Abstract
Although hematopoietic stem cell transplantation (HSCT) has been widely used in the treatment of many diseases, graft-versus-host disease (GVHD) remains a major complication after allogeneic HSCT. Butyrophilin-like 2 (BTNL2) protein has been reported to have the ability to inhibit T cell proliferation in vitro; its ability to inhibit T cell responses in vivo has not been determined. We show here that in vivo administration of recombinant BTNL2-IgG2a Fc (rBTNL2-Ig) fusion protein ameliorates GVHD in mice. This is related to the ability of rBTNL2- Ig to inhibit T cell proliferation, activation and Th1/Th17 cytokine production in vivo. Furthermore, rBTNL2-Ig treatment increases the generation of regulatory T cells. Our results suggest that rBTNL2-Ig has the potential to be used in the prevention and treatment of patients with GVHD.
Keywords: hematopoietic stem cell transplantation, graft-versus-host disease, Butyrophilin-like 2, regulatory T cells, T cell proliferation, activation
1. Introduction
While allogeneic HSCT has been widely used in the treatment of a variety of malignancies and non-malignant conditions, GVHD remains as a serious complication that limits its success [1–3]. GVHD occurs in up to 50% transplant recipients and accounts for 15–30% of deaths following allogeneic HSCT [2]. GVHD, especially acute GVHD, is primarily mediated by donor T cells in the graft [1–8]. Alloreactive donor T cells recognize disparate histocompatibility antigens in the recipients and lead to progressive damage to target organs such as the liver, gastrointestinal tract, skin, and lung. The symptoms of acute GVHD include maculopapular skin rash, nausea, anorexia, diarrhea, abdominal pain, and cholestatic hyperbilirubinemia, etc [2]. Development of new approaches to prevent or treat GVHD thus remains a major clinical goal.
T cell immune responses are regulated by T cell co-stimulatory and co-inhibitory molecules. The B7 family plays a central role in regulating immune responses. Targeting B7 family ligands and receptors has achieved great success in the treatment of autoimmune disease and cancer. For example, the recombinant fusion protein CTLA-4-Fc (Abatacept or Balatacept) has been used in the treatment of rheumatoid arthritis and kidney transplantation rejection.
Butyrophilin (BTN) and BTN-like (BTNL) molecules are emerging as potentially critical immune regulators [9–13]. BTN and BTNL molecules share sequence, structural, and functional similarity with the B7 family members so are thus considered as extended B7 family members [14–16]. It has been reported that BTNL2 can inhibit T cell proliferation and cytokine production in vitro [17, 18]. However, the ability of BTNL2 to inhibit T cell responses in vivo and to treat GVHD has not been reported.
In this study, we first clone and express the BTNL2 gene to produce a high yielding rBTNL2-Ig fusion protein. We then demonstrate that in vivo administration of rBTNL2-Ig attenuates GVHD in mice. This is associated with its ability to inhibit T cell proliferation, activation and cytokine production in vivo. In addition, rBTNL2-Ig treatment significantly increases the generation of regulatory T cells (Tregs) in the GVHD mice.
2. Methods
2.1. Cloning, expression and purification of rBTNL2-Ig
The extracellular domain of mouse BTNL2 (aa27–452) was cloned and fused into a pCMV6-AC-FC-S expression vector containing the constant region of mouse IgG2a (ORIGENE, Rockville, MD). The vectors were transfected into HEK-293 cells. The fusion protein was purified from the supernatant using Protein G Sepharose 4 Fast Flow according to the manufacturer’s instructions (GE Healthcare). Purified proteins were verified by SDS-PAGE, Coomassie Staining and Western blot. Protein was quantified using the Pierce™ BCA Protein Assay Kit (Pierce, Rockford, IL). The endotoxin level was determined by the Limulus Amebocyte Lysate (LAL) assay. Control Ig (recombinant mouse IgG2a Fc protein) was purchased from BXCell (West Lebanon, NH) or produced in our laboratory with the same expression vector, HEK-293 cells, and purification process as rBTNL2-Ig protein.
2.2. SDS-PAGE and Western blot
Purified rBTNL2-Ig was loaded on a 12% SDS-PAGE, and either stained with Coomassie blue or transferred to a polyvinylidene fluoride membrane. The protein-containing membrane was incubated with HRP conjugated anti-mouse IgG2 antibody and developed with Super Signal® West Pico chemiluminescent Substrate (Thermo Scientific).
2.3. LAL assay
The endotoxin level in the purified protein was determined by the endpoint chromogenic LAL test according to the manufacturer’s instructions (Lonza, Walkersville, MD) [19].
2.4. Mice
BALB/c and C57BL/6 mice were purchased from Jackson Laboratory. The mice were used in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Connecticut.
2.5. HSCT Procedure
BM cell suspensions were harvested from mice by flushing the marrow from the femurs and tibias with cold RPMI 1640. Recipients received 900 cGy total body irradiation from a 137Cs source (Gammator-50 Gamma Irradiator; Radiation Machinery Corporation, Parsippany, NJ). Two to four hours later, the mice were injected intravenously (i.v.) with 5 × 106 BM with or without a prescribed dose of splenic cells. Groups of mice were then injected i.v. with prescribed dose of rBTNL2-Ig or control Ig at the time of GVHD induction (day 0) and then intraperitoneally (i.p.) with rBTNL2-Ig or control Ig at indicated times.
2.6. Assessment of GVHD
The severity of GVHD was evaluated with a clinical GVHD scoring system. In brief, HSCT recipients in coded cages were individually scored every week for five clinical parameters on a scale from 0 to 2: weight loss, posture, activity, fur texture and skin integrity. A clinical GVHD index was generated by summation of the five criteria scores (maximum index = 10).
Groups of GVHD mice were euthanized 14 days after HSCT, and GVHD target organs were harvested for histopathological analysis. The organs were formalin-preserved, paraffin-embedded, sectioned and hematoxylin/eosin (H&E)-stained. Assessment of tissue damage was performed based on scoring systems previously described [20]. Briefly, liver GVHD was scored on the number of involved tracts and severity of liver cell necrosis; the maximum score is 10. Gut GVHD was scored on the basis of crypt apoptosis and lamina propria inflammation; the maximum score is 8. Lung GVHD was scored on the periluminal infiltrates, pneumonitis, and the severity of lung tissues involved; the maximum score is 9.
2.7. Flow Cytometry analysis
Single cell suspensions of organs and tumors were stained with the fluorochrome-conjugated antibodies protein as described [21–24]. For intracellular staining, the cells were first permeabilized with a BD Cytofix/Cytoperm solution for 20 minutes at 4 ℃. Direct or indirect staining of fluorochrome-conjugated antibodies included: CD4, CD8, CD44, CD62L, CD69, Foxp3, and H-2Kb, IFNγ, TNFα, and IL-17A (BioLegend, or BD Biosciences, San Jose, CA, San Diego, CA). The samples were analyzed on a FACSCalibur or LSRFortessa X-20 Cell Analyzer (BD Biosciences). Data analysis was done using FlowJo software (Ashland, OR).
2.8. T cell proliferation assay
Murine CD3+ T cells were purified from C57BL/6 mice by an immunomagnetic system (Miltenyi, Auburn, CA), and the purity of the cells was usually >95%. T cells were stimulated with anti-CD3 antibody (Biolegend) in the presence of BTNL2-Ig or control Ig. Proliferative response was assessed by pulsing the culture with 1 µCi of [3H] thymidine (PerkinElmer, Inc., Downers Grove, IL) 12 hours before harvest. Incorporation of [3H] thymidine was measured by liquid scintillation spectroscopy (PerkinElmer, Inc.).
2.9. ELISA
The concentration of cytokines IFNγ, TNFα, and IL-17A was determined by the respective ELISA Kit (Biolegend) according to the manufacturer’s instructions.
3.0. Statistical analysis
P-values were based on the two-sided Student’s t test. A confidence level above 95% (p<0.05) was determined to be significant.
4. Results
4.1. Production and characterization of rBTNL2-Ig fusion protein
We cloned the extracellular domain of mouse BTNL2 to an expression vector that contains the constant region of mouse IgG2a. The vector was then transfected into HEK-293 cells to generate rBTNL2-Ig fusion protein. We found that this system expressed rBTNL2-Ig protein at a high level. This protein was the dominant band in SDS-PAGE for the supernatant before purification. We purified rBTNL2-Ig protein from the supernatant using Protein G. As shown in Figure 1A, a relatively high purity of rBTNL2-Ig protein was obtained, as determined by Coomassie blue-stained SDS-PAGE (lane 2). The identity of the fusion protein was verified by Western blot using an anti-mouse IgG2a antibody (lane 3). The actual molecular weight (MW) of the rBTNL2-Ig protein was higher than the predicted MW, suggesting that the recombinant protein was glycosylated. According to the NetNGlyc 1.0 server prediction, BTNL2 has 3 potential N-glycosylated sites. The endotoxin level was less than 0.01 EU/ml of 1 µg of purified rBTNL2-Ig. In vitro assays show that rBTNL2-Ig protein inhibits anti-CD3-induced T cell proliferation in a dose-responsive manner (Figure 1B), consistent with the previous reports [17, 18].
Figure 1.
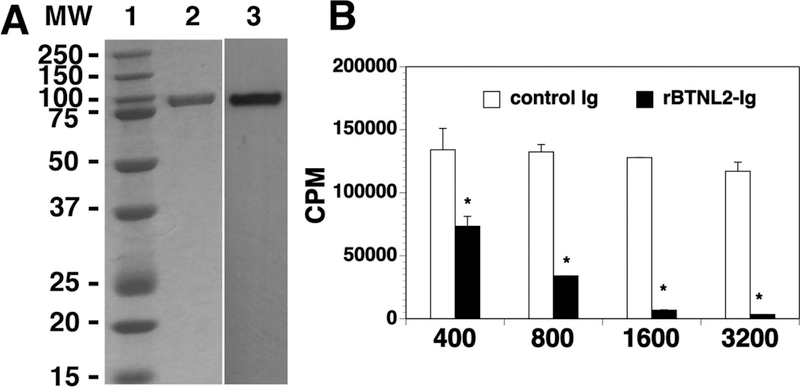
Characterization of purified rBTNL2-Ig protein. (A) Gel and blot show purified rBTNL2-Ig protein; Lane 1: MW markers; 2: Coomassie blue-stained SDS-PAGE; 3: Western blot with an anti-mouse IgG2a antibody. (B) rBTNL2-Ig protein inhibits T cell proliferation in vitro. T cells were purified from splenocytes of C57BL/6 mice by magnetic separation. The cells were cultured on plates pre-coated with anti-CD3 antibody (1 µg/ml) and indicated doses (ng/ml) of rBTNL2-Ig or control Ig for 3 days. [3H] thymidine (1 µCi/well) was added to the cultures 12 hours before harvest. T cell proliferation was measured by [3H] thymidine incorporation. Results are expressed as counts per minute (CPM). The data are expressed as mean ± SD and representative of 3 independent experiments. * P<0.05 compared with control Ig.
4.2. rBTNL2-Ig treatment ameliorates GVHD
To determine whether BTNL2 treatment affects GVHD, we used a well-defined MHC-mismatched HSCT model [C57BL/6 (H2b) → BALB/c (H2d)] [25]. GVHD in this model is mediated by both donor CD4 and CD8 T cells [25]. BALB/c mice were lethally irradiated and injected i.v. with BM and splenic cells from allogeneic C57BL/6 mice. The recipient mice were then treated with rBTNL2-Ig or control Ig. As shown in Figure 2A-C, control Ig-treated recipients revealed gradual body weight loss and all had succumbed by day 35 after HSCT (Figure 2A-C). In contrast, rBTNL2-Ig reduced the mortality and morbidity of GVHD with 50% of the mice still surviving at day 90 post HSCT (Figure 2A-C). GVHD severity was confirmed with pathologic analysis, showing that pathology scores of the liver, lung and small intestine (SI) in rBTNL2-Ig-treated recipients were significantly lower than those in control Ig-treated recipients (Figure 2D, E). It is worth noting that there were not significant differences in the survival, weight change, and clinical GVHD scores between control Ig-treated recipients and untreated recipients (data not shown). The results suggest that in vivo administration of rBTNL2-Ig ameliorates GVHD in mice.
Figure 2.
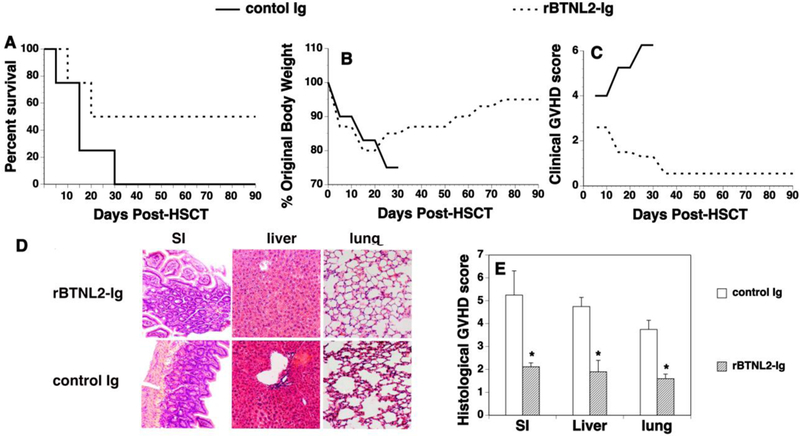
rBTNL2-Ig ameliorates GVHD. Lethally irradiated BALB/c recipients were injected with 5X106 BM and 2.5X106 spleen cells from C57BL/6 mice, as well as 50 μg rBTNL2-Ig or control Ig at day 0. The recipients were then injected i.p. with 50 μg rBTNL2-Ig or control Ig at 3-day intervals for 30 days. (A-C) Recipients were monitored for (A) survival (A Kaplan- Meier survival curve is shown), (B) weight change, and (C) clinical GVHD. (D, E) In separate experiments, recipients given 50 μg rBTNL2-Ig or control Ig at 3-day intervals from days 0–12 were euthanized 2 weeks after HSCT. The SI, liver and lung were analyzed for histologic damage. (D) Representative photomicrographs (the magnification was X200), and (E) mean ± SD of histopathology scores. Pooled data from 3 separate experiments are represented; with 4–5 mice per group in each experiment. * P<0.05 compared with control Ig-treated mice.
4.3. rBTNL2-Ig inhibits T cell proliferation and activation in vivo
We then investigated the mechanisms by which rBTNL2-Ig ameliorates GVHD. Since the severity of GVHD is associated with the proliferation, survival and alloactivation of donor T cells [6, 26–28], we first analyzed the effect of BTNL2 on T-cell proliferation in vivo. Splenocytes from C57BL/6 mice were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), and adoptively transferred into lethally irradiated BALB/c recipients that were also injected i.v. at day 0 and i.p. at day 2 with 50 µg rBTNL2-Ig or control Ig protein. The recipients were analyzed for the proliferation of donor T cells in the spleen at day 4. CFSE dilution analysis revealed that rBTNL2-Ig decreased the percentage of CFSElo proliferating CD4+ and CD8+ T cells (Figure 3A, B), indicating that rBTNL2-Ig inhibits the proliferation of the donor T cells. We then analyzed Annexin V+ 7-ADD-apoptotic donor CD4+ and CD8+ T cells, and found that rBTNL2-Ig treatment did not significantly affect T cell survival, as compared to control Ig treatment (data not shown).
Figure 3.
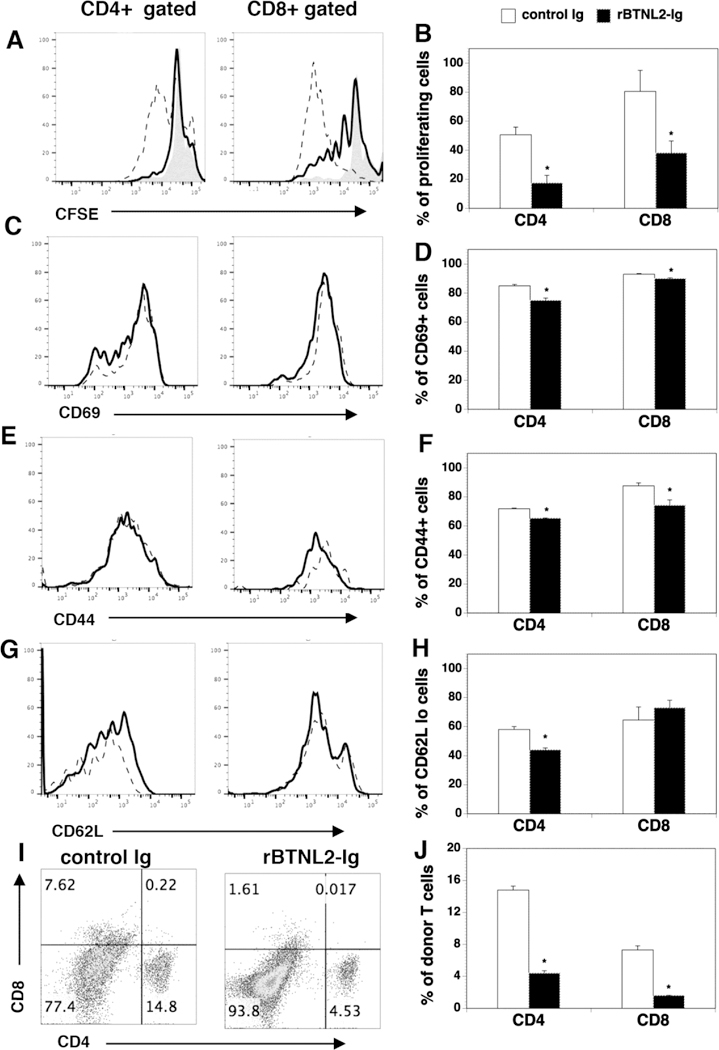
rBTNL2-Ig inhibits T-cell proliferation and activation in response to alloantigens in mice. (A-H) Lethally irradiated BALB/c mice were injected i.v. with 5X106 BM cells and 10X106 splenic cells labelled (A, B) with or (C-H) without CFSE from C57BL/6 mice. The recipients were injected i.v. at day 0 and i.p. at day 2 with 50 μg rBTNL2-Ig or control Ig. At Day 4 post-transplant, the percentage of (A, B) CFSElo (shaded histograms: splenic cells labelled with CFSE before injection into mice), (C, D) CD69+, (E, F) CD44hi, and (G, H) CD62Llo cells in donor CD4 and CD8 T cells (H-2Kb+CD4+, or H-2Kb+CD8+) in the spleen were examined by flow cytometry. Dot lines: control Ig; solid lines: rBTNL2-Ig. (I, J) Lethally irradiated BALB/c mice were injected with BM and splenic cells, and then given 50 μg rBTNL2-Ig or control Ig as in Figure 2D. The percentages of donor CD4+ and CD8+ T cells were analyzed 2 weeks after HSCT. (A, C, E, G, I) Representative flow cytometric profiles and (B, D, F, H, J) mean ± SD from one of three independent experiments with similar results. * P<0.05 compared with control Ig group.
We next examined the expression of activation markers by CD4+ and CD8+ T cells. CD69 is an activation marker. We observed that rBTNL2-Ig decreased the percentages of CD69+ in donor CD4+ and CD8+ T cells (Figure 3C, D). Since naïve T cells are CD44loCD62Lhi, whereas effective memory T cells are CD44hiCD62Llo, we analyzed the percentages of CD44hi and CD62Llo. As shown in Figure 3E-H, rBTNL2-Ig treatment decreased the percentages of CD44hi and CD62Llo cells in CD4+ and CD8+ T cells, although the percentages of CD62Llo cells in CD8+ T cells were not significantly different between control Ig- and rBTNL2-Ig-treated groups. Collectively, our data suggest that rBNTL2-Ig inhibits the proliferation and activation of donor T cells in response to alloantigens in mice.
In order to determine whether reduced T cell proliferation results in decreased donor T cells in rBTNL2-Ig-treated mice, we analyzed the percentages of donor T cells in the allogeneic recipients 14 days after HSCT. At this time point, almost no BM-derived donor T cells were generated. Therefore, the injected T cells could be measured exclusively [20]. As shown in Figure 3I and J, the percentages of CD4+ and CD8+ donor T cells in the spleen of rBTNL2-Ig-treated mice was significantly lower than that in control-treated mice. The results suggest that the reduced T cell expansion results in reduced donor T cells in rBTNL2-Ig-treated mice.
4.4. rBTNL2-Ig reduces Th1 and Th17 cytokine production in vivo
Inflammatory T cells such as Th1/Th17 cells are thought to play a critical role in the development of GVHD. We measured Th1/Th17 cytokine production in the serum of rBTNL2-Ig or control Ig-treated GVHD recipients. We found that the production of Th1 cytokines IFNγ and TNFα, as well as Th17 cytokine IL-17A was significantly reduced in rBTNL2-Ig-treated mice (Figure 4A).
Figure 4. rBTNL2-Ig reduces Th1 and Th17 cytokine production in vivo.
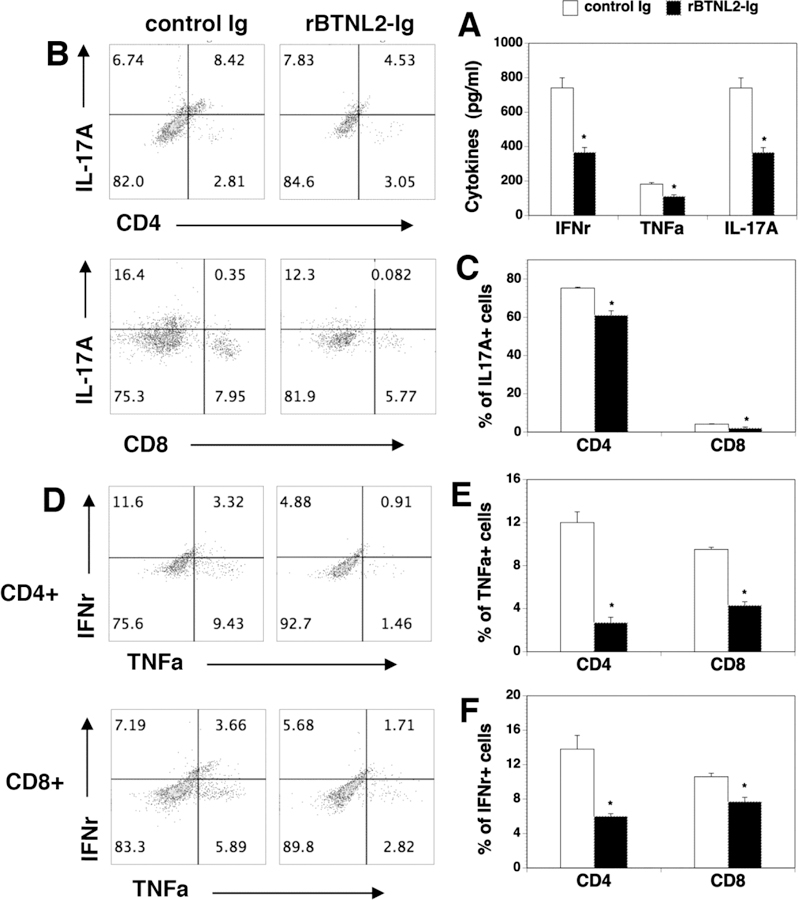
(A) Serum cytokine profile in control- or BTN-treated recipients. Lethally irradiated BALB/c mice were injected with BM and splenic cells, and then injected with 50 μg rBTNL2-Ig or control Ig as in Figure 2D. Serum was harvested at day 14 after HSCT and indicated cytokines were measured by ELISA. (B-F) Lethally irradiated BALB/c recipients were injected i.v. with 5X106 BM and 2.5X106 spleen cells from C57BL/6 mice. The recipients were then injected i.p. with 50 μg rBTNL2-Ig or control Ig at 3-day intervals for 27 days. At Day 30 after BMT, intracellular cytokine profiles of splenic CD4+ and CD8+ T cells were analyzed. (B, D) Representative flow cytometry profiles, (C, E, F) statistical analysis showing the percentages of IL-17A-, TNFα- and IFNγ-producing CD4+ or CD8+ T cells. * P<0.05 compared with control Ig group.
We also analyzed intracellular cytokine profiles in CD4 and CD8 T cells from rBTNL2-Ig and control Ig-treated GVHD recipients. Similar to the serum results, significantly lower percentages of IFNγ-, TNFα-, and IL-17A-producing CD4 and CD8 T cells were observed in rBTNL2-Ig-treated mice, as compared to that in control Ig-treated mice (Figure 4B-F). Collectively, the results suggest that rBTNL2-Ig inhibits Th1 and Th17 cytokine production in the GVHD mice.
4.5. rBTNL2-Ig-treated mice have an increased percentage of Treg cells
Studies have shown that Tregs also play an important role in the prevention of GVHD [29–36]. It has been reported that BTNL2 can induce the development of Foxp3-expressing T cells with a regulatory cell phenotype and function in vitro [37]. We thus analyzed Tregs in rBTNL2-Ig or control Ig-treated GVHD recipients. As shown in Figure 5, rBTNL2-Ig treatment resulted in a significantly higher percentage of Tregs in the spleen.
Figure 5. rBTNL2-Ig treatment increases the percentage of Tregs in GVHD recipients.
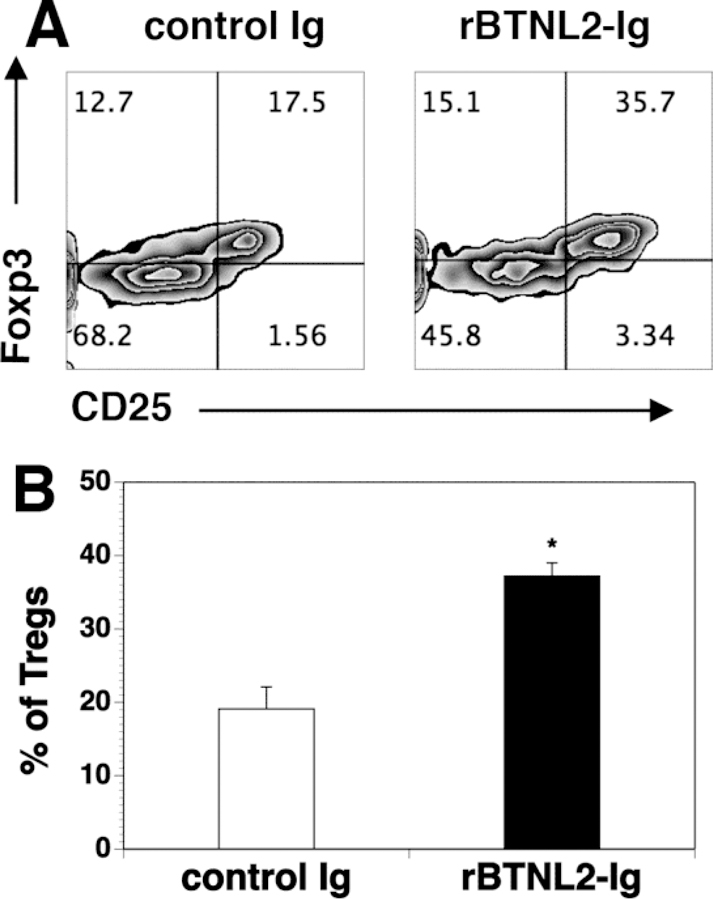
Lethally irradiated BALB/c recipients were injected i.v. with 5X106 BM and 2.5X106 spleen cells from C57BL/6 mice. The recipients were treated with 50 μg rBTNL2-Ig or control Ig at 3- day intervals from days 0–12 as in Figure 2D. Fourteen days after BMT, the spleens were harvested and analyzed for CD4+CD25+Foxp3+ Tregs. (A) Flow cytometry files showing the expression of CD25 and Foxp3 in gated donor CD4+ cells; (B) Mean ± SD for the percentage of Tregs from one of three independent experiments with similar results. * P<0.05 compared with control Ig group.
5. Discussion
We show here that in vivo administration of rBTNL2-Ig attenuates GVHD in mice. This is related to the ability of rBTNL2-Ig to inhibit T cell proliferation, activation and Th1/Th17 cytokine production, and to enhance the generation of Tregs in vivo. To the best of our knowledge, this is the first report that rBTNL2-Ig fusion protein is able to inhibit T cell responses and GVHD in vivo. The results are consistent with our in vitro data and those from others that rBTNL2-Ig inhibits the proliferation and cytokine production of effective T cells, and enhances the generation of Tregs in vitro [17, 18, 37].
The B7 family members typically contain IgV and IgC domains in the extracellular portion. BTNL2 shares sequence and structural similarity with B7 family members. The extracellular region of BTNL2 contains two IgV-IgC pairs (IgVa-IgCa and IgVb-IgCb) [17, 18]. Human and mouse BTNL2 share 63% identity in amino acid sequence. Although human BTNL2 has an isoform that lacks the IgCa domain [17, 38], it is likely that human and mouse BTNL2 proteins function similarly, because in the B7 family it is the IgV domain that mediates receptor binding [39]. BTN molecules typically contain an intracellular B30.2 domain, whereas B7 molecules do not. BTNL2 does not have the B30.2 domain, suggesting that BTNL2 is most similar to B7 molecules. The lack of the B30.2 domain also suggest that BTNL2 may not be capable of signaling itself; rather it may act via delivery of a signal into cells expressing its cognate receptor [40]. However, since BTNL2 has an unusual structure, it is not clear whether it represents a bona fide gene or pseudogene in humans.
The BTNL2 mutation has been associated with inflammatory autoimmune diseases [38, 41, 42]. For example, the sarcoidosis-associated polymorphism rs 2076530 has over-activated T cells and overt inflammation that are caused by a G–A transition in BTNL2 resulting in the loss of its inhibitory function [38]. Studies have also linked BTNL2 polymorphism to increased risk of ulcerative colitis [43–45], tuberculosis [46], rheumatoid arthritis, and systemic lupus erythematosus [47]. It has been reported that BTNL2 is highly expressed in lymphoid tissues including the lymph nodes, spleen and thymus, as well as in immune cells, such as B cells, T cells, and macrophages [17, 18]. BTNL2 is also expressed in some of the GVHD target organs, such as intestine and lung [17, 18, 48]. In addition, BTNL2 expression was upregulated in inflamed colon [17]. Therefore, endogenous BTNL2 may play a role in the prevention of autoimmune disease in physical conditions, but may not be sufficient to prevent GVHD and autoimmune disease in pathological conditions.
The BTNL2 putative receptor is expressed on activated T and B cells [17, 18]. Although the nature of the receptor remains unknown at this stage, it is distinct from CD28, CTLA4, ICOS, BTLA and PD-1 [17, 18]. Like other T cell inhibitory molecules, BTNL2 inhibits proximal TCR signaling events [18], suggesting that BTNL2 may bind to an ITIM-containing receptor [40]. Our results and those from others show that BTNL2 inhibits the proliferation of purified T cells, suggesting that BTNL2 directly acts on T cells. Although the putative BTNL2 receptor is also expressed on B cells, it does not affect B cell proliferation [17, 18]. It remains to be determined whether BTNL2 also indirectly acts on T cells by affecting antigen presenting cells, such as dendritic cells.
In addition to the inhibition of the proliferation, activation and cytokine production of effective T cells, the effect of BTNL2 on Tregs may also play a role in ameliorating GVHD. We have shown that rBTNL2-Ig treatment significantly increased the generation of Tregs in GVHD mice. This is consistent with the results that BTNL2 induces the development of Tregs in vitro [37]. It has also been shown that the BTNL2-induced Tregs are similar to thymus-derived Tregs [37]. It remains to be determined whether the increased production of Tregs in BTNL2-Ig-treated mice is caused by enhanced development of naturally Tregs in the thymus and/or induced Tregs in the periphery.
In summary, we have demonstrated that in vivo administration of rBTNL2-Ig reduces T cell immune responses and ameliorates GVHD in vivo. Our results suggest that rBTNL2-Ig may offer a new tool to prevent and treat GVHD in patients. In addition, targeting the BTNL2 pathway may represent a new strategy to modulate T cell-mediated immunity for the treatment of autoimmune disease and cancer.
Highlights.
In vivo administration of rBTNL2-Ig protein ameliorates GVHD in mice
rBTNL2-Ig protein inhibits T cell proliferation, activation and Th1/Th17 cytokine production in vivo
rBTNL2-Ig protein treatment increases the generation of regulatory T cells
Acknowledgements:
This work was supported by grants from NIH (1R01AI123131-01, to LL), Connecticut Regenerative Medicine Research Fund (16-RMB-UCONN-02, to LL), and a project supported from Science and Technology Department of Liaoning Province, China (grant no. 201602816, to CC).
Abbreviations:
- HSCT
hematopoietic stem cell transplantation
- GVHD
graft-versus-host disease
- BTNL2
Butyrophilin-like 2
- rBTNL2-Ig
recombinant BTNL2-IgG2a Fc
- BTN
Butyrophilin
- BTNL
BTN-like
- Tregs
regulatory T cells
- SI
small intestine
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- LAL
Limulus Amebocyte Lysate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of conflicting interests: The Authors declare that there is no conflict of interest.
References
- [1].Shlomchik WD, Graft-versus-host disease, Nat. Rev. Immunol, 7 (2007) 340–352. [DOI] [PubMed] [Google Scholar]
- [2].Ferrara JL, Levine JE, Reddy P, Holler E, Graft-versus-host disease, Lancet, 373 (2009) 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Welniak LA, Blazar BR, Murphy WJ, Immunobiology of allogeneic hematopoietic stem cell transplantation, Annu. Rev. Immunol, 25 (2007) 139–170. [DOI] [PubMed] [Google Scholar]
- [4].Coghill JM, Sarantopoulos S, Moran TP, Murphy WJ, Blazar BR, Serody JS, Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new, Blood, 117 (2011) 3268–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Warren EH, Deeg HJ, Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies, Tissue Antigens, 81 (2013) 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liang Y, Liu C, Djeu JY, Zhong B, Peters T, Scharffetter-Kochanek K, Anasetti C, Yu XZ, Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects, Blood, 111 (2008) 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Radojcic V, Pletneva MA, Yen HR, Ivcevic S, Panoskaltsis-Mortari A, Gilliam AC, Drake CG, Blazar BR, Luznik L, STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model, Journal of immunology (Baltimore, Md. : 1950), 184 (2010) 764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D, Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease, Blood, 114 (2009) 3101–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abeler-Dorner L, Swamy M, Williams G, Hayday AC, Bas A, Butyrophilins: an emerging family of immune regulators, Trends Immunol, 33 (2012) 34–41. [DOI] [PubMed] [Google Scholar]
- [10].Afrache H, Gouret P, Ainouche S, Pontarotti P, Olive D, The butyrophilin (BTN) gene family: from milk fat to the regulation of the immune response, Immunogenetics, 64 (2012) 781–794. [DOI] [PubMed] [Google Scholar]
- [11].Arnett HA, Viney JL, Immune modulation by butyrophilins, Nat. Rev. Immunol, 14 (2014) 559–569. [DOI] [PubMed] [Google Scholar]
- [12].Guo Y, Wang AY, Novel Immune Check-Point Regulators in Tolerance Maintenance, Front Immunol, 6 (2015) 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rhodes DA, Reith W, Trowsdale J, Regulation of Immunity by Butyrophilins, Annu. Rev. Immunol, 34 (2016) 151–172. [DOI] [PubMed] [Google Scholar]
- [14].Linsley PS, Peach R, Gladstone P, Bajorath J, Extending the B7 (CD80) gene family, Protein Sci, 3 (1994) 1341–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang Y, Liu XK, Nguyen T, Bishop C, Graf D, Dong C, Characterization of B7S3 as a novel negative regulator of T cells, Journal of immunology (Baltimore, Md. : 1950), 178 (2007) 3661–3667. [DOI] [PubMed] [Google Scholar]
- [16].Compte E, Pontarotti P, Collette Y, Lopez M, Olive D, Frontline: Characterization of BT3 molecules belonging to the B7 family expressed on immune cells, European journal of immunology, 34 (2004) 2089–2099. [DOI] [PubMed] [Google Scholar]
- [17].Arnett HA, Escobar SS, Gonzalez-Suarez E, Budelsky AL, Steffen LA, Boiani N, Zhang M, Siu G,Brewer AW, Viney JL, BTNL2, a butyrophilin/B7-like molecule, is a negative costimulatory molecule modulated in intestinal inflammation, J. Immunol, 178 (2007) 1523–1533. [DOI] [PubMed] [Google Scholar]
- [18].Nguyen T, Liu XK, Zhang Y, Dong C, BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation, J. Immunol, 176 (2006) 7354–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Song Y, Liu Y, Hu R, Su M, Rood D, Lai L, In vivo antitumor Activity of a Recombinant IL-7/IL-15 Hybrid Cytokine in Mice, Mol. Cancer Ther, (2016). [DOI] [PubMed]
- [20].Hu R, Liu Y, Song Y, Su M, Lu X, Rood D, Lai L, Recombinant IL-7/HGFbeta hybrid cytokine separates acute graft-versus-host-disease from graft-versus-tumour activity by altering donor T cell trafficking, Br. J. Haematol, 175 (2016) 505–516. [DOI] [PubMed] [Google Scholar]
- [21].Jin J, Goldschneider I, Lai L, In vivo administration of the recombinant IL-7/hepatocyte growth factor beta hybrid cytokine efficiently restores thymopoiesis and naive T cell generation in lethally irradiated mice after syngeneic bone marrow transplantation, Journal of immunology (Baltimore, Md. : 1950), 186 (2011) 1915–1922. [DOI] [PubMed] [Google Scholar]
- [22].Lai L, Zhang M, Goldschneider I, Recombinant IL-7/HGFbeta efficiently induces transplantable murine hematopoietic stem cells, J. Clin. Invest, 122 (2012) 3552–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lai L, Zhang M, Song Y, Rood D, Recombinant IL-7/HGFbeta Hybrid Cytokine Enhances T Cell Recovery in Mice Following Allogeneic Bone Marrow Transplantation, PloS one, 8 (2013) e82998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Song Y, Su M, Panchatsharam P, Rood D, Lai L, c-Met signalling is required for efficient postnatal thymic regeneration and repair, Immunology, 144 (2015) 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schroeder MA, DiPersio JF, Mouse models of graft-versus-host disease: advances and limitations, Disease models & mechanisms, 4 (2011) 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu SX, Holland AM, Na IK, Terwey TH, Alpdogan O, Bautista JL, Smith OM, Suh D, King C, Kochman A, Hubbard VM, Rao UK, Yim N, Liu C, Laga AC, Murphy G, Jenq RR, Zakrzewski JL, Penack O, Dykstra L, Bampoe K, Perez L, Furie B, Furie B, van den Brink MR, Absence of P-selectin in recipients of allogeneic bone marrow transplantation ameliorates experimental graft-versus-host disease, Journal of immunology (Baltimore, Md. : 1950), 185 (2010) 1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu SX, Kappel LW, Charbonneau-Allard AM, Atallah R, Holland AM, Turbide C, Hubbard VM, Rotolo JA, Smith M, Suh D, King C, Rao UK, Yim N, Bautista JL, Jenq RR, Penack O, Na IK, Liu C, Murphy G, Alpdogan O, Blumberg RS, Macian F, Holmes KV, Beauchemin N, van den Brink MR, Ceacam1 separates graft-versus-host-disease from graft-versus-tumor activity after experimental allogeneic bone marrow transplantation, PloS one, 6 (2011) e21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Waldman E, Lu SX, Hubbard VM, Kochman AA, Eng JM, Terwey TH, Muriglan SJ, Kim TD, Heller G, Murphy GF, Liu C, Alpdogan O, van den Brink MR, Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine, Blood, 107 (2006) 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Coghill JM, Carlson MJ, Moran TP, Serody JS, The biology and therapeutic potential of natural regulatory T-cells in the bone marrow transplant setting, Leuk. Lymphoma, 49 (2008) 1860–1869. [DOI] [PubMed] [Google Scholar]
- [30].Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL, CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease, J. Exp. Med, 196 (2002) 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS, CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation, Nat. Med, 9 (2003) 1144–1150. [DOI] [PubMed] [Google Scholar]
- [32].Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S, Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation, J. Exp. Med, 196 (2002) 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Semple K, Yu Y, Wang D, Anasetti C, Yu XZ, Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice, Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation, 17 (2011) 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas PJ, Gress RE, Levine BL, June CH, Serody JS, Blazar BR, L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection, Blood, 104 (2004) 3804–3812. [DOI] [PubMed] [Google Scholar]
- [35].Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon BL, Cohen JL, Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia, J. Clin. Invest, 112 (2003) 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D, In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease, Blood, 112 (2008) 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Swanson RM, Gavin MA, Escobar SS, Rottman JB, Lipsky BP, Dube S, Li L, Bigler J, Wolfson M, Arnett HA, Viney JL, Butyrophilin-like 2 modulates B7 costimulation to induce Foxp3 expression and regulatory T cell development in mature T cells, J. Immunol, 190 (2013) 2027–2035. [DOI] [PubMed] [Google Scholar]
- [38].Valentonyte R, Hampe J, Huse K, Rosenstiel P, Albrecht M, Stenzel A, Nagy M, Gaede KI, Franke A, Haesler R, Koch A, Lengauer T, Seegert D, Reiling N, Ehlers S, Schwinger E, Platzer M, Krawczak M, Muller-Quernheim J, Schurmann M, Schreiber S, Sarcoidosis is associated with a truncating splice site mutation in BTNL2, Nat. Genet, 37 (2005) 357–364. [DOI] [PubMed] [Google Scholar]
- [39].Zhang X, Schwartz JC, Almo SC, Nathenson SG, Crystal structure of the receptor-binding domain of human B7–2: insights into organization and signaling, Proceedings of the National Academy of Sciences of the United States of America, 100 (2003) 2586–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arnett HA, Escobar SS, Viney JL, Regulation of costimulation in the era of butyrophilins, Cytokine, 46 (2009) 370–375. [DOI] [PubMed] [Google Scholar]
- [41].Rybicki BA, Walewski JL, Maliarik MJ, Kian H, Iannuzzi MC, The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites, Am. J. Hum. Genet, 77 (2005) 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Price P, Santoso L, Mastaglia F, Garlepp M, Kok CC, Allcock R, Laing N, Two major histocompatibility complex haplotypes influence susceptibility to sporadic inclusion body myositis: critical evaluation of an association with HLA-DR3, Tissue Antigens, 64 (2004) 575–580. [DOI] [PubMed] [Google Scholar]
- [43].Juyal G, Prasad P, Senapati S, Midha V, Sood A, Amre D, Juyal RC, Bk T, An investigation of genome-wide studies reported susceptibility loci for ulcerative colitis shows limited replication in north Indians, PloS one, 6 (2011) e16565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mochida A, Kinouchi Y, Negoro K, Takahashi S, Takagi S, Nomura E, Kakuta Y, Tosa M, Shimosegawa T, Butyrophilin-like 2 gene is associated with ulcerative colitis in the Japanese under strong linkage disequilibrium with HLA-DRB1*1502, Tissue Antigens, 70 (2007) 128–135. [DOI] [PubMed] [Google Scholar]
- [45].Pathan S, Gowdy RE, Cooney R, Beckly JB, Hancock L, Guo C, Barrett JC, Morris A, Jewell DP, Confirmation of the novel association at the BTNL2 locus with ulcerative colitis, Tissue Antigens, 74 (2009) 322–329. [DOI] [PubMed] [Google Scholar]
- [46].Moller M, Kwiatkowski R, Nebel A, van Helden PD, Hoal EG, Schreiber S, Allelic variation in BTNL2 and susceptibility to tuberculosis in a South African population, Microbes Infect, 9 (2007) 522–528. [DOI] [PubMed] [Google Scholar]
- [47].Orozco G, Eerligh P, Sanchez E, Zhernakova S, Roep BO, Gonzalez-Gay MA, Lopez-Nevot MA, Callejas JL, Hidalgo C, Pascual-Salcedo D, Balsa A, Gonzalez-Escribano MF, Koeleman BP, Martin J, Analysis of a functional BTNL2 polymorphism in type 1 diabetes, rheumatoid arthritis, and systemic lupus erythematosus, Hum. Immunol, 66 (2005) 1235–1241. [DOI] [PubMed] [Google Scholar]
- [48].Stammers M, Rowen L, Rhodes D, Trowsdale J, Beck S, BTL-II: a polymorphic locus with homology to the butyrophilin gene family, located at the border of the major histocompatibility complex class II and class III regions in human and mouse, Immunogenetics, 51 (2000) 373–382. [DOI] [PubMed] [Google Scholar]


