, +DOE; open bars, -DOE. *p<0.05 between groups, #p<0.05 after weight loss.
Figure 4:
Dyspnea descriptors of +DOE group before and after weight loss. Number of subjects who selected the descriptors (left) as one of the “best three” that applied to their DOE during constant-load cycling at 105 W. Filled bars, before weight loss; open bars, after weight loss.
Figure 5:
Unpleasantness and negative emotions related to breathlessness during submaximal exercise decreased after weight loss in +DOE group. *p<0.05
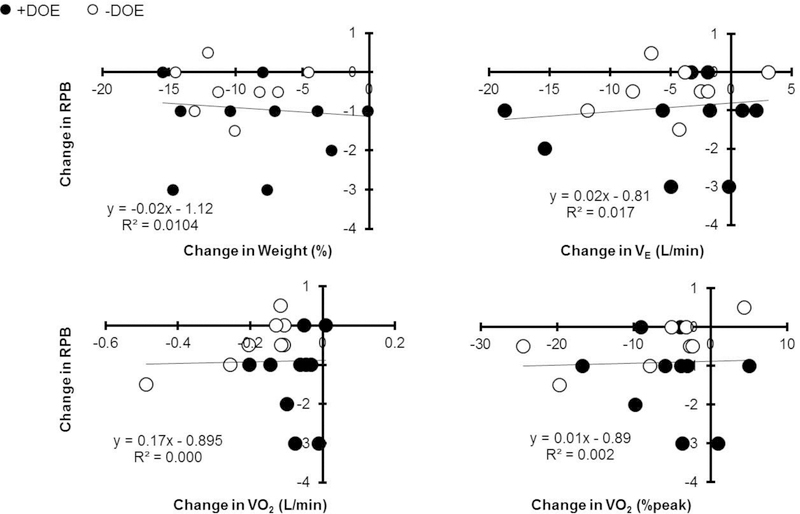
The decrease in RPB was not significantly correlated with decreases in body weight or the improvements in submaximal exercise variables (p > 0.05, Figure 6). The decreased body weight was correlated with increased FRC (r2 = 0.176).
Figure 6:
No significant correlations between change in RPB and change in body composition or cardiorespiratory fitness. Filled circles, +DOE; open circles, -DOE.
During peak exercise, there were group differences at baseline in absolute , , and work rate; but not in relative as % of predicted. On average, subjects were not deconditioned as peak exercise capacity was 99 ± 15% of predicted (range between 75–128%). There were no statistically significant changes in peak exercise capacity following weight loss in either group, except for time to exhaustion which was minimally increased (less than 0.5 seconds) following weight loss.
4. DISCUSSION
The present results confirm that moderate weight loss, without aerobic exercise training, can improve dyspnea ratings during cycling exercise as well as reduce unpleasantness and negative emotions related to the dyspnea in adults with obesity. This improvement in DOE is not dependent on percent body fat before or after weight loss, or on the amount of weight loss, or any change in body composition, pulmonary function, oxygen cost of breathing, or cardiorespiratory measures during submaximal or maximal exercise, which is similar to the findings in women with obesity we previously studied,. Although the exact physiological mechanism(s) of reduced breathlessness ratings remains elusive, our findings provide encouraging evidence that moderate weight loss of “only” ~10 kg (or ~9% of body weight) without aerobic exercise training can help reduce exertional dyspnea in otherwise healthy, adult men with obesity. Exertional dyspnea can be a major barrier for beginning and maintaining an aerobic exercise program, losing weight via a reduced calorie diet seems to be an effective first step in fighting obesity and its associated negative impact on health.
Weight loss reduced all body circumference measurements somewhat proportionately. Body mass index was reduced, but all subjects were still considered overweight/obese at the end of the study. Lean body mass was reduced, even though subjects performed resistance exercises three days per week. However, the loss of fat mass overshadowed the loss lean body mass (~7 kg vs ~3 kg). None of the changes in body composition measurements were correlated with the improvement in RPB during exercise.
It is well established that weight loss alters pulmonary function (Aaron et al., 2004; De Lorenzo et al., 1999; Emirgil and Sobol, 1973; Hakala et al., 1995; Santesson and Nordenstrom, 1978; Thomas et al., 1989; Wadstrom et al., 1991; Wei et al., 2011). Our results are in agreement with a previous study showing increases in resting FVC, FRC, and ERV (Babb et al., 2011) following weight loss in sedentary men with obesity (but not TLC, FEV1, PEF). Similarly, Womack et al (Womack et al., 2000) found increases in FVC, TLC, FRC, and RV (but no changes in FEV1 or DLCO). And in women with obesity, moderate weight loss increased FRC and ERV (Bernhardt and Babb, 2014b). However, none of the observed changes in pulmonary function were significantly correlated with the improved RPB during exercise.
The differences in absolute , , and work rate at peak exercise were mainly due to the +DOE group being older, as there was no difference in as percent of predicted which takes age into account (Wasserman et al., 2005). There was no correlation between RPB and (% pred). As intended, maximal exercise capacity did not change with the intervention, as subjects did not perform any aerobic exercise training in order to solely examine the effects of weight loss. Previously, we have shown that an aerobic exercise training intervention, without weight loss, reduced DOE in women with obesity (Bernhardt et al., 2016). Thus, weight loss and aerobic exercise training appear to have separate and/or synergistic effects on DOE. Further studies on the impacts of a combined intervention is warranted, although the additional effort, time, and commitment required from the subjects may be difficult to maintain. Dropout rates from these types of interventions are notoriously high. In the present study, eight subjects were disqualified for non-compliance or dropped out for personal reasons during the weight loss intervention phase (5 +DOE and 3 –DOE, Figure 1), which is 31% of the 26 subjects who started. In our earlier studies in women with obesity, the dropout rate was 45% (unpublished data). Thus, a weight loss and aerobic exercise training program without a very controlled environment could potentially retain even fewer participants. Our participants had access to a personal trainer, meal plan, dietician, exercise physiologist, and investigators without cost to the participant and yet 31% were unable or unwilling to complete the program.
Only about 29% of the variance in RPB is explained by submaximal intensity ( as % of peak) at 105 W, both before and after weight loss. Thus, over 70% of the variance is due to factors besides exercise intensity. Somewhat surprisingly, submaximal and were decreased in both groups following the intervention, even though maximal values were not. It is possible that the men had very low physical activity levels at baseline and therefore the little resistance exercise they did during the intervention was enough to improve submaximal exercise; although maximal exercise capacity at baseline was normal and did not improve with the weight loss program. The improvements in submaximal exercise, however, were not significantly correlated with the improved RPB.
Feelings of unpleasantness, anxiety, and frustration were elevated in those men with DOE at initial testing and were significantly reduced following weight loss. We previously reported similar findings of higher unpleasantness, anxiety, and fear in women with DOE (Marines-Price et al., 2018). To our knowledge, the present study is the first to show that moderate weight loss can alleviate negative emotions associated with breathlessness during exercise, which is an important factor for maintaining an exercise program.
The work of breathing is greater in obese individuals (Kress et al., 1999; Naimark and Cherniack, 1960; Pelosi et al., 1996) and decreases with weight loss (Bernhardt and Babb, 2014b; Bhammar et al., 2016). Similarly to our previous results in women with obesity, the work of breathing in the present study was significantly reduced following weight loss in the men with obesity, but there was no correlation between this reduction and the improvement in RPB during exercise.
It is likely that a multitude of factors play a role in the reduction of exertional dyspnea; including not only the studied physiological measures, but also psychophysiological ones. For example, it is conceivable that just being more physically active and meeting with a personal trainer several times per week had a positive impact on the previously sedentary men. Similarly, following a meal plan and experiencing steady and continuous weight loss would have a positive psychological effect, which could have influenced the perception of breathlessness. Unfortunately, we did not investigate these potential psychological improvements in this study.
In conclusion, moderate weight loss, without aerobic exercise training, can improve dyspnea ratings during cycling exercise as well as reduce unpleasantness and negative emotions related to the dyspnea in adults with obesity. This improvement appears independent of changes in percent body fat, or amount of weight loss, or changes in body composition, pulmonary function, oxygen cost of breathing, or cardiorespiratory measures during submaximal or maximal exercise. Nevertheless, our findings provide encouraging evidence that moderate weight loss of “only” ~9% of body weight without aerobic exercise training can improve the discomfort of exertional dyspnea in otherwise healthy, adult men with obesity. As such, losing weight via a reduced calorie diet may be an effective first step in fighting the limitations of obesity, its associated negative impact on health, or before beginning an aerobic exercise program.
Highlights:
Weight loss of ~9% effectively reduced dyspnea on exertion (DOE) in obese men
Unpleasantness and negative emotions related to DOE were reduced after weight loss
Exact mechanism(s) of the improved DOE remains unclear
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Jonathon Stickford, J. Todd Bassett, Joseph Genovese, Andreas Kreutzer, Anastasia Pyz, Maria Roman, and Deborah Trevino for their assistance in various stages of this project, and the staff members of the Texas Health Finley Ewing Cardiovascular & Fitness Center Dallas for their expertise in implementing the diet and exercise program, especially Gerry Maness and Susan Rodder. This work was supported by the National Institutes of Health (HL096782), King Charitable Foundation Trust, Cain Foundation, and Texas Health Presbyterian Hospital Dallas. The funding sources had no involvement in the study design; collection, analysis and interpretation of data; writing of the report; nor decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE, 2004. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 125, 2046–2052. [DOI] [PubMed] [Google Scholar]
- Babb TG, 2013. Obesity: Challenges to ventilatory control during exercise-A brief review. Respir. Physiol. Neurobiol [DOI] [PMC free article] [PubMed]
- Babb TG, Buskirk ER, Hodgson JL, 1989. Exercise end-expiratory lung volumes in lean and moderately obese women. Int J Obes 13, 11–19. [PubMed] [Google Scholar]
- Babb TG, DeLorey DS, Wyrick BL, Gardner PP, 2002. Mild obesity does not limit change in end-expiratory lung volume during cycling in young women. J Appl Physiol 92, 2483–2490. [DOI] [PubMed] [Google Scholar]
- Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B, 2008a. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med 178, 116–123. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, Chase PJ, Delorey DS, Rodder SG, Feng MY, Ranasinghe KG, 2011. Weight loss via diet and exercise improves exercise breathing mechanics in obese men. Chest 140, 454–460. [DOI] [PubMed] [Google Scholar]
- Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY, 2008b. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest 134, 704–711. [DOI] [PubMed] [Google Scholar]
- Bernhardt V, Babb TG, 2014a. Respiratory symptom perception differs in obese women with strong or mild breathlessness during constant-load exercise. Chest 145, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Babb TG, 2014b. Weight loss reduces dyspnea on exertion in obese women. Respir Physiol Neurobiol 204, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Bhathia S, Moran RB, Bassett JT, Lorenzo S, Pineda JN, Genovese JP, Babb TG, 2014. Repeatability of Rating of Perceived Breathlessness during Exercise in Obese Women. Med Sci Sports Exerc 46, S529. [Google Scholar]
- Bernhardt V, Stickford JL, Bhammar DM, Babb TG, 2016. Aerobic exercise training without weight loss reduces dyspnea on exertion in obese women. Respir Physiol Neurobiol 221, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt V, Wood HE, Moran RB, Babb TG, 2013. Dyspnea on exertion in obese men. Respir Physiol Neurobiol 185, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhammar DM, Stickford JL, Bernhardt V, Babb TG, 2016. Effect of weight loss on operational lung volumes and oxygen cost of breathing in obese women. Int J Obes (Lond) 40, 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg GA, 1982. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14, 377–381. [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Arbes SJ Jr., Germolec DR, Korach KS, Zeldin DC, 2007. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18, 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo RO, Morris AH, Gardner RM, 1982. Reference values for pulmonary tissue volume, membrane diffusing capacity, and pulmonary capillary blood volume. Bull Eur Physiopathol Respir 18, 893–899. [PubMed] [Google Scholar]
- Dalle Grave R, Calugi S, Centis E, El Ghoch M, Marchesini G, 2010. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. Journal of obesity 2011. [DOI] [PMC free article] [PubMed]
- De Lorenzo A, Petrone-De Luca P, Sasso GF, Carbonelli MG, Rossi P, Brancati A, 1999. Effects of weight loss on body composition and pulmonary function. Respiration 66, 407–412. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Wyrick BL, Babb TG, 2005. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes (Lond) 29, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Emirgil C, Sobol BJ, 1973. The effects of weight reduction on pulmonary function and the sensitivity of the respiratory center in obesity. Am Rev Respir Dis 108, 831–842. [DOI] [PubMed] [Google Scholar]
- Hakala K, Mustajoki P, Aittomaki J, Sovijarvi AR, 1995. Effect of weight loss and body position on pulmonary function and gas exchange abnormalities in morbid obesity. Int J Obes Relat Metab Disord 19, 343–346. [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL, (2017). Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief National Center for Health Statistics, Hyattsville, MD. [PubMed] [Google Scholar]
- Kress JP, Pohlman AS, Alverdy J, Hall JB, 1999. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med 160, 883–886. [DOI] [PubMed] [Google Scholar]
- Lin CK, Lin CC, 2012. Work of breathing and respiratory drive in obesity. Respirology 17, 402–411. [DOI] [PubMed] [Google Scholar]
- Luce JM, 1980. Respiratory complications of obesity. Chest 78, 626–631. [DOI] [PubMed] [Google Scholar]
- Mahler DA, Harver A, Lentine T, Scott JA, Beck K, Schwartzstein RM, 1996. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med 154, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Marines-Price R, Bernhardt V, Bhammar DM, Babb TG, 2018. Dyspnea on exertion provokes unpleasantness and negative emotions in women with obesity. Respiratory Physiology and Neurobiology [DOI] [PMC free article] [PubMed]
- Mead J, 1980. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis 121, 339–342. [DOI] [PubMed] [Google Scholar]
- Meek PM, Schwartzstein RM, Adams L, M.D., A., Breslin EH, Carrieri-Kohlman V, Gift A, Hanley MV, Harver A, Jones PW, 1999. Dyspnea, Mechanisms, Assessment, and Management: A Consensus Statement. Am. J. Respir. Crit. Care Med 159, 321–340. [DOI] [PubMed] [Google Scholar]
- Naimark A, Cherniack RM, 1960. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol 15, 377–382. [DOI] [PubMed] [Google Scholar]
- Ofir D, Laveneziana P, Webb KA, O’Donnell DE, 2007. Ventilatory and perceptual responses to cycle exercise in obese women. J Appl Physiol (1985) 102, 2217–2226. [DOI] [PubMed] [Google Scholar]
- Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE, 2012. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 185, 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L, 1996. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest 109, 144–151. [DOI] [PubMed] [Google Scholar]
- Santesson J, Nordenstrom J, 1978. Pulmonary function in extreme obesity. Influence of weight loss following intestinal shunt operation. Acta Chir Scand Suppl 482, 36–40. [PubMed] [Google Scholar]
- Schaeffer MR, Mendonca CT, Levangie MC, Andersen RE, Taivassalo T, Jensen D, 2014. Physiological mechanisms of sex differences in exertional dyspnoea: role of neural respiratory motor drive. Exp Physiol 99, 427–441. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Guenette JA, 2008. Mechanics of breathing during exercise in men and women: sex versus body size differences? Exerc Sport Sci Rev 36, 128–134. [DOI] [PubMed] [Google Scholar]
- Society AT, 1995. Standardization of Spirometry 1994 Update. Am J Respir Crit Care Med 152, 1107–1136. [DOI] [PubMed] [Google Scholar]
- Thomas PS, Cowen ER, Hulands G, Milledge JS, 1989. Respiratory function in the morbidly obese before and after weight loss. Thorax 44, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM, 1982. Postnatal human lung growth. Thorax 37, 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JB, Dougherty LM, Archer CR, Price DD, 1996. Assessing the stages of pain processing: a multivariate analytical approach. Pain 68, 157–167. [DOI] [PubMed] [Google Scholar]
- Wadstrom C, Muller-Suur R, Backman L, 1991. Influence of excessive weight loss on respiratory function. A study of obese patients following gastroplasty. Eur J Surg 157, 341–346. [PubMed] [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ, 2005. Principles of Exercise Testing and Interpretation, Fourth ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- Wei YF, Tseng WK, Huang CK, Tai CM, Hsuan CF, Wu HD, 2011. Surgically induced weight loss, including reduction in waist circumference, is associated with improved pulmonary function in obese patients. Surg Obes Relat Dis 7, 599–604. [DOI] [PubMed] [Google Scholar]
- Womack CJ, Harris DL, Katzel LI, Hagberg JM, Bleecker ER, Goldberg AP, 2000. Weight loss, not aerobic exercise, improves pulmonary function in older obese men. The journals of gerontology. Series A, Biological sciences and medical sciences 55, M453–457. [DOI] [PubMed] [Google Scholar]