Abstract
Invadopodia, cancer cell protrusions with proteolytic activity, are functionally associated with active remodeling of the extracellular matrix. Here, we show that the invadopodia-related protein TKS5 is expressed in human pancreatic adenocarcinoma lines, and demonstrate that pancreatic cancer cells depend on TKS5 for invadopodia formation and function. Immunofluorescence staining of human pancreatic cancer cells reveals that TKS5 is a marker of mature and immature invadopodia. We also analyze the co-staining patterns of TKS5 and the commonly used invadopodia marker Cortactin, and find only partial co-localization of these two proteins at invadopodia, with a large fraction of TKS5-positive invadopodia lacking detectable levels of Cortactin. Whereas compelling evidence exist on the role of invadopodia as mediators of invasive migration in cultured cells and in animal models of cancer, these structures have never been detected inside human tumors. Here, using antibodies against TKS5 and Cortactin, we describe for the first time structures strongly resembling invadopodia in various paraffin-embedded human tumor surgical specimens from pancreas and other organs. Our results strongly suggest that invadopodia are present inside human tumors, and warrants further investigation on their regulation and occurrence in surgical specimens, and on the value of TKS5 antibodies as pathological research and diagnostic tools.
Keywords: Cancer, Invadopodia, TKS5, Cortactin, Tumor Pathology
1. Introduction
Invadopodia are cancer cell protrusions with localized pericellular proteolytic activity (Chen 1989). These structures are functionally linked to processes involving active extracellular matrix remodeling, such as invasive migration (Murphy and Courtneidge, 2011), proliferation in a three-dimensional protein matrix (Blouw et al., 2015; Iizuka et al., 2016), and angiogenesis (Petropoulos et al., 2018; Blouw et al., 2008). Accordingly, invadopodia are regulated in vitro by proinvasive signals such as EGFR ligands and TGFβ (Diaz et al., 2013; Zhou et al., 2014; Mandal et al., 2008), adhesion to the extracellular matrix (Branch et al., 2012), hypoxia (Diaz et al., 2013; Lucien et al., 2011; Hanna et al., 2013), and extracellular matrix rigidity (Alexander et al., 2008) among other signals. Invadopodia have been extensively studied in cell culture, and in various cancer cell implantation models in vivo (Blouw et al., 2015; Leong et al., 2014; Eckert et al., 2011). However, these structures have never been detected inside intact tumors.
Analysis of invadopodia assembly using time-lapse microscopy have described three stages in the invadopodia life cycle: invadopodia precursor, immature invadopodia, and mature invadopodia (Beaty and Condeelis, 2014). The mature invadopodia stage is defined by the presence of extracellular protein degradation, an activity measured using an in vitro assay that detects focal digestion of a fluorescently-labeled protein substrate (Mueller et al., 1992). This extensively used assay, easily performed in vitro in cultured cell lines, has been used to detect invadopodia in primary cancer cells freshly isolated from human surgical head-and-neck tumors (Clark et al., 2007). However, detecting focal invadopodia-dependent proteolysis inside intact tumors remains technically challenging. Therefore, the identification of invadopodia inside tumors still relies on the use of suitable invadopodia markers.
The adaptor protein TKS5 (Lock et al., 1998) is functionally linked to invadopodia formation and activity. Cancer cells with reduced levels of TKS5 fail to elaborate invadopodia, and have decreased invasive ability in vitro and in vivo (Blouw et al., 2008, 2015; Iizuka et al., 2016; Eckert et al., 2011; Seals et al., 2005). The TKS5 protein contains a PHOX homology domain (PX domain), and five SH3 domains (Lock et al., 1998). The PX domain is necessary for phosphoinositide binding and membrane localization, and the SH3 domains fulfill the scaffolding function of TKS5 (Abram et al., 2003; Oikawa et al., 2008). Recently, we and others have identified additional forms of mouse Tks5 lacking the PX domain, which are the result of transcription from intronic initiation sites (Li et al., 2013; Cejudo-Martin et al., 2014). Different PX domain-lacking Tks5 isoforms were named Tks5short and Tks5β, and the canonical PX-containing Tks5 form was named Tks5α/Tks5long. All forms of Tks5 are detected in most normal mouse tissues, whereas increased expression of Tks5α/Tks5long is associated with malignant transformation, metastasis and invadopodia formation (Li et al., 2013; Cejudo-Martin et al., 2014). TKS5 is highly enriched at invadopodia when compared with other cellular compartments, and it is present at all stages from precursor to mature (Abram et al., 2003; Di Martino et al., 2014; Sharma et al., 2013). In breast cancer cells stimulated with EGF, TKS5 is recruited to invadopodia precursors shortly after Cortactin, an actin nucleation-promoting factor, which is also necessary for invadopodia formation and activity (Clark et al., 2007). Cortactin is also present in invadopodia at all stages, and promotes F-actin polymerization (Oser et al., 2009). In addition, Cortactin is also abundant at other subcellular localizations such as lamellipodia, membrane ruffles and sites of exocytosis (Schnoor et al., 2018).
Here, using immunofluorescence for TKS5 and Cortactin on human cell lines and tumor surgical specimens, we analyze the co-localization patterns of these two proteins in invadopodia formed by cancer cells in vitro, and describe for the first time the presence of invadopodia-like structures inside human tumors. This work refines the tools to identify and study these cancer cellular structures. The possibility to detect invadopodia in paraffin-embedded archived samples will greatly expand future studies on the pathology of invadopodia-associated tumor biology.
2. Materials and methods
2.1. Tumor surgical specimens
In this study, we evaluated archived paraffin-embedded blocks from 25 patients from Harbor-UCLA Medical Center at Los Angeles County, who underwent surgical resections for varying tumor pathologies from pancreas, stomach, colon, rectum, gallbladder, breast, and brain. Los Angeles Biomedical Research Institute Human Subjects Committee reviewed and approved the study (IRB Protocol number 31363).
2.2. Cell lines
BxPC-3, PANC-1, MIAPaCa-2, CAPAN-1, SU86.86 and MRC-5 cells were from ATCC. L3.6pl cells were obtained from J. Summy (MD Anderson, Orlando). BxPC-3, MIAPaCa-2, CAPAN-1 and SU86.86 cells were grown in RPMI containing 10% FBS. PANC-1 were grown in DMEM containing 10% FBS. MRC-5 cells were grown in ATCC-formulated IMEM containing 10% FBS.
2.3. Antibodies
Polyclonal antibody against human TKS5 was from LSBio (cat. no. LS-C383498), monoclonal antibody against human TKS5 (clone 13H6.3) and Cortactin antibody (clone 4F11) were from MilliporeSigma. Polyclonal antibody against mouse Tks5 (1737) was a generous gift from Dr. Sara Courtneidge (OHSU, Portland, OR, US). Cytokeratin antibody (CAM 5.2) was from LSBio (LS-C312027). Anti-β actin antibody (D6A8) was from Cell Signaling Technology.
2.4. Immunofluorescence of tumor samples
Paraffin-embedded formalin-fixed specimens from patients who underwent tumor surgical resection were freshly cut into 5 μm thickness sections. After deparaffinization, samples were subjected to heat-induced epitope retrieval for 15 min using antigen retrieval solution (Citra Buffer pH 6, cat. no. HK086 from Biogenex). After three washes with PBS, samples were blocked in 5% normal donkey serum for 1 h at room temperature, washed with PBS and incubated with TKS5 antibody (LSBio) at 1:50 overnight at 4 °C. Signal was developed with donkey anti-rabbit IgG antibody coupled with Alexa 488 (Jackson ImmunoResearch) at 1:100. Selected samples were co-stained with anti-TKS5 and either anti-Cortactin (1:50) or Phalloidin-Alexa 568 (1:100). Cortactin signal was developed using donkey anti-mouse IgG antibody coupled with Alexa 594 (Jackson ImmunoResearch) at 1:200. Images were captured from regions containing TKS5-positive cancer cells using 100× oil-immersion objective in a Nikon eclipse E400 microscope equipped with an Excelitas X-Cite 120 fluorescence illuminator, a Nikon Y-IDP Double port, a Nikon DS-Fi2 camera head, and Nikon NIS elements D imaging software.
2.5. Invadopodia formation and staining
BxPC-3 cells were plated on coverslips coated with a layer of 2% type B gelatin (MilliporeSigma) or with Oregon Green-labeled gelatin (ThermoFisher Scientific), essentially as described before (Diaz et al., 2013; Díaz, 2013); PANC-1 were plated on coverslips coated with type B gelatin and treated with 10% NuSerum supplement (Corning) for 24 h. Cells were fixed with 4% paraformaldehyde for 10 min, blocked in PBS containing 0.1% Triton X-100 and 3% BSA, and incubated overnight at 4 °C in primary antibody diluted 1:500 in PBS containing 0.1% Triton X-100 and 0.3% BSA. Secondary antibodies conjugated with Alexa 488 or Alexa 594, as well as Phalloidin-Alexa 568 (ThermoFisher Scientific) were used at 1:500. Samples were mounted using vectashield containing DAPI (Vector Labs). Images were obtained using a 63× objective in a Zeiss AxioImager A1 equipped with a PRIOR Lumen 200, Axiocam 503 mono camera and ZEN2 software (Zeiss).
2.6. RNA interference
BxPC-3 cells were transfected with a human TKS5-specific siRNA pool (Dharmacon) at a final concentration of 80 nM using lipofectamine 2000 (ThermoFisher Scientific). Invadopodia formation and activity were quantified 72 h after transfection, essentially as described previously (Diaz et al., 2013). Briefly, 24 h after transfection, cells were plated on Oregon-green gelatin-coated coverslips, fixed and stained with Phalloidin-Alexa 568. Cells forming invadopodia were quantified in 15 randomly selected microscope fields (representing at least 80 total cells), and data represented as percent of cells forming invadopodia. Gelatin degradation was measured as relative degraded area normalized to cell number. Image J software (Schneider et al., 2012) was used to calculate the relative degraded area on 15 randomly selected fields corresponding to at least 80 total cells. Experiments were repeated three times. Statistical significance was calculated using Student’s t-test.
2.7. Immunoblotting
Cells were lysed in NP40-containing lysis buffer and proteins separated in an 8% acrylamide gel. After electrotransfer, membranes were blocked in 5% milk in PBS and incubated overnight at 4 °C in primary antibody diluted in PBS containing 0.1% Tween 20 and 0.3% BSA. TKS5 antibody (13H6.3) was used at 1:500 and β actin at 1:10,000. Membranes were washed and incubated with HRP-conjugated anti-mouse or anti-rabbit antibodies (GE Healthcare) and imaged using enhanced chemiluminescence (Supersignal West Pico PLUS, ThermoFisher Scientific) and a film developer. Films were scanned to digital files.
3. Results
3.1. Expression of TKS5 in human pancreatic tumors
Our initial goal was to analyze TKS5 protein expression by immunofluorescence in pancreatic surgical tumor samples of various degrees of malignancy (from benign to highly malignant) to evaluate whether TKS5 expression levels correlated with the invasive/malignant potential of these lesions. To that end, we analyzed 15 archived formalin-fixed paraffin embedded tumor specimens from patients that underwent surgery at Harbor-UCLA Medical Center. Pancreatic tumors included mucinous cystadenoma (benign, but with malignant transformative potential), solid pseudopapillary tumors (relatively good prognosis), ampullary invasive adenocarcinomas (medium prognosis), and pancreatic invasive adenocarcinomas (worse prognosis). Pathological and clinical information of the analyzed specimens is detailed in Table 1 and Supplementary Table 1. TKS5 staining was positive in 9 of the 15 samples analyzed (60%). TKS5 was not detected in the analyzed benign tumor, but was detected in samples from all other tumors types. Ampullary invasive adenocarcinomas displayed the highest level of TKS5 expression, where TKS5-positive cancer cells were usually scarce and preferentially localized at the tumor invasive front (Fig. 1). In addition, pseudopapillary tumor cells and centriacinar cells from preserved acini structures, were also positive (see Fig. 1 in (Chen et al., n.d.)).
Table 1.
TKS5 staining and Invadopodia detection in pancreatic tumors.
| Specimen | Pathology | location | Grade | Number of positive lymph nodes | Stage | LVI | PNI | TKS5 Staining | Invadopodia |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mucinous Cystadenoma Solid | Pancreas tail | n.a. | n.a. | n.a. | n.a. | n.a. | Negative | No |
| 2 | Solid pseudopapillary | Pancreas tail | 1 | 0 | pT2N0M0 | No | No | Positive | No |
| 3 | Solid pseudopapillary | Pancreas head | - | 0 | pT2N0M0 | No | No | Positive | Yes |
| 4 | Solid pseudopapillary | Pancreas head | - | 0 | pT2N0M0 | - | - | Positive | Yes |
| 5 | Invasive Adenocarcinoma | Ampulla of Vater | 2 | 0 | pT3N0M0 | Yes | - | Pegative | No |
| 6 | Invasive Adenocarcinoma | Ampulla of Vater | 2 | 0 | pT1N0M0 | No | - | Positive | Yes |
| 7 | Invasive Adenocarcinoma | Ampulla of Vater | 2 | 1 | pT2N1M0 | No | - | Positive | Yes |
| 8 | Invasive Adenocarcinoma | Ampulla of Vater | 1 | 0 | pT1N0M0 | No | Yes | Positive | Yes |
| 9 | Invasive Adenocarcinoma | Ampulla of Vater | 2 | 4 | pT4N1M0 | Yes | Yes | Positive | Yes |
| 10 | Invasive Adenocarcinoma | Pancreas body | - | - | cT3N1M0 | - | - | Negative | No |
| 11 | Invasive Adenocarcinoma | Pancreas head | 1 | 0 | pT4N0M0 | No | Yes | Positive | No |
| 12 | Invasive Adenocarcinoma | Pancreas head | 1 | 17 | pT2N1M0 | Yes | No | Negative | No |
| 13 | Invasive Adenocarcinoma | Pancreas head | 2 | 1 | ypT3N1M0 | Yes | Yes | Negative | No |
| 14 | Invasive Adenocarcinoma | Pancreas head | - | - | cTxNxM1 | - | - | Negative | No |
| 15 | Invasive Adenocarcinoma | Pancreas head | 3 | - | cT3N1M1 | - | - | Positive | Yes |
n.a., not applicable; LVI, lymphovascular invasion; PNI, perineural invasion; “p” pathologic stage; “c”, clinical stage; “yp” pathologic stage following neoadjuvant therapy.
Fig. 1.

TKS5 expressing cells localize at the tumor invasive front. Representative images from similar (not consecutive) regions of an ampullary invasive adeno-carcinoma (Specimen 6, Table 1) stained with H&E (A, B); Cytokeratin (CAM 5.2) followed by immunoperoxidase reaction to detect pancreatic cancer cells (C, D); and TKS5 followed by immunofluorescence detection (E, F). Lower panels represent magnification of the regions indicated by a square in the upper panel images. Arrowheads in C and D point to cytokeratine-positive pancreatic adenocarcinoma cells at the tumor invasive front. Arrowheads in E and F point to TKS5-positive cells at the tumor invasive front. Bar, 200 μm in upper panels, 100 μm in lower panels.
Strikingly, careful microscopical analysis of TKS5-positive samples under high magnification (100× objective), revealed the presence of TKS5-positive puncta associated with epithelial tumor cells in solid pseudopapillary tumors, ampullary invasive adenocarcinomas, and pancreatic invasive adenocarcinomas (Fig. 2). Size and polarized distribution of TKS5-positive puncta was highly reminiscent of invadopodia elaborated by cancer cells in culture. This finding suggests that cancer cells elaborate invadopodia in vivo inside human tumors, and that TKS5 is a suitable marker for their identification. This unexpected and potentially relevant observation prompted us to further evaluate whether these structures might indeed be invadopodia.
Fig. 2.

TKS5-positive invadopodia-like puncta in human pancreatic tumor specimens. (A-G) Human pancreatic tumor specimens were stained with anti-human TKS5 antibody (LSBio), and DAPI. Pictures show tumor cells containing TKS5-positive puncta in solid pseudopapillary tumors (A, B), invasive ampullary adenocarcinomas (C-F), and pancreatic adenocarcinoma (G, H). Panels A and B correspond to patients 3 and 4; C to F to patients 6–to 9 respectively; G and H to patient 15 (see Table 1). TKS5-positive invadopodia-like structures are marked by arrowheads. Bar, 5 μm.
3.2. Expression of TKS5 protein in human pancreatic cancer cell lines
To select the most appropriate pancreatic cell line for in vitro experiments, we evaluated total TKS5 protein expression levels in a small panel of human pancreatic carcinoma cell lines, including PANC-1, BxPC-3, MIAPaCa-2, SU86.86, L3.6pl, and Capan-1. Protein extracts from these lines, along with a control non-cancer cell line (immortal human fibroblasts, MRC5), were separated by electrophoresis, followed by western blotting for TKS5. Out of the three TKS5 antibodies used in this study, only clone 13H6.3 was suitable for detection of the human protein by western blotting. As predicted by the TKS5 region used to generate 13H6.3, this antibody detected the PX-containing form TKS5α/TKS5long (~150 kDa), as well as non-PX containing forms TKS5β and TKS5short (~135–140 kDa) in the non-cancer line MRC-5 (Fig. 3). None of the pancreatic cancer cells analyzed expressed detectable levels of isoforms Tks5β or TKS5short, in agreement with these forms being preferentially expressed by non-cancer cells. The highest expression of TKS5α/TKS5long (TKS5 hereinafter) corresponded to BxPC-3 and L3.6pl cells, a highly metastatic variant selected from COLO357 cells (Bruns et al., 1999). All other pancreatic cancer cells analyzed expressed moderate to low levels of TKS5. This analysis indicates that TKS5 is expressed in pancreatic adenocarcinoma cell lines at different levels, and that TKS5α/TKS5long is the only protein isoform detected by western blot.
Fig. 3.
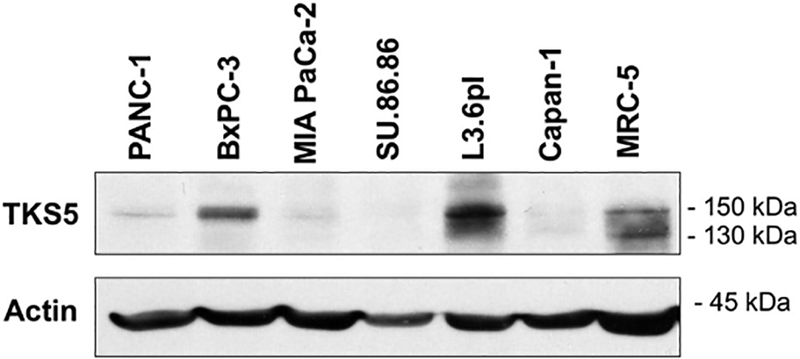
TKS5 protein expression in pancreatic adenocarcinoma cells. Protein lysates from the indicated pancreatic adenocarcinoma cell lines, and from the human fibroblast line MRC-5, were subjected to immunoblotting with the TKS5 antibody 13H6.3. Actin was used as loading control. The electrophoretic mobility of the TKS5 isoforms is 150 kDa (TKS5α/TKS5long) and ~135–140 kDa (TKS5β and Tks5short).
3.3. TKS5 localizes at invadopodia in pancreatic adenocarcinoma cells
Based on the above results, we selected the pancreatic cancer cell line BxPC-3, which readily forms invadopodia in culture (Diaz et al., 2013), to investigate the subcellular localization of TKS5 in human pancreatic adenocarcinoma cells. We plated BxPC-3 on gelatin B-coated coverslips and process them for TKS5 immunofluorescence along with fluorescent phalloidin staining to detect F-actin. Whereas TKS5 polyclonal antibody from LSBio was not suitable for this application, immunofluorescence with 13H6.3 revealed a strong discrete punctate pattern co-localizing with ventral F-actin-rich puncta (Fig. 4 A), indicative of invadopodia. Most F-actin rich puncta (95%) were positive for TKS5; however, some TKS5-positive puncta lacked detectable levels of F-actin. Analysis of a different pancreatic adenocarcinoma cell line (PANC-1) revealed similar results (Fig. 4 A). To evaluate whether TKS5 localizes at mature or immature invadopodia, we plated BxPC-3 cells on coverslips coated with Oregon-green labeled gelatin and incubated them for 24 h. After fixation and staining with TKS5 antibody 13H6.3, fluorescence microscopy visualization revealed that most TKS5-positive puncta co-localized with extracellular degradation areas, an indication of mature invadopodia. In addition, some TKS5-positive puncta were not associated with gelatin degradation, confirming TKS5 as a marker of both immature and mature invadopodia (Fig. 4 B). TKS5 is, therefore, a valuable invadopodia marker in human cancer cells because preferentially associates with invadopodia, while being largely undetectable by conventional fluorescence microscopy at other cellular locations. Notably, TKS5 staining pattern in pancreatic cancer cells in culture highly resembles TKS5-positive puncta found in tumor specimens (compare Fig. 2 with middle panels in Fig. 4 A).
Fig. 4.
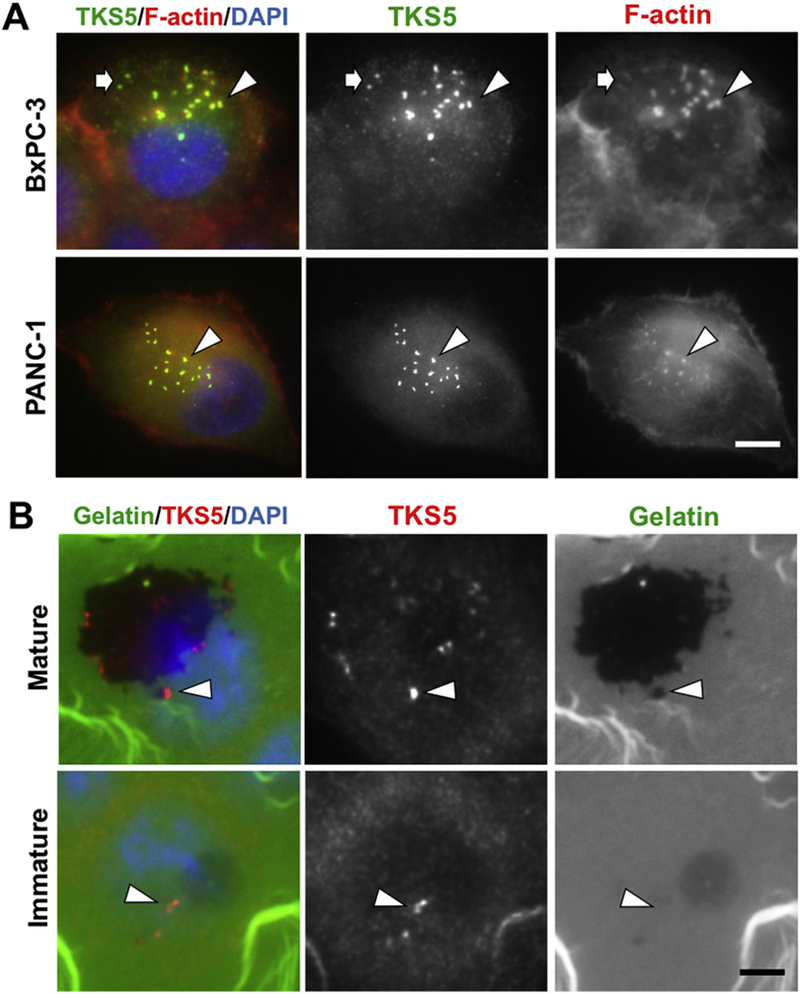
TKS5 localizes at mature and immature invadopodia in pancreatic adenocarcinoma cells.(A) BxPC-3 and PANC-1 cells were processed for immunofluorescence with TKS5 antibody 13H6.3 (green) and AlexaFluor 546-Phalloidin (red) to visualize F-actin. Most-TKS5 positive-puncta co-localized with F-actin (arrowheads); some TKS5-positive puncta contain little to undetectable F-actin (arrows). (B) BxPC-3 cells were grown on Oregon Green-labeled gelatin and processed for immunofluorescence with TKS5 antibody 13H6.3 (red). TKS5 is present at mature and immature invadopodia (B, top and bottom panels respectively). Top panels show TKS5-positive puncta with gelatin degradation activity (arrowhead). Bottom panels show TKS5-positive puncta without associated degradation activity (arrowheads). Bars, 5 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Invadopodia formation and function in pancreatic cancer cells requires TKS5
To investigate the functional relevance of TKS5 in pancreatic cancer cells, we transfected BxPC-3 cells with a control non-targeting siRNA pool or with a TKS5-specific siRNA pool (Fig. 5 A), and compare invadopodia formation and activity. BxPC-3 cells with reduced levels of TKS5 were significantly impaired in their ability to form invadopodia (Fig. 5 B) and to digest fluorescent gelatin (Fig. 5 C). These data indicate that TKS5 is required in pancreatic cancer cells to elaborate functional invadopodia, and suggest that TKS5 expression in pancreatic tumors is largely associated with active extracellular matrix remodeling.
Fig. 5.
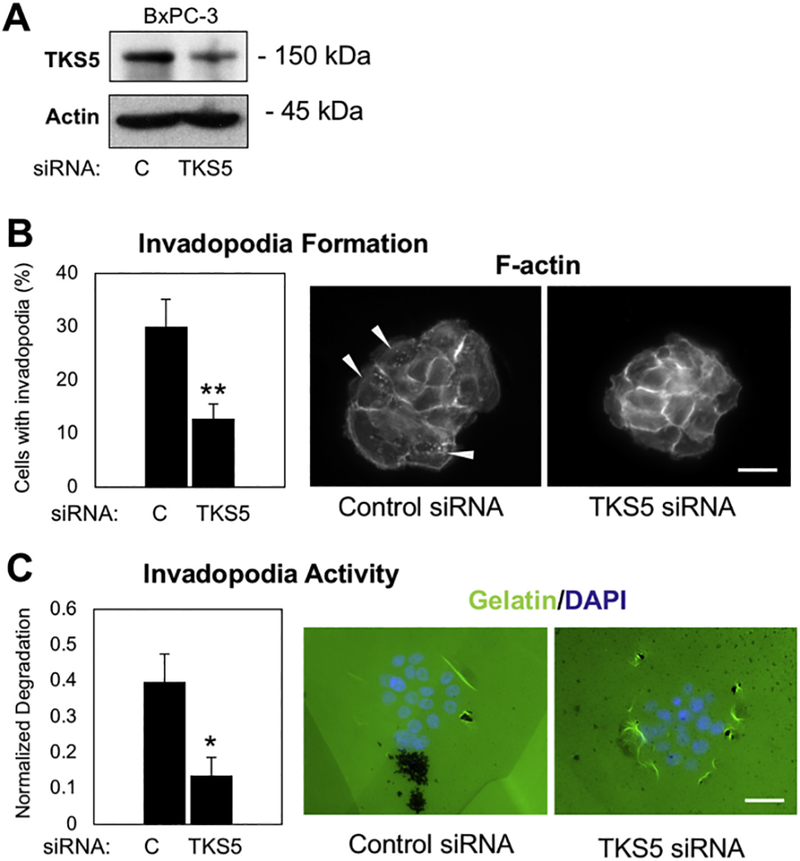
TKS5 is necessary for invadopodia formation and activity in pancreatic cancer cells. (A) BxPC-3 transfected with non-targeting siRNA (control) or a TKS5-specific siRNA pool were subjected to western blotting to verify TKS5 knockdown. Actin was used as loading control. (B) BxPC-3 control and TKS5 knockdown cells were stained for F-actin and the number of cells forming F-actin positive invadopodia was quantified. Pictures show representative images of the quantification indicated in the graph. (C) BxPC-3 control and TKS5 knockdown cells were plated on Oregon Green-labeled gelatin. Invadopodia activity (gelatin degradation) was quantified and represented as normalized degraded area. Pictures show representative images of the quantification indicated in the graph. Both graph represents average and SEM. (*) P < .010; (**) P < .00002 (Student’s t-test). Bars, 15 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. TKS5 and Cortactin show variable co-localization patterns at invadopodia in cultured cells
Next, we sought to evaluate whether an additional invadopodia marker would be suitable for co-staining and marking invadopodia-like structures in human tumor specimens. Cortactin is a commonly used invadopodia marker, which marks all invadopodia stages, and localizes at invadopodia in pancreatic cancer cells (Razidlo et al., 2014). We analyzed Cortactin expression by western blotting in the pancreatic cancer cells PANC-1, BxPC-3, MIAPaCa-2, SU86.86, L3.6pl, and Capan-1. All pancreatic cancer lines expressed Cortactin, albeit to different levels (see Fig. 2 in (Chen et al., n.d.)). No obvious correlation between Cortactin and TKS5 expression levels was observed (Fig. 2 in (Chen et al., n.d.) and Fig. 3). To evaluate whether TKS5 and Cortactin co-localize at invadopodia in cancer cells, we stained BxPC-3 cells with a Cortactin monoclonal antibody (4F11) in combination with a Tks5 polyclonal antibody (1737) that has been characterized previously (Lock et al., 1998). TKS5 and Cortactin-positive puncta showed either complete co-localization or close apposition (Fig. 6 A and Fig. 3 in (Chen et al., n.d.) respectively) in 24.6% of the cells analyzed. Notably, we observe a surprisingly high number of cells containing TKS5-positive puncta with low or undetectable Cortactin staining (69.5%). The cases in which Cortactin-positive puncta contained low or undetectable TKS5 staining represented the remaining 5.9% of cells. The fact that a significant number of invadopodia were only TKS5-positive, suggests that Cortactin staining might only identify a subset of all the invadopodia present in a cell. Although both TKS5 and Cortactin have been detected at immature and mature invadopodia (Sharma et al., 2013), our finding suggests that Cortactin and TKS5 are not always equivalent markers of invadopodia. This finding might reflect a cell-type specific dynamic association of these proteins with the invadopodium during the life cycle of this structure. Based on this finding, we expect that only a fraction of TKS5-positive puncta stain for Cortactin inside human tumors.
Fig. 6.
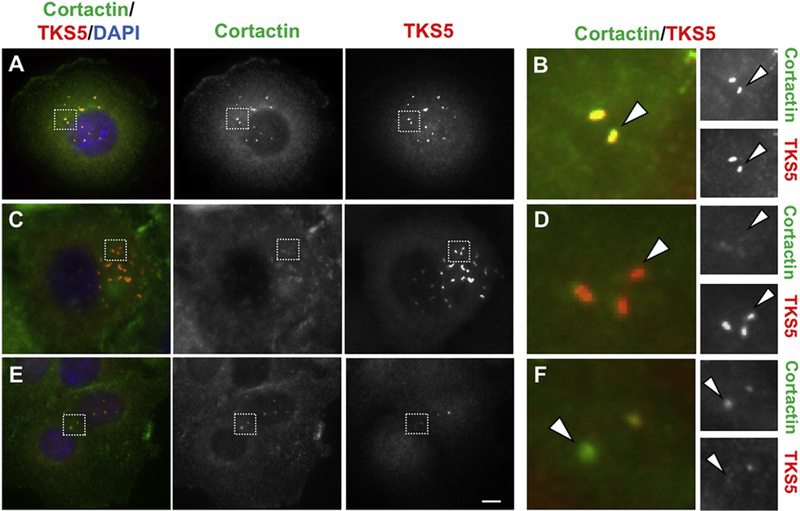
Cortactin and TKS5 display different co-localization patterns within the same cell line. BxPC-3 cells were co-stained with Cortactin (green) and TKS5 (red). Different invadopodia-associated co-localization patterns were observed in the same slide: Co-localization of TKS5 and Cortactin (A, B); TKS5-positive puncta with low or undetectable Cortactin (C, D); and Cortactin-positive puncta with low or undetectable TKS5 (E, F). Squares in A, C and E, indicate the areas magnified in B, D and F respectively. Bar, 0.5 μm in A, C, E and 0.05 μm in B, D, F. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.6. Cortactin and F-actin co-localize with TKS5 at invadopodia-like structures in human pancreatic tumors
To evaluate whether additional invadopodia markers are present in TKS5-positive puncta resembling invadopodia observed in human tumors (Fig. 2), we co-stained selected pancreatic surgical specimens with phalloidin or Cortactin along with TKS5. Phalloidin staining was only successful in a few specimens, likely due to variability in sample fixation or post-fixation processing. Co-localization between F-actin and TKS5 was detected in some specimens (see Fig. 4 in (Chen et al., n.d.)). Furthermore, some TKS5 and Cortactin-positive puncta in close apposition were also identified (Fig. 4 in (Chen et al., n.d.)). Most of the Cortactin-positive signal, however, localized at the cellular periphery, as expected from previous studies (Weed et al., 2000). These results further support the idea that TKS5-positive puncta detected inside pancreatic human tumors are invadopodia.
3.7. TKS5-positive invadopodia-like structures in human cancer specimens from different organs
To investigate whether malignant tumors from other organs also contain TKS5-positive puncta resembling invadopodia, we processed cancer samples from stomach, colon, rectum, gallbladder, breast and brain (see Table 2 and Supplementary Table 2) for TKS5 and Cortactin staining. As summarized in Table 2, TKS5 staining was positive in 8 out of 10 tumors (80%), and TKS5-positive puncta were detected in 7 out of 8 TKS5-positive samples (87%) (Fig. 7). Similarly to pancreatic tumors, some TKS5-positive puncta also contained Cortactin (Fig. 8). Collectively, our results strongly suggest that TKS5-positive puncta represent invadopodia, revealing for the first time the presence of these structures inside intact human tumors. Our findings underscore the physiological relevance of invadopodia for human tumor biology. The tools described here should allow a systematic analysis of different aspects of invadopodia-related biology in archived tumor samples.
Table 2.
TKS5 staining and Invadopodia detection in various malignant tumors.
| Specimen | Organ | Pathology | Location | Grade | Number of positive lymph nodes | Cancer Stage | LVI | PNI | TKS5 Staining | Invadopodia |
|---|---|---|---|---|---|---|---|---|---|---|
| 16 | Stomach | Invasive Adenocarcinoma | Peritoneal Implant | - | n.a. | cTxNxM1 | - | - | Negative | No |
| 17 | Stomach | Invasive Adenocarcinoma | Stomach | 3 | 1 | ypT3N1M0 | Yes | No | Positive | No |
| 18 | Stomach | Invasive Adenocarcinoma | Stomach | - | - | cTxNxM1 | - | - | Positive | Yes |
| 19 | Stomach | Invasive Adenocarcinoma | Stomach | 3 | 2 | ypT1N1M0 | Yes | No | Positive | Yes |
| 20 | Colon | Invasive Adenocarcinoma | Sigmoid Colon | Low | 2 | T3N1bM0 | Yes | No | Positive | Yes |
| 21 | Rectal | Invasive Adenocarcinoma | Rectum | - | - | cT3N1M0 | - | - | Positive | Yes |
| 22 | Gallbladder | Invasive Adenocarcinoma | Gallbladder | 3 | 1 | pT2N0M0 | No | No | Positive | Yes |
| 23 | Breast | Invasive Adenocarcinoma | Breast | 2 | 12 | ypT2N3M0 | Yes | - | Negative | No |
| 24 | Breast | Invasive Adenocarcinoma | Breast | 3 | 27 | ypT4bN3aM0 | Yes | - | Positive | Yes |
| 25 | Brain | Astrocytic Glioblastoma | Brain | - | - | - | - | - | Positive | Yes |
n.a., not applicable; LVI, lymphovascular invasion; PNI, perineural invasion; “p” pathologic stage; “c”, clinical stage; “yp” pathologic stage following neoadjuvant therapy.
Fig. 7.

TKS5-positive invadopodia-like puncta in human malignant tumor specimens.
Fig. 8.
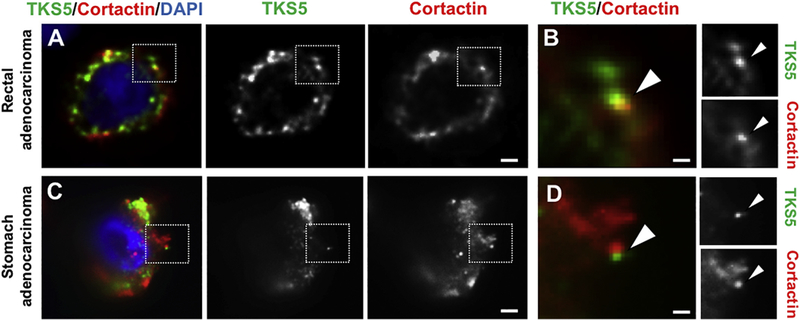
TKS5 and Cortactin-positive invadopodia-like puncta in human malignant tumor specimens.
4. Discussion
Increased levels of SH3PXD2A transcript (the gene encoding for TKS5) are associated with cancer progression and/or poor prognosis in a number of human malignancies including glioblastoma, schwannoma, and breast cancer (Blouw et al., 2015; Stylli et al., 2014; Stylli et al., 2012). TKS5 transcripts are overexpressed in pancreatic adenocarcinoma epithelium when compared to normal pancreas (Badea et al., 2008), but TKS5 protein levels in pancreatic adenocarcinoma have not been evaluated, and no information on transcript or protein levels is available for pancreatic neoplastic lesions with comparative better prognosis, such as papillary tumors (Dinarvand, 2017) or ampullary adenocarcinomas (Talamini et al., 1997). In this study, we observe that TKS5 protein expression levels are comparable between pancreatic tumors of better and worse prognosis. Although our study did not include enough sample size to evaluate the existence of correlations, it suggests the possibility that TKS5 expression is associated with the likeliness of recurrence in solid pseudopapillary tumors and/or ampullary adenocarcinomas. Within invasive adenocarcinomas, TKS5-positive cells were interspersed at the tumor invasive front (Fig. 1), in agreement with previous studies linking TKS5-dependent invadopodia formation with epithelial-to-mesenchymal transition (Eckert et al., 2011) and metastatic colonization (Blouw et al., 2008).
TKS5 is necessary for the assembly and activity of invadopodia in Src-transformed mouse fibroblasts (Seals et al., 2005), as well as human breast cancer cells and melanoma (Iizuka et al., 2016; Seals et al., 2005). Our finding that TKS5 is necessary for invadopodia formation and activity in pancreatic adenocarcinoma cells is in line with these studies. TKS5 accumulates at invadopodia in several human cancer cell lines including breast and melanoma (Seals et al., 2005; Weidmann et al., 2016). Additionally, mouse Tks5 localizes at invadopodia in Src-transformed mouse fibroblasts (Blouw et al., 2008; Abram et al., 2003). However, the presence of TKS5 at invadopodia has not been systematically evaluated, and this information is lacking for most human cancer cell lines. A limitation to these studies has been the availability of TKS5 antibodies suitable for immunofluorescence on human cells. The commercially available TKS5 monoclonal antibody 13H6.3, raised against human TKS5, seems an excellent tool for immunofluorescence studies in human cells. We observed that some TKS5-positive puncta lacked detectable levels of F-actin (Fig. 4 A), which is consistent with F-actin nucleation taking place after TKS5 recruitment to the nascent invadopodium (Oser et al., 2009).
Cortactin is one of the most commonly used invadopodia markers, but the degree of co-localization between Cortactin and TKS5 is unknown. To avoid the technical limitations of using two antibodies raised in mouse for co-staining studies, we used a polyclonal Tks5 antibody raised against mouse Tks5 (Lock 1998) that has been extensively characterized. Interestingly, a surprisingly large number of TKS5-positive puncta contained low or undetectable Cortactin signal. This does not seem in agreement with invadopodia assembly studies in which TKS5 and Cortactin are present at all stages of invadopodia from precursors to mature invadopodia, with Cortactin arriving earlier than TKS5 (Sharma et al., 2013). Instead, it seems more in line with a role for TKS5 in the recruitment of Cortactin (Crimaldi et al., 2009). It is possible that the co-occurrence of these two markers at invadopodia is cell-type specific. Alternatively, it is possible that during invadopodia disassembly, a less studied process, Cortactin leaves invadopodia before TKS5 does. We observe that most invadopodia within a given cell display similar TKS5/Cortactin co-localization patterns. Perhaps, the different TKS5/Cortactin co-staining patterns that we observe are due to heterogeneity in the relative expression levels of both proteins among individual cells within a cell population. Although unlikely, it could also be plausible that, when highly phosphorylated, the Cortactin antibody 4F11 binds weakly to invadopodia-associated Cortactin. In any case, our results indicate that TKS5 and Cortactin are not always equivalent invadopodia markers, and indicate that, when possible, TKS5 staining should also be used for invadopodia identification.
The presence of TKS5-positive puncta associated with tumor cells in surgical specimens strongly suggests that tumor cells elaborate invadopodia in vivo. The size and polarized subcellular distribution of the TKS5-positive puncta detected inside tumors do not resemble any other structure elaborated by cells, and the staining pattern that we observed is striking and very unique. Some TKS5-positive puncta contained F-actin or Cortactin, further supporting our hypothesis. Cells with associated TKS5-positive puncta were not abundant in any specimen, perhaps reflecting the difficulty to identify these structures in thick sections vs. cells in two-dimensional culture conditions. Indeed, invadopodia in various three-dimensional culture conditions, which better mimic their natural environment, are thin and elongated finger-like cell protrusions (Mueller et al., 1992; Schoumacher et al., 2010; Lizarraga et al., 2009; Bowden et al., 1999). It is not clear yet whether TKS5, Cortactin, or other invadopodia components localize at the tip, base, or everywhere along the finger-like invadopodium. It would be conceivable that invadopodia proteins dynamically associate with different portions of the finger-like invadopodium at different stages of the invadopodia life cycle. This might explain the observation that, when both are present at invadopodia in cultured cells, TKS5 and Cortactin either co-localize or appear in close apposition (Fig. 6A and Fig. 3 in (Chen et al., n.d.)).
Surprisingly, tumors with low malignancy such as pancreatic solid papillary tumors, also contain TKS5-positive puncta. One possible explanation is that these represent immature invadopodia with low matrix remodeling activity. Since the presence of TKS5 or Cortactin cannot differentiate between mature and immature invadopodia, testing this hypothesis would require the evaluation of active proteolysis. It is also possible that TKS5-positive puncta in tumors of low invasive potential are associated with additional functions of invadopodia such as growth in three-dimensional culture (Blouw et al., 2015; Iizuka et al., 2016). It would be plausible that additional oncogenic signals are necessary to confer degradative ability to invadopodia-containing pre-malignant tumor cells, such as those in solid papillary tumors. Indeed, Cortactin and TKS5 are direct substrates of the non-receptor tyrosine kinase and proto-oncogene product Src (Wu et al., 1991; Lock et al., 1998). Tyrosine phosphorylation of Cortactin by Src is linked to its role in promoting migration and invasion (reviewed in Schnoor et al., 2018). Notably, Src activity promotes the stabilization of the pro-invasive isoform Tks5α/Tks5long concomitantly with the destabilization of the Tks5β and Tks5short isoforms (Cejudo-Martin et al., 2014). Increased TKS5long/TKS5short ratio predicts worse survival in early stage lung adenocarcinoma patients (Li et al., 2013). Interestingly, TKS5α is the only protein isoform detected in the pancreatic adenocarcinoma cells analyzed (Fig. 3). This fact, along with the prominent role of Src activity in pancreatic adenocarcinoma malignancy, (Lutz et al., 1998; Yezhelyev et al., 2004; Hilbig, 2008) suggest that Cortactin and TKS5 are important effectors of Src in invadopodia-mediated pancreatic adenocarcinoma invasion. Whether Src activity is associated with worse prognosis/recurrence in other pancreatic tumors such as ampullary and pseudopapillary tumors remains to be tested. Likewise, it would be interesting to evaluate the relative expression of TKS5 isoforms in pancreatic tumors with relatively low malignant potential.
Collectively, our data strongly support the existence of invadopodia inside human tumors, an idea that deserves further attention. The possibility to identify invadopodia in archived human tumors should greatly expand our understanding of the biology of these cancer cell structures.
5. Conclusions
Our study demonstrate that human pancreatic cancer cells depend on the adaptor protein TKS5 for invadopodia formation and function, and that TKS5 is an excellent marker of mature and immature invadopodia in human pancreatic cancer cells. Cortactin and TKS5 co-localize at invadopodia only in a fraction of pancreatic cancer cells, suggesting that Cortactin and TKS5 are not equivalent markers of invadopodia, and that Cortactin staining alone might not identify all the invadopodia formed by cells. Importantly, our study reveals for the first time the presence of invadopodia-like structures inside intact human tumors. This finding underscores the physiological relevance of invadopodia for human tumor biology, and should allow a systematic analysis of different aspects of invadopodia-related biology in archived tumor samples. Furthermore, it opens the possibility to use similar tools to analyze podosomes (the counterparts of invadopodia in non-cancer cells) in a variety of normal and diseased human tissues.
Supplementary Material
Acknowledgements
We are grateful to Dr. Sara A. Courtneidge (OHSU) for sharing the Tks5 antibody 1737 and for useful comments and advice, and to Barbara French and Isabel Mejia (LA BioMed) for excellent technical support.
Funding
This work has been supported by Los Angeles Biomedical Research Institute Grant, and by NIH National Center for Advancing Translational Science (NCATS) UCLA, CTSI Grant Number UL1TR001881 (B. Díaz).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yexmp.2018.11.005.
References
- Abram CL, Seals DF, Pass I, Salinsky D, Maurer L, Roth TM, Courtneidge SA, 2003. The adaptor protein fish associates with members of the ADAMs family and localizes to podosomes of Src-transformed cells. J. Biol. Chem 278, 16844–16851. [DOI] [PubMed] [Google Scholar]
- Alexander NR, Branch KM, Parekh A, Clark ES, Iwueke IC, Guelcher SA, Weaver AM, 2008. Extracellular matrix rigidity promotes invadopodia activity. Curr. Biol 18, 1295–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I, 2008. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepato-Gastroenterology 55, 2016–2027. [PubMed] [Google Scholar]
- Beaty BT, Condeelis J, 2014. Digging a little deeper: the stages of invadopodium formation and maturation. Eur. J. Cell Biol 93, 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA, 2008. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur. J. Cell Biol 87, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouw B, Patel M, Iizuka S, Abdullah C, You WK, Huang X, Li JL, Diaz B, Stallcup WB, Courtneidge SA, 2015. The invadopodia scaffold protein Tks5 is required for the growth of human breast cancer cells in vitro and in vivo. PLoS One 10, e0121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC, 1999. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18, 4440–4449. [DOI] [PubMed] [Google Scholar]
- Branch KM, Hoshino D, Weaver AM, 2012. Adhesion rings surround invadopodia and promote maturation. Biol Open 1, 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ, 1999. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia 1, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejudo-Martin P, Yuen A, Vlahovich N, Lock P, Courtneidge SA, Diaz B, 2014. Genetic disruption of the sh3pxd2a gene reveals an essential role in mouse development and the existence of a novel isoform of tks5. PLoS One 9, e107674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT, 1989. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool 251, 167–185. [DOI] [PubMed] [Google Scholar]
- Chen Y, Baik M, Byers JT, Chen KT, French SW, Diaz B. TKS5 Expression and Co-Localization with Additional Invadopodia Markers in Pancreatic Cancer Cells and Human Tumor Specimens. Data in Brief (in press). [Google Scholar]
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM, 2007. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227–4235. [DOI] [PubMed] [Google Scholar]
- Crimaldi L, Courtneidge SA, Gimona M, 2009. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp. Cell Res 315, 2581–2592. [DOI] [PubMed] [Google Scholar]
- Di Martino J, Paysan L, Gest C, Lagree V, Juin A, Saltel F, Moreau V, 2014. Cdc42 and Tks5: a minimal and universal molecular signature for functional invadosomes. Cell Adhes. Migr 8, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz B, 2013. Invadopodia Detection and Gelatin Degradation Assay. Bio-protocol 3(24), e997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA, 2013. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia.J. Cell Biol 201, 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarvand P, Lai J, 2017. Solid Pseudopapillary Neoplasm of the Pancreas: a rare Entity with Unique Features. Arch Pathol Lab Med 141, 990–995. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J, 2011. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell 19, 372–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna SC, Krishnan B, Bailey ST, Moschos SJ, Kuan PF, Shimamura T, Osborne LD, Siegel MB, Duncan LM, O’Brien ET 3rd, Superfine R, Miller CR, Simon MC, Wong KK, Kim WY, 2013. HIF1alpha and HIF2alpha independently activate SRC to promote melanoma metastases. J. Clin. Invest 123, 2078–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbig A, 2008. Src kinase and pancreatic cancer. Recent Results Cancer Res. 177,179–185. [DOI] [PubMed] [Google Scholar]
- Iizuka S, Abdullah C, Buschman MD, Diaz B, Courtneidge SA, 2016. The role of Tks adaptor proteins in invadopodia formation, growth and metastasis of melanoma. Oncotarget 7, 78473–78486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, Hague MN, Ablack A, Carmine-Simmen K, McPherson VA, Postenka CO, Turley EA, Courtneidge SA, Chambers AF, Lewis JD, 2014. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep. 8, 1558–1570. [DOI] [PubMed] [Google Scholar]
- Li CM, Chen G, Dayton TL, Kim-Kiselak C, Hoersch S, Whittaker CA, Bronson RT, Beer DG, Winslow MM, Jacks T, 2013. Differential Tks5 isoform expression contributes to metastatic invasion of lung adenocarcinoma. Genes Dev. 27, 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P, 2009. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 69, 2792–2800. [DOI] [PubMed] [Google Scholar]
- Lock P, Abram CL, Gibson T, Courtneidge SA, 1998. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 17, 4346–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucien F, Brochu-Gaudreau K, Arsenault D, Harper K, Dubois CM, 2011. Hypoxia-induced invadopodia formation involves activation of NHE-1 by the p90 ribosomal S6 kinase (p90RSK). PLoS One 6, e28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H, Buchler MW, Adler G, 1998. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem. Biophys. Res. Commun 243, 503–508. [DOI] [PubMed] [Google Scholar]
- Mandal S, Johnson KR, Wheelock MJ, 2008. TGF-beta induces formation of F-actin cores and matrix degradation in human breast cancer cells via distinct signaling pathways. Exp. Cell Res 314, 3478–3493. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Yeh Y, Chen WT, 1992. Tyrosine phosphorylation of membrane proteins mediates cellular invasion by transformed cells. J. Cell Biol 119, 1309–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA, 2011. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat. Rev. Mol. Cell Biol 12, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T, Itoh T, Takenawa T, 2008. Sequential signals toward podosome formation in NIH-src cells. J. Cell Biol 182, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J, 2009. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J.Cell Biol 186, 571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulos C, Guichet PO, Masliantsev K, Wager M, Karayan-Tapon L, 2018. Functional invadopodia formed in glioblastoma stem cells are important regulators of tumor angiogenesis. Oncotarget 9, 20640–20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razidlo GL, Schroeder B, Chen J, Billadeau DD, McNiven MA, 2014. Vav1 as a central regulator of invadopodia assembly. Curr. Biol 24, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M, Stradal TE, Rottner K, 2018. Cortactin: Cell Functions of a MultifacetedActin-Binding Protein. Trends Cell Biol. 28, 79–98. [DOI] [PubMed] [Google Scholar]
- Schoumacher M, Goldman RD, Louvard D, Vignjevic DM, 2010. Actin, micro-tubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol 189, 541–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Azucena EF Jr., Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA, 2005. The adaptor protein Tks5/fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell 7, 155–165. [DOI] [PubMed] [Google Scholar]
- Sharma VP, Eddy R, Entenberg D, Kai M, Gertler FB, Condeelis J, 2013. Tks5 and SHIP2 regulate invadopodium maturation, but not initiation, in breast carcinoma cells. Curr. Biol 23, 2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylli SS, I ST, Kaye AH, Lock P, 2012. Prognostic significance of Tks5 expression in gliomas. J. Clin. Neurosci 19, 436–442. [DOI] [PubMed] [Google Scholar]
- Stylli SS, Luwor RB, Kaye AH, I ST, Hovens CM, Lock P, 2014. Expression of the adaptor protein Tks5 in human cancer: prognostic potential. Oncol. Rep 32, 989–1002. [DOI] [PubMed] [Google Scholar]
- Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL, 1997. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann. Surg 225, 590–599 (discussion 9–600). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA,Parsons JT, 2000. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol 151, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann MD, Surve CR, Eddy RJ, Chen X, Gertler FB, Sharma VP, Condeelis JS, 2016. Mena(INV) dysregulates cortactin phosphorylation to promote invadopodium maturation. Sci. Rep 6, 36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT, 1991. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol 11, 5113–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A, Barge A,Green T, Fennell M, Bruns CJ, 2004. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin. Cancer Res 10, 8028–8036. [DOI] [PubMed] [Google Scholar]
- Zhou ZN, Sharma VP, Beaty BT, Roh-Johnson M, Peterson EA, Van Rooijen N, Kenny PA, Wiley HS, Condeelis JS, Segall JE, 2014. Autocrine HBEGF expression promotes breast cancer intravasation, metastasis and macrophage-independent invasion in vivo. Oncogene 33, 3784–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


