Abstract
Premigratory neural crest cells arise within the dorsal neural tube and subsequently undergo an epithelial-to-mesenchymal transition (EMT) to leave the neuroepithelium and initiate migration. Draxin is a Wnt modulator that has been shown to control the timing of cranial neural crest EMT. Here we show that this process is accompanied by three stages of remodeling of the basement membrane protein laminin, from regression to expansion and channel formation. Loss of Draxin results in blocking laminin remodeling at the regression stage, whereas ectopic maintenance of Draxin blocks remodeling at the expansion stage. The latter effect is rescued by addition of Snail2, previously shown to be downstream of Draxin. Our results demonstrate an essential function for the Wnt modulator Draxin in regulating basement membrane remodeling during cranial neural crest EMT.
Keywords: Neural crest, Laminin, Draxin, Snail2, Epithelial-to-Mesenchymal Transition
1. INTRODUCTION
Neural crest (NC) cells undergo a spatiotemporally regulated epithelial-to-mesenchymal transition (EMT) to separate from the neuroepithelium and become migratory. The process of EMT involves first breaking cell-cell and cell-basement membrane connections to detach from the neuroepithelium and delaminate (Gouignard et al., 2018; Nieto et al., 2016). Delamination and onset of migration involves intrinsic changes in cell polarity and cell surface proteins, including cadherins and integrins, as well as remodeling of the basement membrane and interactions with interstitial extracellular matrix (ECM) components (Christian et al., 2013; Duband, 2006; Perris, 1997). In particular, laminin is a major component of both the basement membrane and interstitial ECM that plays an essential role in regulating cranial NC migration (Coles et al., 2006; Duband and Thiery, 1987; Perris and Perissinotto, 2000).
During EMT, cells begin the delamination process by down-regulating laminin (Zeisberg and Neilson, 2009). Interestingly, members of the Snail family of transcription factors have been shown to directly repress transcription of laminin alpha5 (Takkunen et al., 2008), which has a critical function in cranial NC migration (Coles et al., 2006). Snail genes are well-known regulators of EMT via transcriptional repression in both cancer cells and neural crest cells (Cano et al., 2000; Strobl-Mazzulla and Bronner, 2012; Taneyhill et al., 2007). Thus, there appears to be an important link between intrinsic NC EMT factors and laminin expression. However, the mechanisms underlying the interplay between Snail, laminin, and basement membrane remodeling during neural crest EMT remain unclear.
We recently characterized the role of the Wnt signaling antagonist, Draxin, in regulating the timing of onset of cranial NC EMT (Hutchins and Bronner, 2018). Interestingly, the transcription factor Snail2, the only Snail gene family member expressed by chick cranial NC (del Barrio and Nieto, 2002), emerged as a likely target downstream of a transient pulse of Wnt signaling. Given that Snail genes are known to repress expression of laminin in other contexts (Takkunen et al., 2008) and laminin is an important component of the basement membrane surrounding the neural tube during NC EMT, we asked whether Draxin may play a role in the modulation of laminin during EMT. The results show that Draxin perturbation impedes basement membrane remodeling during cranial NC EMT via changes to laminin expression and/or organization. We further demonstrate that Draxin’s effect on laminin is mediated, at least in part, by Snail2. Thus, modulation of Wnt signaling via Draxin and its downstream effector Snail2 plays a critical role in regulating basement membrane remodeling during neural crest EMT.
2. MATERIALS AND METHODS
2.1. Cryosectioning and Immunohistochemistry
Chicken embryos (Gallus gallus) were obtained commercially and incubated at 37°C to reach the desired Hamburger-Hamilton (Hamburger and Hamilton, 1951) stage as indicated. Embryos were fixed overnight at 4°C in 4% paraformaldehyde in sodium phosphate buffer, then cryosectioned and immunostained as described (Hutchins and Bronner, 2018). Following gelatin removal, cryosections were incubated in primary antibodies overnight at 4°C and secondary antibodies for one hour at room temperature. Primary antibodies and counterstains used are listed in the Key Resources Table. Species-specific secondary antibodies were labeled with Alexa Fluor 488, 568, and 647 (Invitrogen). Prior to imaging, coverslips were mounted with Fluoromount-G (SouthernBiotech). Because Draxin expression has no effect on the early neural crest marker Pax7 (Hutchins and Bronner, 2018), we elected to use Pax7 throughout as a general neural crest label to assess effects of gene perturbation on basement membrane remodeling in the context of neural crest migration defects. Thus, defects in EMT could be attributed to changes to neural crest migration in general, and not to changes to a known/established target.
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Pax7; Species: Mouse IgG1 | Developmental Studies Hybridoma Bank | Cat# pax7, RRID:AB_528428 |
| Pan-Laminin; Species: Rabbit | Sigma-Aldrich | Cat# L9393 |
| Fluorescein; Species: Goat | Novus Biologicals | Cat# NB600–493 |
| FLAG M2; Species Mouse IgG1 | Sigma-Aldrich | Cat# F1804 |
| V5; Species: Mouse IgG2a | Thermo | Cat# R960–25 |
| Slug (C19G7); Species: Rabbit | Cell Signaling | Cat# 9585 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Fluoromount-G | SouthernBiotech | Cat# 0100–01 |
| Wheat Germ Agglutinin, Alexa Fluor™ 488 Conjugate | Thermo | W11261 |
| DAPI (4’,6-Diamidino-2-Phenylindole, Dihydrochloride) | Thermo | D1306 |
| Experimental Models: Organisms/Strains | ||
| Gallus gallus | Sun State Ranch (Monrovia, CA, USA) | N/A |
| Oligonucleotides | ||
| Draxin morpholino w/ 3’Fluorescein: AAGGTGGAAGAAGCTGCCATAATCC | Hutchins and Bronner, 2018; GeneTools | N/A |
| Standard Control morpholino w/ 3’Fluorescein: CCTCTTACCTCAGTTACAATTTATA | GeneTools | N/A |
| Snail2 morpholino w/ 3’Fluorescein: TCTTGACCAGGAAGGAGC | Taneyhill et al., 2007; GeneTools | N/A |
| Nhel-V5-Snail2_F Primer: ATAGCTAGCGCCACCATGGGTAAGCCTATCCCTAAC | This paper; IDT | N/A |
| Xbal-V5-Snail2_R Primer: ACCTCTAGATCAGTGTGCTACGCAGCAG | This paper; IDT | N/A |
| Recombinant DNA | ||
| pCI-H2B-RFP | Betancur et al., 2010 | N/A |
| Draxin-FLAG | Hutchins and Bronner, 2018 | N/A |
| NC1.1 M3:eGFP | Simoes-Costa et al., 2012 | N/A |
| pCIG-V5-Snail2-IRES-nls-GFP | Liu et al., 2013 | Addgene Plasmid #44282 |
| pCIG | Megason and McMahon, 2002 | N/A |
| NC1.1M3-Snail2 | This paper | N/A |
| Software and Algorithms | ||
| ImageJ64 | NIH | N/A |
| Fiji | Schindelin et al., 2012 | N/A |
| Zen 2 Blue | Zeiss | N/A |
| Photoshop CC | Adobe | N/A |
2.2. Electroporations
As described previously (Hutchins and Bronner, 2018; Sauka-Spengler and Barembaum, 2008; Simoes-Costa et al., 2015), dissected embryos were electroporated ex ovo at Hamburger-Hamilton (HH) stage HH4. Following injection of expression constructs or morpholino oligomers, embryos were electroporated using platinum electrodes (5 pulses, 6.0V, 30ms duration at 100ms intervals), and cultured at 37°C in albumin/1% penicillin/streptomycin to indicated HH stages.
2.3. Expression constructs and Morpholino Oligomers
Draxin and Snail2 knockdown using translation-blocking antisense morpholinoelectroporation (Draxin MO and Snail2 MO, respectively, Key Resources Table; GeneTools; co-electroporated with pCIG), and Draxin overexpression using the Draxin-FLAG expression construct, were performed as described (Hutchins and Bronner, 2018; Taneyhill et al., 2007). The Draxin-FLAG construct contains coding for the secreted, full-length chick Draxin protein with a C-terminal FLAG epitope tag (Hutchins and Bronner, 2018), and a downstream internal ribosomal entry site (IRES) that drives expression of a separate Histone H2B-RFP. RFP nuclear fluorescence is used only as an indicator of successful electroporation. Control reagents were described previously (Hutchins and Bronner, 2018), and are listed in the Key Resources Table. In order to generate the NC1-Snail2 construct, the coding for V5-tagged Snail2 was PCR amplified from pCIG-V5-Snail2-IRES-nls-GFP (Liu et al., 2013). This V5-Snail2 fragment was then cloned into the NC1.1 M3 construct (Simoes-Costa et al., 2012), following excision of GFP by NcoI and XbaI. All constructs and primers (Integrated DNA Technologies) are listed in the Key Resources Table.
2.4. Microscope image acquisition
Confocal-like images were acquired with structured illumination microscopy to generate optical sections, using a Zeiss Imager.M2 with an ApoTome.2 module and Zen 2 Blue software, with a Zeiss Plan-Apochromat 20x objective/0.8 NA, and a Zeiss EC Plan-NEOFLUAR 40x oil objective/1.3 NA. Z-stacks were taken at 0.55μm (20x objective) and 0.325μm (40x objective) intervals and displayed as maximum intensity projections. Images were minimally processed for brightness and contrast using Adobe Photoshop CC.
2.5. Statistical analysis
Statistical analyses were performed using GraphPad Prism 7. P values were calculated using an unpaired, two-tailed t-test and are indicated in the text. Data are presented as the percentage of the total number of sections displaying the specified phenotype (n≥8 sections among 5 embryos, per condition). An “incomplete channel” was scored if laminin appeared to partially block channel opening, but Pax7+ neural crest cells were able to pass laterally beyond the laminin blockage. “No channel” was scored if laminin formed a complete barrier beyond which Pax7+ cells could not migrate. Multiple sections from multiple embryos were factored into our analysis to account for section-to-section differences and biological variability. Further, this variation is why we chose not to quantify fluorescence intensity in the neural crest region, but rather characterize gross morphological changes to the organization of the basement membrane (i.e. channel or no channel) in order to simplify phenotypic interpretation and reduce the impact of technical and biological variability.
3. RESULTS
3.1. Cranial neural crest migrates through a laminin channel during EMT
In the avian cranial NC, the events of EMT and neural tube closure progress simultaneously. As the neural tube undergoes separation from the non-neural ectoderm, premigratory NC cells in this region delaminate and complete EMT to migrate dorsolaterally between the newly-formed overlying dorsal ectoderm and the underlying ventral mesoderm (Baker et al., 1997; Duband, 2006; Theveneau and Mayor, 2012). The separation of the neural tube from the non-neural ectoderm occurs via a remodeling of the laminin-rich basement membrane from a single, continuous structure into distinct dorsal ectodermal and neural tube basement membranes (Duband and Thiery, 1987).
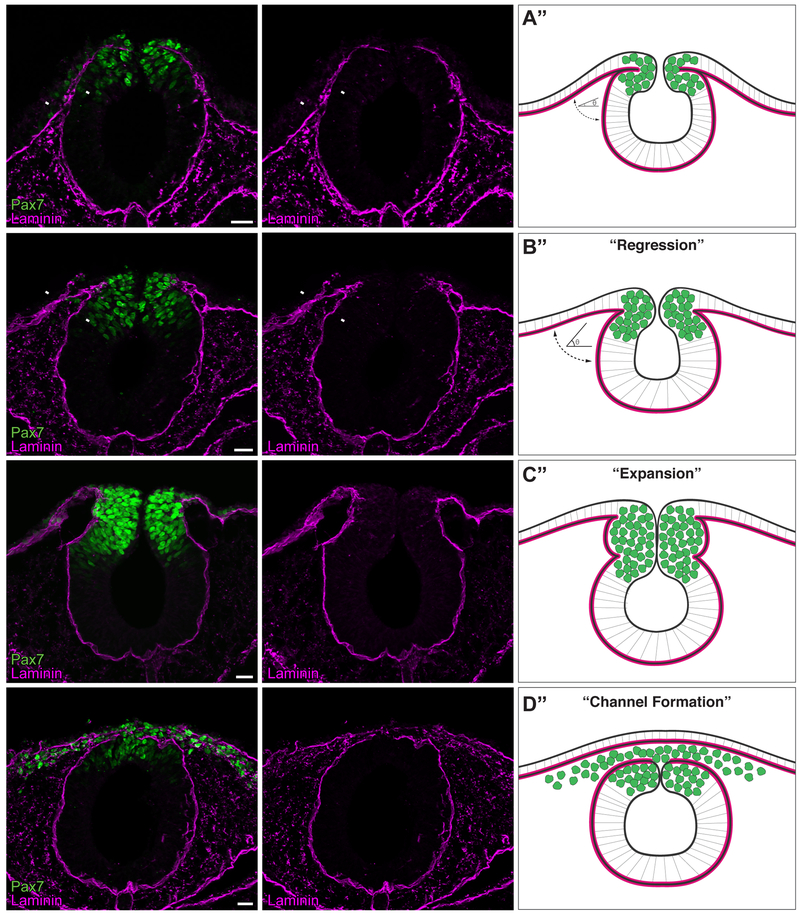
In order to correlate events of cranial NC EMT with reorganization of the basement membrane, we examined the expression of laminin (as a marker for the basement membrane) and the neural crest marker Pax7 across several developmental time points (Fig. 1, Fig. S1). Immunohistochemical analysis of transverse sections through wild-type chick mesencephalon and rostral hindbrain revealed dynamic rearrangement of the basement membrane correlating with discrete stages of cranial neural crest EMT. Between Hamburger-Hamilton stages 8+ and 9− (HH8+/9−), as specified premigratory NC cells were localized to the dorsal aspect of the neural tube, the basement membrane was remodeled to form a space between the non-neural ectoderm and neural tube, which were tightly apposed at HH8+. This remodeling served to increase the angle between the non-neural ectoderm and neuroepithelium basement membranes (Fig. 1A–B, arrowheads). At HH9, as cranial NC began delaminating from the neuroepithelium, the basement membrane at the junction of the neural tube and ectoderm remained continuous, despite expanding to encapsulate newly delaminated NC, apparently creating a barrier that blocked NC entry into the dorsolateral migratory route (Fig. 1C). By HH9+, the lateral laminin barrier disappeared to generate separate ectodermal and neural tube basement membranes. Concomitantly, cranial NC cells completed EMT and mesenchymalized to migrate away from the neural tube (Hutchins and Bronner, 2018). Because the basement membranes of the ectoderm and neural tube remained in close proximity, this separation formed a laminin-lined “channel”, through which the cranial NC migrated (Fig. 1D). Thus, during cranial NC EMT, basement membrane remodeling occurs in three successive stages, which we term “Regression” (Fig. 1B”), “Expansion” (Fig. 1C”), and “Channel Formation” (Fig. 1D”).
Figure 1. The basement membrane is remodeled during cranial neural crest EMT.
(A-D) Immunostaining for Pax7 (green) and laminin (magenta) in cross sections of HH8+ (A), HH9- (B), HH9 (C), and HH9+ (D) embryos. Arrowheads in (A’) and (B’) indicate apposed and non-apposed basement membranes, respectively. Scale bar, 20 μm. Schematics (A”-D”) summarize immunostaining data (n=12 sections, 3 embryos per stage), where green circles represent neural crest cells and magenta lines indicate the laminin-rich basement membrane. As development progresses from HH8+ to HH9-, a “regression” occurs, where the angle (θ) between the non-neural ectoderm basement membrane and the neuroepithelium basement membrane increases (A”-B”). As Pax7+ neural crest cells undergo EMT, a laminin channel forms by HH9+, creating a passage through which the cells are able to migrate.
3.2. Draxin knockdown impedes basement membrane remodeling during EMT
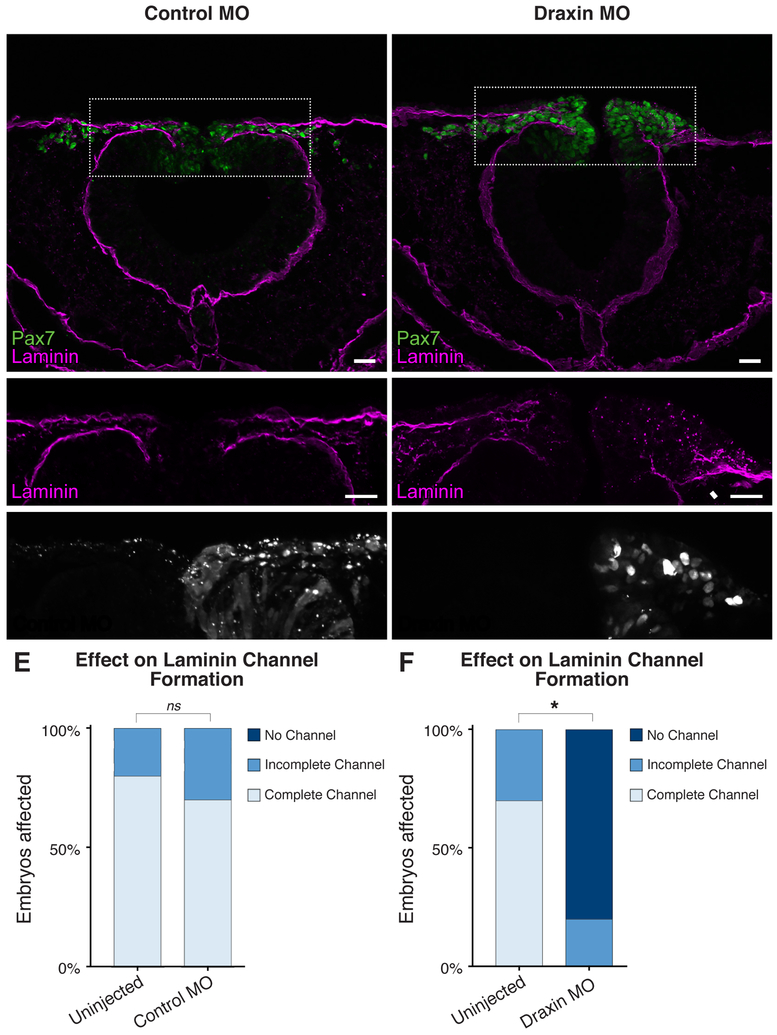
We previously identified a critical role of the Wnt antagonist, Draxin, in the regulation of cranial NC EMT; loss of Draxin induced premature NC exit from the neuroepithelium, though the cells failed to complete EMT and become migratory (Hutchins and Bronner, 2018). To determine whether Draxin played a role in basement membrane remodeling, we examined transverse sections of embryos unilaterally electroporated with either control morpholino (MO) (Fig. 2A–B) or a translation-blocking Draxin MO (Fig. 2C–D). Whereas control MO electroporation yielded no significant change in the incidence of laminin channel formation compared to the contralateral uninjected side (p = 0.63; Fig. 2E), loss of Draxin significantly inhibited laminin channel formation (p < 0.0001; Fig. 2F). Examination of the Draxin knockdown phenotype at stage HH9+ showed a continuous basement membrane (Fig. 2D, arrowhead), with laminin expression resembling a stage HH9- wild type phenotype (Fig. 1B), with neural tube closure appearing to progress normally; interestingly, this is also the stage of onset of Draxin expression (Hutchins and Bronner, 2018). We further examined earlier stages of development (HH9-/9), and observed defects in laminin organization as early as HH9 (Fig. S2). These data suggest that Draxin knockdown halts basement membrane remodeling at the “Regression” stage. Thus, Draxin is necessary for complete basement membrane reorganization during cranial neural crest EMT.
Figure 2. Draxin knockdown inhibits basement membrane remodeling during EMT.
(A-D) Representative images for Pax7 (green) and laminin (magenta) immunostaining in embryos unilaterally electroporated with FITC-labeled control (A-B) or translation-blocking Draxin (C-D) morpholino (MO). Scale bar, 20 μm. (E-F) Quantification of the effect of MO electroporation indicated Draxin knockdown significantly reduced laminin channel formation, whereas control MO did not (p = 0.63). Boxes in (A,D) indicate enlarged areas in (B,E), as indicated. White arrowhead highlights area of altered laminin distribution. *, p < 0.0001, two-tailed t-test. ns, nonsignificant.
3.3. Ectopic maintenance of Draxin blocks laminin channel formation during EMT
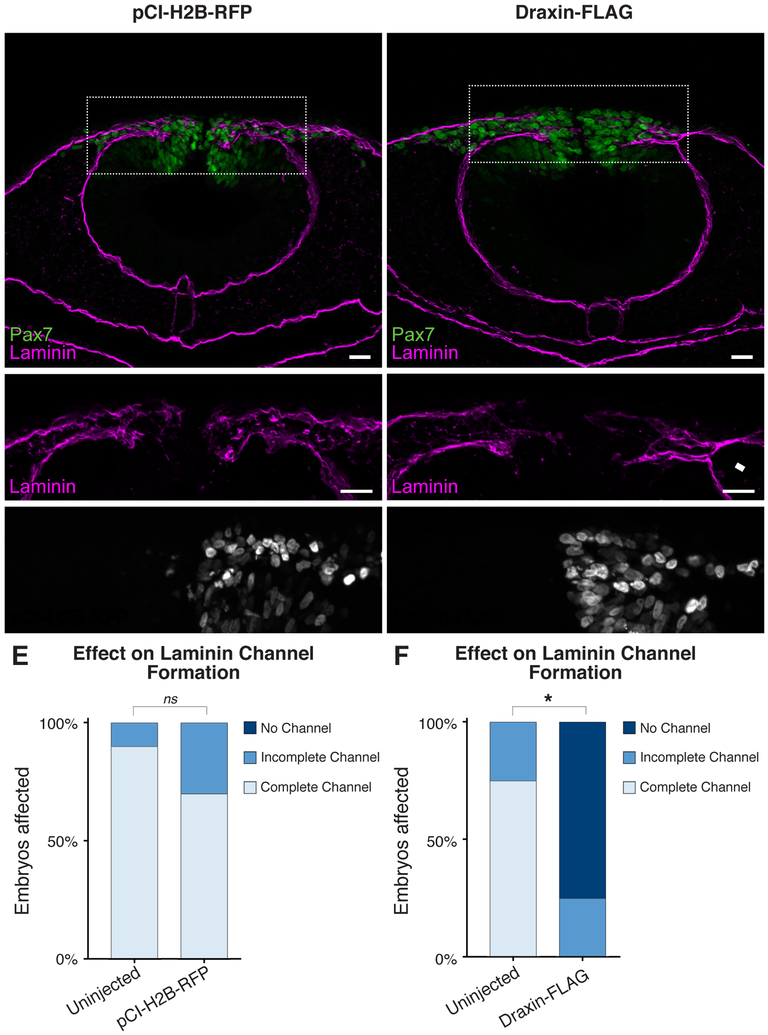
Draxin is expressed in a transient head-to-tail wave within the premigratory NC, and its downregulation mirrors initiation of cranial NC emigration. Furthermore, ectopic overexpression of Draxin after its endogenous downregulation results in inhibited cranial NC delamination and migration (Hutchins and Bronner, 2018). Thus, we sought to assess the effects of ectopic Draxin overexpression on laminin expression during EMT. Following unilateral electroporation of pCIH2B-RFP as a control (Fig. 3A–B) or a Draxin-FLAG overexpression construct (Hutchins and Bronner, 2018; Fig. 3C–D), we examined transverse sections of embryos for effects on laminin channel formation. Control pCI-H2B-RFP electroporations had no significant effect on laminin channel formation (P = 0.29; Fig. 3E). In contrast, ectopic Draxin overexpression significantly reduced the incidence of complete channel formation (P < 0.0001; Fig. 3F); examination of the Draxin-FLAG phenotype at stage HH9+ revealed a physical blockage of the laminin channel due to aberrant laminin organization (Fig. 3D, arrowhead), resembling a stage HH9 wild type phenotype (Fig. 1C). These data suggest that ectopic maintenance of Draxin halts basement membrane remodeling at the “Expansion” stage, inhibiting the dissolution of the lateral laminin barrier, which creates a physical block to the migration of cranial NC away from the neural tube.
Figure 3. Ectopic maintenance of Draxin impedes laminin channel formation.
(A-D) Representative images for Pax7 (green) and laminin (magenta) immunostaining in embryos unilaterally electroporated with pCI-H2B-RFP control (A-B) or a Draxin-FLAG overexpression construct (C-D). Scale bar, 20 μm. (E-F) Quantification of laminin channel formation indicated that, whereas pCI-H2B-RFP electroporation had no effect on channel formation (p = 0.29), Draxin-FLAG overexpression significantly inhibited channel completion due to altered laminin distribution. Boxes in (A,D) indicate zoomed areas in (B,E), as indicated. White arrowhead highlights area of altered laminin distribution. *, p < 0.0001, two-tailed t-test. ns, nonsignificant.
3.4. Perturbation of Snail2 alters laminin channel formation during EMT
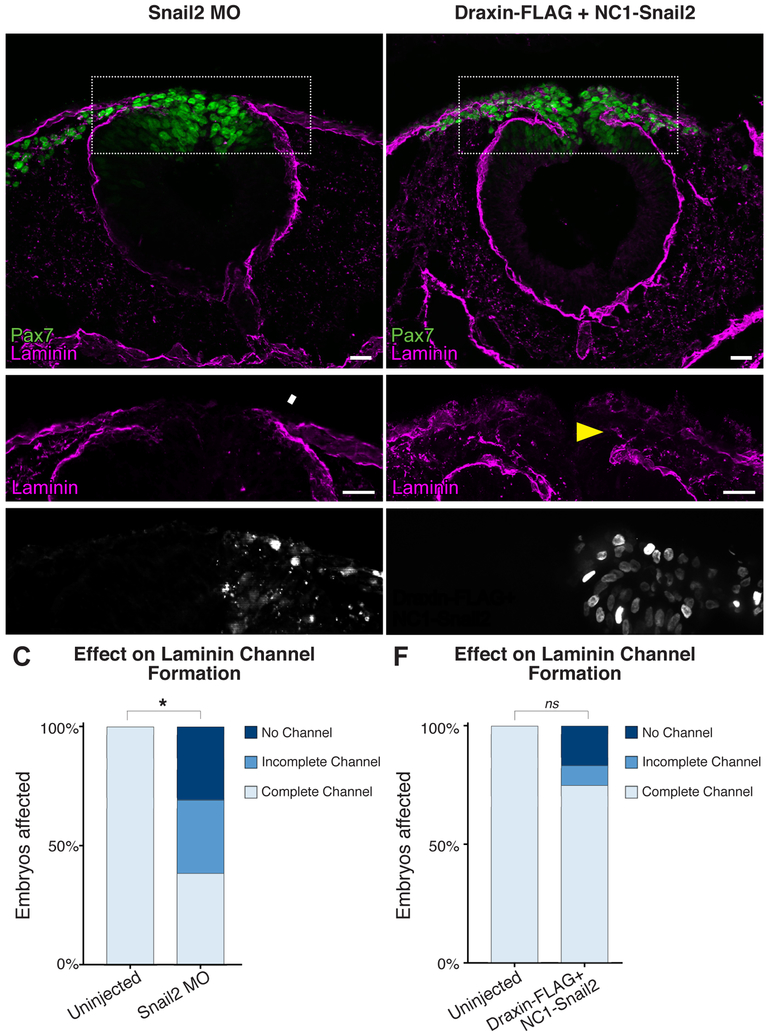
We have previously demonstrated that Draxin overexpression inhibited Snail2 in cranial NC (Hutchins and Bronner, 2018). Given that Snail family members have been implicated in both EMT and the transcriptional control of laminin (Cano et al., 2000; Takkunen et al., 2008; Zeisberg and Neilson, 2009), we asked whether the effects of Draxin on laminin channel formation and basement membrane remodeling in cranial NC are mediated by Snail2. To this end, we performed unilateral electroporation of a translation-blocking Snail2 MO (Taneyhill et al., 2007) and observed a significant reduction in the number of Snail2+ cells on the MO-electroporated side (Fig. S3A–C; *, P = 0.004, two tailed t-test). We next examined transverse sections of Snail2 MO-electroporated embryos for effects on laminin channel formation, and found that Snail2 knockdown significantly impeded laminin channel formation (Fig. 4A–C, Fig. S3D–G; *, P = 0.0007, two tailed t-test). To further confirm that Draxin’s effect on basement membrane remodeling is mediated at least in part by Snail2, we generated a Snail2 overexpression construct (NC1-Snail2) in which the FoxD3 NC1 enhancer (Simoes-Costa et al., 2012) drives expression of V5-tagged, full-length chick Snail2 (Fig. S4). This construct restricts expression of Snail2 to the cranial NC following specification, in order to bypass potential deleterious effects on earlier induction and specification events. When co-electroporated with Draxin-FLAG (Fig. D-E), we observed complete rescue of laminin channel formation (Fig. 4F; compare to Fig. 3). Together, these data suggest that Draxin controls the progression of basement membrane remodeling, in large part, via modulation of Snail2.
Figure 4. Modulating Snail2 alters laminin channel formation.
(A-B) Representative images for Pax7 (green) and laminin (magenta) immunostaining in embryos unilaterally electroporated with FITC-labeled translation-blocking Snail2 morpholino (MO). Scale bar, 20 μm. (C) Quantification of the effect of MO electroporation indicated Snail2 knockdown significantly reduced laminin channel formation. Box in (A) indicates enlarged area in (B), as indicated. White arrowhead highlights area of altered laminin distribution. *, p < 0.001, two-tailed t-test. ns, nonsignificant. (D-E) Representative images for Pax7 (green) and laminin (magenta) immunostaining in embryos unilaterally co-electroporated with NC1-Snail2 and Draxin-FLAG. Nuclear fluorescence in E’ indicates electroporated side. Scale bar, 20 μm. Box in (D) indicates enlarged area in (E), as indicated. Yellow arrowhead highlights rescue of channel formation. (F) Quantification of laminin channel formation indicated that NC1-Snail2 rescued the effect of Draxin-FLAG overexpression on channel formation. ns, nonsignificant (p = 0.082).
4. DISCUSSION
The canonical Wnt antagonist, Draxin, has critical functions in regulating the progression of cranial NC EMT. A transient pulse of Draxin expression tightly coordinates the events of cranial NC delamination and mesenchymalization, to allow successful migration away from the neural tube (Hutchins and Bronner, 2018). Here, we demonstrate a novel function of Draxin during EMT—in regulation of basement membrane remodeling. Our data provide a mechanistic link between Wnt signaling modulation and progressive laminin reorganization to restructure the basement membrane.
Combined with our prior study (2018), our results suggest the following model for the interplay between Snail2, laminin, and basement membrane remodeling during cranial NC EMT. Under wild-type conditions, endogenous Draxin expression within the premigratory cranial neural crest attenuates Wnt signaling, and by extension, the Wnt target gene Snail2; reduced Wnt signaling maintains the basement membrane in a single, continuous layer that is able to progress through the “Regression” and “Expansion” stages. As Draxin is endogenously downregulated, Wnt signaling increases, allowing intrinsic NC EMT factors (e.g. Snail2, etc.) to dissolve the lateral laminin barrier (presumably via both transcriptional and post-translational regulation) and complete channel formation.
With loss of Draxin, Wnt signaling increases prematurely and halts laminin reorganization. We hypothesize this abrogation of basement membrane remodeling may be in part due to reduced Cadherin6B (Cad6B) expression. Cad6B is a target of proteolytic cleavage, and it was recently shown that its cleaved fragments promote laminin degradation and basement membrane remodeling during EMT (Schiffmacher et al., 2018; Schiffmacher et al., 2014). Thus, it is possible the premature reduction of Cad6B found with loss of Draxin (Hutchins and Bronner, 2018) subsequently reduces the availability of Cad6B cleavage fragments to modulate laminin. Furthermore, because Snail1 directly represses transcription of a subtype of laminin during EMT (Takkunen et al., 2008), loss of Draxin may affect laminin expression in multiple ways to impede basement membrane remodeling. With ectopic maintenance of Draxin, Wnt signaling fails to increase following NC delamination, and as such, Snail2 expression remains suppressed as Cad6B is inappropriately maintained (Hutchins and Bronner, 2018). This results in a failure to remove the laminin blockage to complete channel formation, despite an increase in Cad6B. We hypothesize that the inability to remove the laminin blockage may be due the loss of Snail2, and the resulting inability to transcriptionally repress laminin and/or degrade the physical barrier to migration. This argument is strengthened by the ability of Snail2 overexpression to rescue laminin channel formation from Draxin overexpression. However, there may also be a reduction in the cleavage of Cad6B, subsequently impeding laminin degradation; examination of whether Draxin perturbation also affects expression of A Disintegrin and Metalloproteinases (ADAMs) or matrix metalloproteinases (MMPs), which themselves target Cad6B (Schiffmacher et al., 2014), would help resolve this question. In addition, laminin channel formation may require not only modification to the ECM components of the basal lamina via proteolytic cleavage, but also the force of migratory neural crest cells pushing against the laminin barrier to facilitate remodeling (Clay and Halloran, 2010, 2011). Parsing whether Draxin perturbation alters proteolytic cleavage of Cad6B, tensile strength of the basement membrane, or motile force of the neural crest cells themselves may provide further mechanistic insights into the effects on basement membrane remodeling during EMT.
We believe the interplay between basement membrane remodeling and neural crest migration we describe here fits nicely with recent work identifying the importance of neural crest/ECM interactions in other systems. For example, cephalic neural crest cells in Xenopus have been shown to directly interact with the ECM, are responsive to matrix/environmental stiffening, and that these mechanical cues are required for successful neural crest EMT and subsequent contact inhibition of locomotion (Barriga et al., 2018; Scarpa et al., 2015). Furthermore, it is interesting that the presence of the ECM proteoglycan versican acts as a nonpermissive substrate to confine neural crest, providing a mechanism of stream formation and directional migration (Szabo et al., 2016). This model of in vivo confinement correlates with our description of laminin channel formation, and suggests an important, conserved role for the ECM and basement membrane in both facilitating and directing neural crest EMT and migration.
In summary, our results suggest an essential function for Draxin in regulating basement membrane remodeling during cranial NC EMT. The progressive reorganization of laminin within the basement membrane correlates with the endogenous transient pulse of Draxin expression. Draxin’s role as a canonical Wnt antagonist thus suggests that the events of cranial NC EMT and basement membrane remodeling are inextricably linked, and that they are tightly controlled and coordinated with canonical Wnt signaling.
Supplementary Material
Supplemental Figure S1. Higher magnification images of basement membrane remodeling during cranial neural crest EMT. (A-C) Cross sections of wild type embryos at HH9- (A), HH9 (B), and HH9+ (C), imaged with 40x objective. Immunostaining for Pax7 (green) and laminin (magenta) with DAPI (gray) and wheat germ agglutinin (WGA, blue) counterstains to label nuclei and membranes, respectively. Scale bar, 20 μm.
Supplemental Figure S2. Draxin knockdown inhibits basement membrane remodeling by HH9. (A-D) Representative images for Pax7 (green) and laminin (magenta) immunostaining in embryos unilaterally electroporated with FITC-labeled translation-blocking Draxin morpholino (MO) at HH9- (A-B) and HH9 (C-D). Scale bar, 20 μm. Boxes in (A,C) indicate enlarged areas in (B,D), as indicated. White arrowhead highlights area of altered laminin distribution.
Supplemental Figure S3. Snail2 knockdown inhibits basement membrane remodeling. (A-B) Representative images for Snail2 (cyan) immunostaining in embryos unilaterally electroporated with FITC-labeled translation-blocking Snail2 morpholino (MO; gray). Scale bar, 20 μm. (C) Quantitation of Snail2+ cells on uninjected (left) versus Snail2 MO (right) sides of embryo (three sections/embryo; n = 3 embryos/condition) yielded a significant decrease (*, p = 0.004; two-tailed t-test) in total Snail2+ cells on the Snail2 MO-electroporated side compared with the contralateral uninjected side. Data are presented as mean ± SEM. (D-G) Representative images for incomplete channel (D-E) and no channel (F-G) phenotypes of Pax7 (green) and laminin (magenta) immunostained embryos unilaterally electroporated with Snail2 MO. Scale bar, 20 μm. Boxes in (D,F) indicate enlarged areas in (E,G), as indicated. White arrowheads highlight areas of altered laminin distribution.
Supplemental Figure S4. Validation of the NC1-Snail2 overexpression construct with Draxin-FLAG co-electroporation. (A-B) Electroporation of Draxin-FLAG yielded expression of H2B-RFP (magenta), which co-localized with nuclear expression of NC1-driven V5-tagged Snail2 (NC1-Snail2, green). Immunostaining for endogenous Snail2 (cyan) or FLAG (yellow) revealed areas of high expression of Draxin-FLAG in vivo near reduced levels of endogenous Snail2.
HIGHLIGHTS.
Cranial neural crest migrate through a laminin-rich basement membrane channel
Perturbation of Draxin, a Wnt antagonist, alters laminin channel formation
Draxin’s effect on laminin channel formation is largely mediated by Snail2
ACKNOWLEDGMENTS
We thank Dr. M. Simoes-Costa for providing the NC1.1 M3:EGFP construct, and Dr. M. Cheung for providing the pCIG-V5-Snail2-IRES-nls-GFP construct (Addgene plasmid # 44282). This work was supported by a National Institutes of Health grant (R01DE024157 and PO1HD037105 to M.E. Bronner), and a Ruth L. Kirschstein National Research Service Award (F32DE026355 to E.J. Hutchins). The authors declare no competing interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baker CV, Bronner-Fraser M, Le Douarin NM, Teillet MA, 1997. Early- and late-migrating cranial neural crest cell populations have equivalent developmental potential in vivo. Development 124, 3077–3087. [DOI] [PubMed] [Google Scholar]
- Barriga EH, Franze K, Charras G, Mayor R, 2018. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 554, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA, 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature cell biology 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Christian L, Bahudhanapati H, Wei S, 2013. Extracellular metalloproteinases in neural crest development and craniofacial morphogenesis. Crit Rev Biochem Mol Biol 48, 544–560. [DOI] [PubMed] [Google Scholar]
- Clay MR, Halloran MC, 2010. Control of neural crest cell behavior and migration: Insights from live imaging. Cell Adh Migr 4, 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MR, Halloran MC, 2011. Regulation of cell adhesions and motility during initiation of neural crest migration. Curr Opin Neurobiol 21, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, Bronner-Fraser M, 2006. Abnormalities in neural crest cell migration in laminin alpha5 mutant mice. Developmental biology 289, 218–228. [DOI] [PubMed] [Google Scholar]
- del Barrio MG, Nieto MA, 2002. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 129, 1583–1593. [DOI] [PubMed] [Google Scholar]
- Duband JL, 2006. Neural crest delamination and migration: integrating regulations of cell interactions, locomotion, survival and fate. Adv Exp Med Biol 589, 45–77. [DOI] [PubMed] [Google Scholar]
- Duband JL, Thiery JP, 1987. Distribution of laminin and collagens during avian neural crest development. Development 101, 461–478. [DOI] [PubMed] [Google Scholar]
- Gouignard N, Andrieu C, Theveneau E, 2018. Neural crest delamination and migration: Looking forward to the next 150 years. Genesis 56, e23107. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL, 1951. A series of normal stages in the development of the chick embryo. J Morphol 88, 49–92. [PubMed] [Google Scholar]
- Hutchins EJ, Bronner ME, 2018. Draxin acts as a molecular rheostat of canonical Wnt signaling to control cranial neural crest EMT. The Journal of cell biology 217, 3683–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JA, Wu MH, Yan CH, Chau BK, So H, Ng A, Chan A, Cheah KS, Briscoe J, Cheung M, 2013. Phosphorylation of Sox9 is required for neural crest delamination and is regulated downstream of BMP and canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America 110, 2882–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, Thiery JP, 2016. Emt: 2016. Cell 166, 21–45. [DOI] [PubMed] [Google Scholar]
- Perris R, 1997. The extracellular matrix in neural crest-cell migration. Trends Neurosci 20, 23–31. [DOI] [PubMed] [Google Scholar]
- Perris R, Perissinotto D, 2000. Role of the extracellular matrix during neural crest cell migration. Mechanisms of development 95, 3–21. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M, 2008. Gain- and loss-of-function approaches in the chick embryo. Methods in cell biology 87, 237–256. [DOI] [PubMed] [Google Scholar]
- Scarpa E, Szabo A, Bibonne A, Theveneau E, Parsons M, Mayor R, 2015. Cadherin Switch during EMT in Neural Crest Cells Leads to Contact Inhibition of Locomotion via Repolarization of Forces. Developmental cell 34, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmacher AT, Adomako-Ankomah A, Xie V, Taneyhill LA, 2018. Cadherin-6B proteolytic N-terminal fragments promote chick cranial neural crest cell delamination by regulating extracellular matrix degradation. Developmental biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmacher AT, Padmanabhan R, Jhingory S, Taneyhill LA, 2014. Cadherin-6B is proteolytically processed during epithelial-to-mesenchymal transitions of the cranial neural crest. Mol Biol Cell 25, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, Stone M, Bronner ME, 2015. Axud1 Integrates Wnt Signaling and Transcriptional Inputs to Drive Neural Crest Formation. Developmental cell 34, 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa MS, McKeown SJ, Tan-Cabugao J, Sauka-Spengler T, Bronner ME, 2012. Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest is Encrypted in the genome. PLoS genetics 8, e1003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl-Mazzulla PH, Bronner ME, 2012. A PHD12-Snail2 repressive complex epigenetically mediates neural crest epithelial-to-mesenchymal transition. The Journal of cell biology 198, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Melchionda M, Nastasi G, Woods ML, Campo S, Perris R, Mayor R, 2016. In vivo confinement promotes collective migration of neural crest cells. The Journal of cell biology 213, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkunen M, Ainola M, Vainionpaa N, Grenman R, Patarroyo M, Garcia de Herreros A, Konttinen YT, Virtanen I, 2008. Epithelial-mesenchymal transition downregulates laminin alpha5 chain and upregulates laminin alpha4 chain in oral squamous carcinoma cells. Histochem Cell Biol 130, 509–525. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA, Coles EG, Bronner-Fraser M, 2007. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development 134, 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theveneau E, Mayor R, 2012. Neural crest delamination and migration: from epithelium-tomesenchyme transition to collective cell migration. Developmental biology 366, 34–54. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG, 2009. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Higher magnification images of basement membrane remodeling during cranial neural crest EMT. (A-C) Cross sections of wild type embryos at HH9- (A), HH9 (B), and HH9+ (C), imaged with 40x objective. Immunostaining for Pax7 (green) and laminin (magenta) with DAPI (gray) and wheat germ agglutinin (WGA, blue) counterstains to label nuclei and membranes, respectively. Scale bar, 20 μm.
Supplemental Figure S2. Draxin knockdown inhibits basement membrane remodeling by HH9. (A-D) Representative images for Pax7 (green) and laminin (magenta) immunostaining in embryos unilaterally electroporated with FITC-labeled translation-blocking Draxin morpholino (MO) at HH9- (A-B) and HH9 (C-D). Scale bar, 20 μm. Boxes in (A,C) indicate enlarged areas in (B,D), as indicated. White arrowhead highlights area of altered laminin distribution.
Supplemental Figure S3. Snail2 knockdown inhibits basement membrane remodeling. (A-B) Representative images for Snail2 (cyan) immunostaining in embryos unilaterally electroporated with FITC-labeled translation-blocking Snail2 morpholino (MO; gray). Scale bar, 20 μm. (C) Quantitation of Snail2+ cells on uninjected (left) versus Snail2 MO (right) sides of embryo (three sections/embryo; n = 3 embryos/condition) yielded a significant decrease (*, p = 0.004; two-tailed t-test) in total Snail2+ cells on the Snail2 MO-electroporated side compared with the contralateral uninjected side. Data are presented as mean ± SEM. (D-G) Representative images for incomplete channel (D-E) and no channel (F-G) phenotypes of Pax7 (green) and laminin (magenta) immunostained embryos unilaterally electroporated with Snail2 MO. Scale bar, 20 μm. Boxes in (D,F) indicate enlarged areas in (E,G), as indicated. White arrowheads highlight areas of altered laminin distribution.
Supplemental Figure S4. Validation of the NC1-Snail2 overexpression construct with Draxin-FLAG co-electroporation. (A-B) Electroporation of Draxin-FLAG yielded expression of H2B-RFP (magenta), which co-localized with nuclear expression of NC1-driven V5-tagged Snail2 (NC1-Snail2, green). Immunostaining for endogenous Snail2 (cyan) or FLAG (yellow) revealed areas of high expression of Draxin-FLAG in vivo near reduced levels of endogenous Snail2.