Abstract
Under resting normoxic conditions, the healthy lung has ample oxygen uptake capacity relative to oxygen demand, but during exercise, increased oxygen demand and utilization become increasingly dependent on ventilation-perfusion matching. A mathematical model is used to investigate the effect of pulmonary flow heterogeneity, as characterized by the coefficient of variation (CV) of capillary blood flow, on pulmonary oxygen uptake in exercise. The model reveals that any level of heterogeneity up to a CV of 3 is consistent with the observed level of arterial oxygen tension under resting conditions, but that such high levels of heterogeneity are incompatible with the levels of oxygen uptake observed during exercise. If a normal diffusing capacity is assumed, the best fit to literature data on arterial oxygen content of exercising humans under normoxic and hypoxic conditions is found with a relatively low CV of 0.48, suggesting that local flow regulation mechanisms such as hypoxic pulmonary vasoconstriction play an important role in ventilation-perfusion matching during exercise.
Keywords: Diffusing capacity, exercise, oxygen uptake, heterogeneity, mathematical model
1. INTRODUCTION
The rate at which oxygen can be taken up into the bloodstream in the lung is a critical determinant of functional capacity, and can be affected not only by alterations in lung oxygen diffusing capacity but also by heterogeneity of pulmonary blood flow, leading to maldistribution and poor ventilation-perfusion matching. The capacity for oxygen transport of healthy individuals is higher than necessary to achieve high arterial oxygen saturation at rest under normoxic conditions, but saturation can become reduced under conditions of exercise, hypoxia, or disease either due to ventilation-perfusion mismatch, decreased gradients across the pulmonary-capillary membrane, or limitations in diffusing capacity.
The diffusing capacity of the lung for oxygen, a major determinant of the maximum rate of pulmonary oxygen uptake, can be defined as
| (1) |
where is the oxygen uptake and PA and are alveolar and mean capillary We previously derived an estimate for this diffusing capacity in terms of the geometry of the pulmonary capillary and the diffusive mass transfer properties of the alveolar-capillary membrane, plasma and erythrocytes (Roy and Secomb, 2014). This value is independent of blood flows, ventilation, and oxygen tension and reflects the maximum theoretical diffusing capacity of the lung.
Heterogeneity in blood flow implies a distribution of flows through capillaries of the lung, which enter with the same venous oxygen content but leave with different values of arterial content. Vessels with higher flows may reach lower arterial saturation values than vessels with lower flows. Since vessels with higher flows are disproportionally represented in the flow- weighted average, flow heterogeneity has a significant potential to decrease the oxygen content of blood exiting the lung.
In order to quantify the effect of flow heterogeneity on pulmonary oxygen uptake, a mathematical model is utilized in this study. The model is used to estimate the degree to which increased heterogeneity in pulmonary blood flow affects values of arterial and venous oxygen levels under conditions of rest and exercise. Oxygen transport in the lung is dependent on the venous content, which is in turn affected by the systemic circulation. Likewise, the oxygen content in the systemic circulation is dependent on arterial oxygen content exiting the lung. For this reason, a combined pulmonary and systemic model is used in this study. A model for systemic oxygen consumption with Michaelis-Menten kinetics is included to account for the fact that consumption may be significantly less than oxygen demand, especially in extreme cases (e.g. high intensity exercise under hypoxic conditions). This model is used to investigate the hypothesis that high levels of heterogeneity would preclude the high oxygen uptake in the lung observed during exercise. The results are discussed in terms of the role of local active regulation of pulmonary capillary flow in reducing the heterogeneity of blood flow in the lung.
2. METHODS
A model reflecting the oxygen uptake and oxygen consuming characteristics of the human body is used to explore the hypothesis outlined above. Briefly, for given values of venous oxygen content, cardiac output and lung diffusing capacity, the model for pulmonary oxygen uptake is used to predict a value for arterial oxygen content. In simulations to test the effects of varying pulmonary flow heterogeneity, the arterial oxygen content is used as an input value for the model of systemic oxygen consumption to generate a value of venous oxygen content; this procedure is iterated to convergence to provide values of and . The lung compartment, consisting of multiple identical capillary compartments with a distribution of flows, is connected with a non-perfused tissue compartment and two oxygen-consuming compartments arranged in parallel (Figure 1). The non-skeletal muscle compartment is assumed to have a fixed resting oxygen demand, whereas the skeletal muscle compartment isassigned a demand dependent on exercise intensity. Parameters used for resting conditions are selected from a range of commonly reported physiological values: cardiac output Q = 6 L min−1, oxygen consumption cm3 O2 min−1, arterial and venous oxygen tension and mmHg respectively, , and respiratory quotient (Hall, 2015; Levick, 2013). Dissolved oxygen is taken into account in calculations of oxygen content and the Hill equation is used for calculations of hemoglobin oxygen saturation. In simulations to compare predicted arterial oxygen content with experimental values, venous oxygen content is set to measured values and the model for systemic oxygen consumption is not used.
Figure 1.
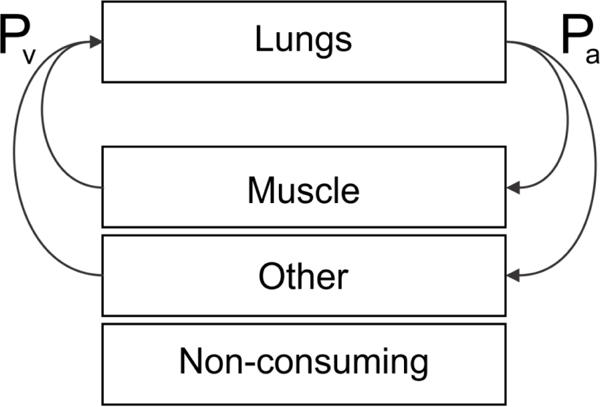
Model overview. A model for pulmonary oxygen uptake is coupled with a model for tissue oxygen utilization in which three compartments are considered: (i) a non-oxygen consuming compartment (9.2 kg); (ii) a skeletal muscle compartment (10.8 kg) with oxygen consumption dependent on exercise intensity; (iii) a non-skeletal (“other”) muscle compartment (50 kg) with oxygen consumption fixed at resting values.
2.1. Pulmonary model
To represent the heterogeneity of perfusion in pulmonary capillaries, cardiac output is non-uniformly divided among 101 otherwise identical capillary compartments arranged in parallel in accordance with a lognormal distribution of blood flow corresponding to a specified coefficient of variation. Example flow distributions are shown in Figure 2. In other studies (Beck et al., 2012; Rice et al., 1999), heterogeneity of pulmonary perfusion and ventilation have been discussed in terms of the quantity LogSD, defined as the standard deviation of the logarithm (base 10) of the quantity in question. The relationship between CV and LogSD is shown in the inset to Figure 2.
Figure 2.
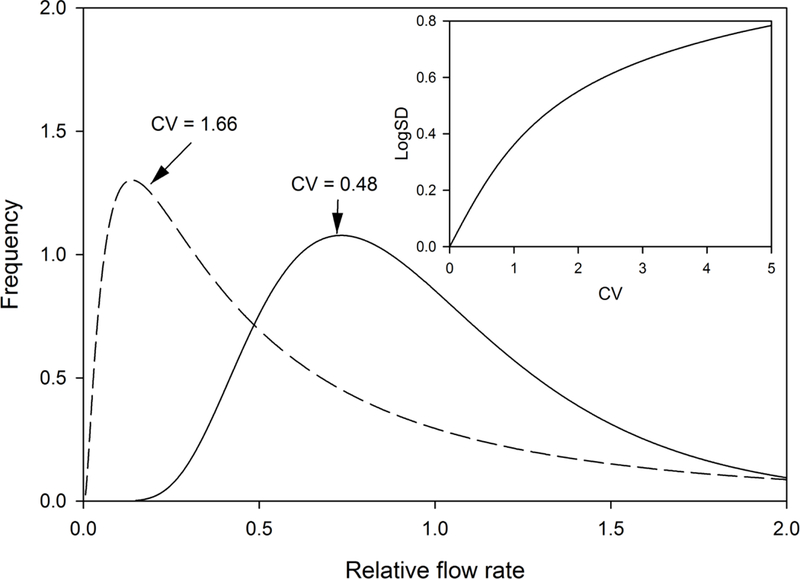
Examples of lognormal flow distributions. A CV of 1.66 corresponds to a LogSD of 0.5. The inset shows the relationship between CV and LogSD.
All capillaries are assumed to have an identical diffusing capacity, corresponding to a total pulmonary diffusing capacity as calculated previously (Roy and Secomb, 2014). Oxygen loading in the lung is simulated by assuming that the entire cardiac output Q passes through parallel compartments of length Lcap with total length Ltot (Wagner, 1996). In each capillary i, the flow is (Qcap)i, and conservation of mass gives:
| (2) |
where x is position along the capillary and Pb represents the blood as a function of x. The oxygen carrying capacity of whole blood is assumed to be proportional to the hematocrit HD (see Table 1) and is approximately 0.2 cm3 O2 (cm3 blood)−1 under normal conditions. Alveolar is computed as a function of the ambient partial pressure of oxygen, reported values of the respiratory quotient R, and alveolar (assumed to be the same as arterial from the alveolar gas equation (Story, 1996). Values of PA thus range from 99.7 mmHg under normoxic conditions to 57.0 and 35.6 mmHg at 15% and 12% oxygen respectively. The average flow in each capillary is equal to . Setting and using the fact that (the total number of capillaries), we obtain:
| (3) |
independent of the number and length of pulmonary capillaries, where represents the slope of the oxyhemoglobin dissociation curve at . This equation is integrated from to , with to obtain . The flow-weighted average of the oxygen content from each capillary compartment is used to calculate the arterial saturation, which is then used as the input to the systemic model.
Table 1.
Parameter values.
| Parameter | Value | Units | Reference |
|---|---|---|---|
| P50 | 26.3 | mmHg | (Hsia, 1998) |
| n | 2.7 | - | (Hsia, 1998) |
| CHb | 1.39 | cm3 O2/g | (McGuire and Secomb, 2001) |
| HD | 14.39 | g/dL | (McGuire and Secomb, 2001) |
| Sh | 2.5 | - | (Heliums et al., 1996) |
| Kpl | 8.3 × 10−10 | (cm2 s−1)(cm3 O2 cm−3 mmHg−1) | (Heliums et al., 1996) |
| DLO2 | 102 | cm3 O2 min−1 mmHg−1 | (Roy and Secomb, 2014) |
| P0 | 10.5 | mmHg | (Golub and Pittman, 2012) |
| rc | 2.5 | μm | (Roy and Secomb, 2014) |
| NA(c,f)skel | 1288 | mm−2 | [Calculated] |
| NA(c,f)nonskel | 308 | mm−2 | (Pasarica et al., 2009) |
| Kt | 9.4 × 10−10 | (cm2 s−1)(cm3 O2 cm−3 mmHg−1) | (Bentley et al., 1993) |
| Dt | 2.41 × 10−5 | cm2 s−1 | (Bentley et al., 1993) |
| αt | 3.89 × 10−5 | cm3 O2 cm−3 mmHg−1 | (Bentley et al., 1993) |
| CMb | 3.83 × 10−7 | mol cm−3 | (Meng et al., 1993) |
| DMb | 1.73 × 10−7 | cm2 s−1 | (Jurgens et al., 1994) |
2.2. Systemic model
In the systemic model, cardiac output is divided between two compartments (Figure 1). For a body mass of 70 kg, the non-skeletal muscle (“other”) compartment is assigned a mass of 50 kg and the muscle compartment is assigned a mass of 10.8 kg. For each compartment, the oxygen uptake characteristics are predicted using a Krogh cylinder model with geometrical parameters appropriate to each compartment (Table 1). In this model, blood flows through a capillary surrounded by a cylindrical tissue region with radius dependent on capillary density. Blood enters with a specified Pa and the oxygen level falls along the capillary as oxygen is consumed in each slice of the cylinder. Intracapillary resistance is characterized by the Sherwood number Sh, assumed to be 2.5 in this study (see Table 1). Michaelis-Menten oxygen kinetics are used to calculate the oxygen consumption based on the assigned oxygen demand in each compartment, resulting in radial gradients of oxygen in the tissue cylinder. This assumption means that local oxygen consumption is less than oxygen demand. In cases when the consumption is prescribed according to observed values, the value of oxygen demand is not known a priori, but must be calculated to correspond to the prescribed consumption. The P50 of blood is assumed to remain constant along the length of the capillary. The oxygen demand of the non-skeletal compartment is fixed at its rest value, whereas the demand of the skeletal muscle compartment is varied with exercise intensity. Facilitated diffusion by myoglobin is taken into account in the tissue compartment (McGuire and Secomb, 2001). As noted above, the actual consumption in the muscle compartment may fall significantly below the level of demand under hypoxic conditions. Since the oxygen demand of each compartment is not known a priori, the following procedure is utilized to calculate the parameters appropriate for each case (rest, moderate, and maximal exercise): (i) The blood flow and oxygen consumption of the non- skeletal compartment are calculated under resting conditions based on its mass fraction. (ii) The oxygen demand for the non-skeletal compartment is calculated to be consistent with its consumption obtained from the calculation above assuming a value for its capillary density (see Table 1). (iii) An oxygen demand of 80 cm3 O2 cm−3 min−1 (corresponding to conditions of ) is used to calculate a value for the capillary density of the skeletal muscle compartment (McGuire and Secomb, 2003). (iv) The blood flow and oxygen consumption for the skeletal muscle compartment are calculated for each case (rest, moderate, and maximal exercise) by subtracting the resting non-skeletal compartment blood flow and oxygen consumption values obtained in (i) from the measured values in each case. The demand for the skeletal muscle compartment is then calculated based on this value of the consumption in conjunction with the capillary density calculated in (iii).
Once these parameters for the systemic model have been derived, they are used to explore the effect of pulmonary perfusion heterogeneity in the combined model. The flow- weighted oxygen content from both compartments is used as the mixed venous input to the pulmonary circulation. The simulation of pulmonary and systemic oxygen transport is repeated iteratively until convergence of and is achieved.
3. RESULTS
The model was initially used to investigate the impact of CV on arterial and saturation (Figure 3). Under resting conditions, the CV was found to have little effect on calculated arterial values, which are seen to be consistent with typical observed levels of approximately 90 to 100 mmHg (Rice et al., 1999) for CV values from zero up to approximately 3. In this range of , arterial saturation is close to 1 and shows only slight changes with varying CV. Due to this low sensitivity, the actual perfusion heterogeneity under resting conditions cannot be reliably estimated from comparisons of predicted and observed arterial oxygen content.
Figure 3.
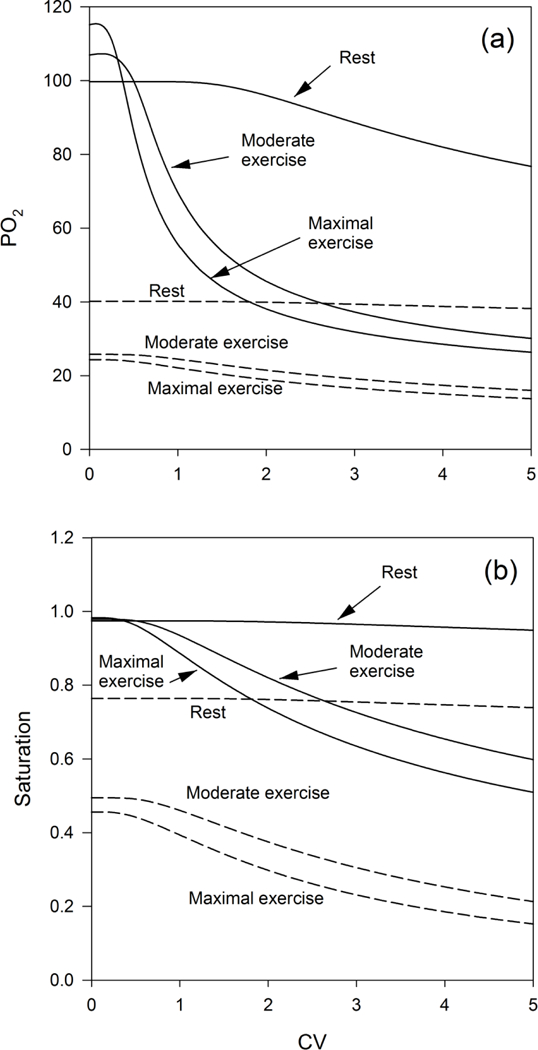
(a) Calculated values of arterial (solid lines) and venous (dashed lines) for simulated conditions of rest, moderate, and maximal exercise as a function of the CV of pulmonary blood flow. (b) Calculated values of arterial saturation (solid lines) and venous saturation (dashed lines) for conditions of rest, moderate, and heavy exercise as a function of the CV of pulmonary blood flow. The results imply that in exercise, the CV has to be in the range of 0.5 to 1 to maintain adequate tissue oxygen supply and to support the observed arteriovenous saturation differences.
Under conditions of moderate or heavy exercise, the predicted arterial and venous values for values of CV exceeding 1 are not consistent with reported values. In experiments under normoxic conditions, the observed arterial remains above 90 mmHg, with venous values in the range of 20–30 mmHg (Roca et al., 1989). Although the predicted venous values are similar, the predicted arterial drops dramatically at values of CV greater than 1, suggesting that a degree of heterogeneity above this would lead to an inability to maintain arterial and to sustain the observed levels of oxygen demand. The corresponding panel illustrating the drop in arterial and venous saturation demonstrates that the arteriovenous decrease in oxygen content declines substantially over the range of CV investigated in the cases of moderate exercise (from 0.48 to 0.39) and maximal exercise (from 0.53 to 0.36). These changes are directly proportional to the predicted declines in systemic oxygen consumption resulting from increasing CV of pulmonary capillary blood flow. The results imply that flow regulation in the lung plays a major role in maintaining oxygen consumption relative to demand. In order to obtain a specific estimate of CV in exercise, aggregated data from five experimental studies were used to estimate the CV that yielded the best fit to observations of arterial saturation in exercise under conditions of normoxia and hypoxia. All five studies used cycle ergometry and included measurements of arterial and mixed or femoral venous at a range of work rates. In each case, measured values of FiO2, cardiac output, oxygen consumption and respiratory quotient were used. Observed venous were used and the pulmonary model was used to predict corresponding arterial and saturation values. The predicted values of were compared to the observed values by regression through the origin on values of . When a value of CV = 0 was used, the slope was found to differ substantially from the line of identity (Figure 4A). The difference in slope from 1 was used as a basis to adjust CV until convergence to a slope of 1 was achieved. With the assumption of a normal diffusing capacity, the best fit overall to aggregated data from five studies was found with a CV of 0.48 (Figure 4B), corresponding to a LogSD of 0.2, lower than the values of 0.3 to 0.6 reported at rest (Beck et al., 2012) and the values of 0.33 to 0.64 reported in young healthy subjects (Wagner et al., 1974).
Figure 4.
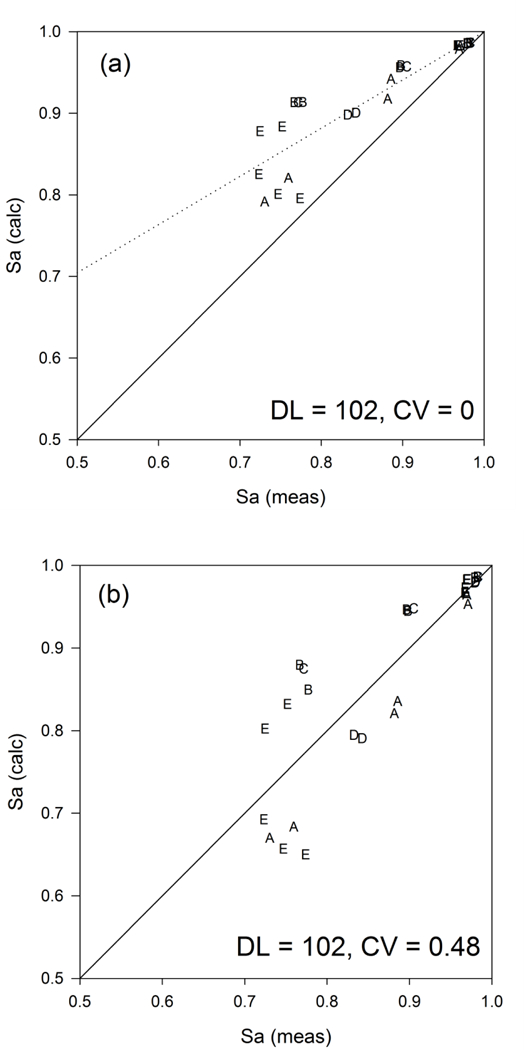
Calculated vs. reported values of arterial oxygen saturation based on data obtained under conditions of exercise and hypoxia from a number of studies based on DLO2 = 102 cm3 O2 min−1 mmHg−1. Regression lines (dashed) shown compared to lines of identity. (a) CV = 0; (b) CV = 0.48. Letters corresponding to references: A (Roca et al., 1989); B (Roca et al., 1992); C (Cardus et al., 1998); D (Schaffartzik et al., 1993); E (Knight et al., 1992).
4. DISCUSSION
Heterogeneity is inherent in microcirculation of all tissues, including systemic and pulmonary (Duling and Damon, 1987; Pries et al., 2009; Pries et al., 1995; Rice et al., 1999). In the lung, heterogeneity of ventilation also exists (Beck et al., 2012; West, 1969). It has long been understood that heterogeneity in matching can impair oxygen uptake in the lung (Wagner, 1992; West, 1969). Many theoretical studies of pulmonary oxygen transport consider a single homogeneous oxygen exchanging compartment or a small number of homogeneous compartments or zones. However, the relationship between heterogeneity and oxygen transport characteristics is not fully understood. There is a lack of quantitative information on the effects of heterogeneity at the microvascular level on overall oxygen transport characteristics of the lung, both at rest and under conditions of physiologic stress. In a study of the contributions of perfusion and ventilation heterogeneity in matching (Wilson and Beck, 1992), it was found that inhomogeneities in flow contribute approximately two-thirds and inhomogeneities in ventilation contribute approximately one-third of the variance to the heterogeneity of the distribution.
The model developed herein for exploring the effect of perfusion heterogeneity in the lung involves several simplifying assumptions. In reality, both ventilation and perfusion exhibit heterogeneity, but in the model the heterogeneity is attributed to perfusion and alveolar is assumed to be uniform within the lung. The results obtained here may approximately represent the behavior in the presence of heterogeneous ventilation, if the CV in the present model is interpreted as representing the distribution of deviations of capillary blood flows from ideal matching with alveolar ventilation. The model for systemic oxygen transport uses a Krogh cylinder model for both the muscle and non-muscle compartments, and does not include the effects of heterogeneity in systemic blood flow.
Our results show a striking difference between rest and exercise with regard to the effects of heterogeneity. In contrast to the minimal effect of pulmonary flow heterogeneity at rest, in exercise the degree of flow heterogeneity must be much lower in order to maintain oxygen uptake and tissue oxygenation. At rest, the levels of oxygen uptake required to maintain observed values of arterial are consistent with values of CV of approximately 3, whereas in exercise we find that the CV needs to be below 1 with a value of 0.48 estimated from experimental data. This is consistent with measurements of the perfusion distribution width obtained by open-circuit wash-in during rest and exercise in humans (Beck et al., 2012; Wilson and Beck, 1992), in which the LogSD of the perfusion is noted to fall from a value of 0.47 at rest to approximately 0.33 during heavy exercise, corresponding to values of CV of approximately 1.5 at rest and 0.9 during heavy exercise.
The implication of these results is that for sufficient uptake in exercise, relatively tight regulation of blood flow is needed, whereas at rest a high degree of heterogeneity can be tolerated. Hypoxic vasoconstriction is an important mechanism for local regulation of pulmonary blood flow. For vessels with high flows, leading to inadequate blood oxygenation, the effect of hypoxic vasoconstriction (HPV) is to reduce flow in these vessels, thereby increasing the saturation of the exiting blood. In this way, HPV reduces the heterogeneity in matching. At rest, high saturations are achieved in virtually all capillaries despite heterogeneity, so this mechanism is not activated. However, HPV has been shown to be active at the low levels of venous oxygen tension seen in exercise and hypoxia (Sylvester et al., 2012). Our results suggest that intact HPV is necessary to achieve moderate or maximal exercise.
Highlights.
A theoretical model is used to investigate effects of pulmonary flow heterogeneity
The normal lung can tolerate a high degree of pulmonary flow heterogeneity at rest
Pulmonary flow heterogeneity decreases arterial oxygen saturation during exercise
Observed arterial oxygen levels in exercise imply limits on flow heterogeneity
Flow regulation must mitigate ventilation-perfusion mismatch during exercise
ACKNOWLEDGEMENTS
Supported by NIH U01 HL133362.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beck KC, Johnson BD, Olson TP, Wilson TA, 2012. Ventilation-perfusion distribution in normal subjects. J Appl Physiol (1985) 113, 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley TB, Meng H, Pittman RN, 1993. Temperature dependence of oxygen diffusion and consumption in mammalian striated muscle. Am J Physiol 264, H1825–1830. [DOI] [PubMed] [Google Scholar]
- Cardus J, Marrades RM, Roca J, Barbera JA, Diaz O, Masclans JR, Rodriguez-Roisin R, Wagner PD, 1998. Effects of F(I)O2 on leg VO2 during cycle ergometry in sedentary subjects. Med Sci Sports Exerc 30, 697–703. [DOI] [PubMed] [Google Scholar]
- Duling BR, Damon DH, 1987. An examination of the measurement of flow heterogeneity in striated muscle. Circ Res 60, 1–13. [DOI] [PubMed] [Google Scholar]
- Golub AS, Pittman RN, 2012. Oxygen dependence of respiration in rat spinotrapezius muscle in situ. Am J Physiol Heart Circ Physiol 303, H47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, 2015. Guyton and Hall Textbook of Medical Physiology E-Book. Elsevier Health Sciences. [Google Scholar]
- Hellums JD, Nair PK, Huang NS, Ohshima N, 1996. Simulation of intraluminal gas transport processes in the microcirculation. Ann Biomed Eng 24, 1–24. [DOI] [PubMed] [Google Scholar]
- Hsia CC, 1998. Respiratory function of hemoglobin. N Engl J Med 338, 239–247. [DOI] [PubMed] [Google Scholar]
- Jurgens KD, Peters T, Gros G, 1994. Diffusivity of myoglobin in intact skeletal muscle cells. Proc Natl Acad Sci U S A 91, 3829–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DR, Poole DC, Schaffartzik W, Guy HJ, Prediletto R, Hogan MC, Wagner PD, 1992. Relationship between body and leg VO2 during maximal cycle ergometry. J Appl Physiol 73, 1114–1121. [DOI] [PubMed] [Google Scholar]
- Levick JR, 2013. An Introduction to Cardiovascular Physiology. Elsevier Science. [Google Scholar]
- McGuire BJ, Secomb TW, 2001. A theoretical model for oxygen transport in skeletal muscle under conditions of high oxygen demand. J Appl Physiol 91, 2255–2265. [DOI] [PubMed] [Google Scholar]
- McGuire BJ, Secomb TW, 2003. Estimation of capillary density in human skeletal muscle based on maximal oxygen consumption rates. Am J Physiol Heart Circ Physiol 285, H2382–2391. [DOI] [PubMed] [Google Scholar]
- Meng H, Bentley TB, Pittman RN, 1993. Myoglobin content of hamster skeletal muscles. J Appl Physiol (1985) 74, 2194–2197. [DOI] [PubMed] [Google Scholar]
- Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR, 2009. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Cornelissen AJ, Sloot AA, Hinkeldey M, Dreher MR, Hopfner M, Dewhirst MW, Secomb TW, 2009. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput Biol 5, e1000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P, 1995. Structure and hemodynamics of microvascular networks: heterogeneity and correlations. Am J Physiol 269, H1713–1722. [DOI] [PubMed] [Google Scholar]
- Rice AJ, Thornton AT, Gore CJ, Scroop GC, Greville HW, Wagner H, Wagner PD, Hopkins SR, 1999. Pulmonary gas exchange during exercise in highly trained cyclists with arterial hypoxemia. J Appl Physiol (1985) 87, 1802–1812. [DOI] [PubMed] [Google Scholar]
- Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD, 1992. Effects of training on muscle O2 transport at VO2max. J Appl Physiol 73, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, Wagner PD, 1989. Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol 67, 291–299. [DOI] [PubMed] [Google Scholar]
- Roy TK, Secomb TW, 2014. Theoretical analysis of the determinants of lung oxygen diffusing capacity. J Theor Biol 351, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffartzik W, Barton ED, Poole DC, Tsukimoto K, Hogan MC, Bebout DE, Wagner PD, 1993. Effect of reduced hemoglobin concentration on leg oxygen uptake during maximal exercise in humans. J Appl Physiol 75, 491–498; discussion 489–490. [DOI] [PubMed] [Google Scholar]
- Story DA, 1996. Alveolar oxygen partial pressure, alveolar carbon dioxide partial pressure, and the alveolar gas equation. Anesthesiology 84, 1011. [DOI] [PubMed] [Google Scholar]
- Sylvester JT, Shimoda LA, Aaronson PI, Ward JP, 2012. Hypoxic pulmonary vasoconstriction. Physiol Rev 92, 367–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD, 1992. Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc 24, 54–58. [PubMed] [Google Scholar]
- Wagner PD, 1996. A theoretical analysis of factors determining VO2 MAX at sea level and altitude. Respir Physiol 106, 329–343. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Laravuso RB, Uhl RR, West JB, 1974. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. The Journal of clinical investigation 54, 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, 1969. Ventilation-perfusion inequality and overall gas exchange in computer models of the lung. Respir Physiol 7, 88–110. [DOI] [PubMed] [Google Scholar]
- Wilson TA, Beck KC, 1992. Contributions of ventilation and perfusion inhomogeneities to the VA/Q distribution. J Appl Physiol (1985) 72, 2298–2304. [DOI] [PubMed] [Google Scholar]


