Abstract
This study aims to evaluate the safety, acceptability, and pharmacokinetics (PK) of an increased dose of nelfinavir during the third trimester of pregnancy. The study was registered as part of the International Maternal Pediatric Adolescent AIDS Clinical Trials network (IMPAACT-P1026s), an ongoing multi-center prospective cohort study of antiretroviral pharmacokinetics (PK) during pregnancy (NCT00042289). Nelfinavir intensive PK evaluations were performed at steady state during the third trimester of pregnancy and 2–3 weeks postpartum. Plasma concentrations of nelfinavir and its active metabolite, hydroxyl-tert-butylamide (M8) were measured using high-performance liquid chromatography (HPLC) with ultraviolet detection. A total of 18 women are included in the analysis. Nelfinavir AUC with the increased dose during the third trimester was nearly identical to standard dose postpartum, with a geometric mean ratio for third trimester to postpartum AUC of 0.98 (90% CI 0.71–1.35). Despite the increased dose, M8 AUC was lower during the third trimester compared to postpartum (0.53, IQR [0.38–0.75]), as was the M8/NFV AUC ratios (0.51, IQR [0.42–0.63]). NFV AUC0–12 was above target in 15 of 18 (83%) of participants during the third trimester compared to 14 of 16 (88%) postpartum. No major safety concerns were noted. Increasing the nelfinavir dose to 1875 mg twice daily during the third trimester achieved similar concentrations postpartum compared to standard dosing (1250mg twice daily). Increased dose nelfinavir regimens may still have some benefit to HIV positive pregnant women living in countries where novel protease inhibitors are currently unavailable, or in individuals who are intolerant to ritonavir-boosted HIV medications.
Keywords: Nelfinavir, pregnancy, hydroxyl-tert-butylamide (M8), postpartum
INTRODUCTION:
Nelfinavir mesylate (NFV) is a protease inhibitor (PI) with moderately potent activity against HIV.1–3 While nelfinavir is no longer recommended for use in the US for prevention of mother to child transmission of HIV, it may still be of benefit to HIV positive individuals living in low-resource countries where novel protease inhibitors are currently unavailable, or in individuals who are intolerant to ritonavir-boosted HIV medications.4,5,6,7,8 Nelfinavir has also been shown to have in vitro efficacy against a wide range of malignancies by causing apoptosis and non-apoptotic cell death, and is under clinical investigation as a cancer therapeutic agent in humans.9–11
Pregnant women living with HIV receive antiretrovirals for their own health and to prevent HIV transmission to their infants.6 Physiologic changes associated with pregnancy may have a large impact on drug disposition, with effects on drug absorption, distribution, metabolism and elimination of antiretrovirals. Nelfinavir is metabolized by CYP2C19 to hydroxyl-tert-butylamide (M8), which has similar potency against HIV as nelfinavir. Both nelfinavir and M8 are both further metabolized by CYP3A4 and CYP 2D6 to less active moieties.2,3,12 Activity of CYP2C19 has been shown to decrease in pregnancy while activity of CYP3A4 and CYP2D6 increase. 12–16,17,18
In a previous study, use of the standard adult nelfinavir dose of 1250 mg twice daily was associated with a 30% reduction in nelfinavir and a 75% reduction in M8 plasma concentrations.19 The goal of the current study was to evaluate nelfinavir and M8 exposures with use of an increased dose during the third trimester.
METHODS:
The study protocol, the informed consent documents, and all subsequent modifications were reviewed and approved by the local institutional review board (IRB)/Ethics Committee responsible for oversight of the study at all 36 institutions/hospitals within the IMPAACT 1026s network (see supplemental table for a complete list of hospitals). The study followed all relevant human subject research guidelines. All participants provided signed informed consent before participation, and the study was registered in http://ClinicalTrials.gov [NCT00042289]. Data were then collected as part of International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1026s, an ongoing, multicenter, non-blinded, prospective Phase IV study of the pharmacokinetics and safety of selected ARVs in HIV infected pregnant women that included an arm for pregnant women at sites receiving nelfinavir (the nelfinavir arm of the study recruited only HIV infected women within the US).
Pregnant women living with HIV were eligible for enrollment if they were receiving nelfinavir as part of clinical care according to the following dosing schedule: 1250mg twice daily until 30 weeks of gestation, then 1875mg twice daily until 2–4 days postpartum, then 1250mg twice daily until 2–3 weeks postpartum. The samples were collected between May 12th 2009 and December 14th 2014. All antiretroviral medications were prescribed by primary care providers and dispensed by local pharmacies, as per the sites’ standard of care. Maternal exclusion criteria were current use of medications known to interfere with nelfinavir metabolism, including lopinavir/ritonavir, atorvastatin, ritonavir and atazanavir, history of hemophilia, liver disease, diabetes mellitus, hyperlipidemia, phenylketonuria, and other clinical or laboratory toxicity that, per site investigators, would require a change in the antiretroviral regimen. Mothers and their infants continued in the study until 6 months after delivery. Infant HIV status was evaluated at 6 months of life by physical examination and chart abstraction.
Clinical and laboratory monitoring
Maternal demographic and clinical information were extracted from the medical record, including maternal HIV-1 RNA, CD4+ lymphocyte count, maternal age, ethnicity, weight and concomitant medications. Background regimens were similar for all women throughout the evaluation period. Plasma HIV-1 RNA assays were performed locally. Study mothers and infants were followed for clinical and laboratory toxicities through six months after delivery. Neonatal gestational age at the time of delivery, birth weight and HIV infection status data were collected from the infant’s medical record. Physical examinations were performed on neonates after delivery, and infant laboratory evaluations were performed only as clinically indicated.
Sample collection and drug assays
Plasma NFV samples for intensive PK sampling was drawn pre-dose and at 1, 2, 4, 6, 8, and 12 hours post-dose. Samples were collected at 20–26 weeks gestation for 2nd trimester PK evaluation; at 30–36 weeks gestation for 3rd trimester PK evaluation and at 2–3 weeks for postpartum evaluation. Maternal and cord blood samples were collected at delivery and infant washout PK samples were collected at 2–10, 18–28, 36–72 hours after birth, and at 5–9 days of life.
Plasma nelfinavir and M8 concentrations were determined simultaneously by high-performance liquid chromatography (HPLC) with ultraviolet detection at the University of California, San Diego Pediatric Pharmacology Laboratory. Briefly, plasma proteins were precipitated using acetonitrile (ACN) and supernatant injected directly onto a LUNA C-18 reversed phase HPLC column (Phenomenex Inc., Torrance, CA, USA). Drugs were separated isocratically using a mobile phase consisting of 10mM potassium phosphate buffer pH 4.2: ACN (62:38 v/v). The flow rate was 1.2 mL/min and ultraviolet (UV) detection was at 206 nm. The detection limit for both NFV and M8 was 0.039 mg/mL. The mean inter and intra-assay coefficients of variation based on validation data (quality control samples were run at multiple different concentrations over the control range of 0.039–8.5 mg/mL). NPV was stable in plasma stored at −20°C. For NFV/M8 HPLC assays, the detection limit was 0.039 μg/mL. Concentrations below the detection limit were treated as half this limit for analysis.
Pharmacokinetic and statistical analysis:
Nelfinavir and M8 plasma concentrations were analyzed using standard descriptive statistics and are presented as medians with interquartile ranges. Areas under the concentration time curve (AUC) for plasma from pre-dose concentration (C0) to 12 hours post dose (AUC0–12) were estimated using the trapezoidal rule, with apparent clearance as dose/AUC0–12. Target AUC was 18.5 μg h/mL, which is the 10th percentile NFV AUC0–12 in non-pregnant historical controls. Within-participant comparisons (third trimester versus postpartum) was performed for continuous outcome measures using the Wilcoxon signed-rank test and for dichotomous outcome measures using the McNemar’s test. Between-participant comparisons was performed for continuous outcome measures using the Wilcoxon rank-sum test and for dichotomous outcome measures using the chi-square or Fisher exact test. 90% confidence limits for the geometric mean ratio of the PK exposure parameters were calculated to describe the range of values that were consistent with the observed data to assess whether there was a clinically significant difference in exposure.
The 90% confidence interval was used to match the usual practice in the pharmacokinetic literature. If the 90% CI is entirely outside the limits of (0.8, 1.25), the pharmacokinetic parameter was deemed different for the two time-points. If, on the other hand, the 90% CI is entirely within the limits (0.8, 1.25), the parameter is not different between the two time-points. If the 90% CI overlaps with (0.8, 1.25), these data alone do not support any conclusions regarding the pharmacokinetic parameter. Pairwise comparisons of plasma AUC and their ratio within each subject during the third trimester was compared to postpartum were performed using a two-sided Wilcoxon signed rank test with p < 0.01 considered statistically significant. Data analysis was done using WinNonlin (version 7.0; Pharsight Corporation, Mountain View, CA, USA).
RESULTS:
Plasma concentration data are available for 18 women in the third trimester and 16 postpartum. Maternal demographic and clinical characteristics of the participants, pregnancy and fetal outcomes are described in Table 1. The median age of the mothers participating in this study was 28.9 years (interquartile range [IQR] 19.8–39.6). Ten of 18 (56.0%) of the mothers were black, six of the mothers were Hispanic (33%), and two women (11%) were white (non-Hispanic). The mean maternal weight at the time of sampling in the 2nd, 3rd trimester and postpartum was 86 kg (IQR 79–100.9); 93 kg (48.5–173.0); and 93.2 kg (48.2–155.6) respectively. The mean gestational age at the time of sampling in the 2nd trimester was 26 weeks (IQR, 24 to 28 weeks), in the 3rd trimester was 34 weeks (IQR, 30 to 38 weeks), and median postpartum sampling time was 3 weeks after delivery (IQR, 2 to 4 weeks postpartum), Table 1.
Table 1:
Participant demographics – Table 1 describes the demographics of participants that were recruited into the NFV pharmacokinetic study.
| Age (years) | 28.9 (19.8–39.6) |
| Weight (kg) | |
| 2nd trimester | 86.0 (71.0–100.9) |
| 3rd trimester | 93.0 (48.5–173.0) |
| 3rd trimester | 93.2 (48.2–155.6) |
| Race/Ethnicity | |
| White, non-Hispanic | 2 (11%) |
| Black, non-Hispanic | 10 (56%) |
| Hispanic | 6 (33%) |
| Gestational age (weeks) | |
| 2nd trimester | 26.1 (24.4–27.9) |
| 3rd trimester | 34.7 (30.3–38.0) |
| Postpartum | 3.0 (2.0–4.0) |
| Timing of PK visit (weeks) | |
| 2nd trimester | 26.1 (24.4–27.9) |
| 3rd trimester | 34.5 (30.3–38.0) |
| Postpartum | 2.7 (1.9–7.3) |
| HIV-1 RNA (≤ 50 copies/mL ) | |
| 2nd trimester | 58.5 (48.0–69.0) |
| 3rd trimester | 48.0 (42.0–51.0) |
| Postpartum | 48.0 (43.0–52.0) |
| CD4+ cells (cells/mm3) | |
| 2nd trimester | 605.5 (521.0–690.0) |
| 3rd trimester | 505.5 (346.0–690.0) |
| Postpartum | 600.0 (346.0–709.0) |
| Infant outcomes | |
| Gestational age at delivery (weeks) | 39.1 (38.0–40.4) |
| Birth weight (grams) | 3165 (2910–3515) |
| Length (cm) | 49.0 (48.3–51.0) |
| Infant infection status* | |
| Confirmed uninfected | 11/17 (61%) |
| Indeterminate | 4/17 (22%) |
| Uninfected by best available data | 3/17 (17%) |
Interquartile ranges (IQR) are in brackets. 90% confidence intervals (CIs) were used for analysis.
One infant was still birth and did not have newborn form entered.
The median maternal plasma HIV-1 RNA was 58.5 (IQR, 48–69) during the second trimester, 48 (42–51) during the third trimester and 48 (IQR 43–52) postpartum. The median CD4 count (cells/mL) was 606 (IQR, 521–690) during the second trimester, 506 (346–690) during the third trimester and 600 (IQR 346–709) postpartum. The mean gestational age at delivery was 39.1 weeks (IQR, 38.0–40.4), with an average birth weight of 3165 grams (IQR, 2910–3515). One infant was still born. Eleven infants (61%) in the cohort were confirmed uninfected, four infants (22%) had indeterminate HIV testing results, and 3 infants (17%) were uninfected by the best available data.
Nelfinavir and M8 pharmacokinetic data are shown in Table 2 and Table 3 respectively. M8 AUC0–12 (geometric mean ratio 0.53 (IQR 0.38–0.75), p=0.03 was lower in the third trimester compared to postpartum, but this did not reach statistical significance (P<0.01), Table 3. M8 Cmax (geometric mean ratio 0.54 (IQR 0.40–0.73), p=0.005 and M8/NFV AUC0–12 ratio - 0.51 (IQR 0.42–0.63) were significantly lower during the third trimester compared to postpartum (P<0.01), Table 3. Nelfinavir Cl/F was higher during the third trimester compared to postpartum (geometric mean ratio 1.54 (IQR 1.12–2.11), p=0.04), Table 2. Nelfinavir + M8 drug exposure were similar during the third trimester of pregnancy compared to postpartum, with a geometric mean ratio of 0.93 (IQR, 0.68–1.26). Individual concentration–time curves of nelfinavir and M8 plasma concentrations during the third trimester of pregnancy and postpartum are shown in Figure 1. Median nelfinavir AUC0–12 was approximately 50% lower in the third trimester compared to postpartum (Figure 1b). However, the median molar sum of nelfinavir plus M8 plasma concentrations were similar during the third trimester vs. postpartum (Figure 1c). Nelfinavir area under the plasma concentration–time (AUCs) and Cl/F curves during pregnancy (third trimester) and postpartum are shown in Figure 2. Nelfinavir AUC0–12 in 15 of the 18 pregnant patients (83%) met AUC target (18.5 μg h/mL, indicated by dashed line) in 3rd trimester, while 14/16 (88%) met this target postpartum. For nelfinavir apparent clearance, there were 11 out of 18 pregnant patients who had increased Cl/F during 3rd trimester compared to the postpartum period.
Table 2:
NFV PK Comparison of 3rd Trimester vs. Postpartum (N=16) – Table 2 describes the pharmacokinetics of NFV during the 3rd trimester, compared to postpartum.
| Parameter | 3rd trimester: median (IQR) (n = 18) 1875mg BID |
Postpartum: median (IQR) (n=16) 1250mg BID |
Geometric mean ratio 3rd
trimester/ postpartum (90% CI) |
P-value* |
|---|---|---|---|---|
| NFV AUC0–12 μg-h/mL [μM-h] |
34.2 (27.2–46.9) [60.2 (47.9–82.6)] |
33.5 (28.6–43.5) [58.9 (50.3–76.6)] |
0.98 (0.71–1.35) |
0.78 |
| NFV Cmin (μg/mL) | 0.47 (0.35–1.33) | 0.52 (0.22–0.80) | 0.90 (0.71–1.16) | 0.49 |
| NFV Cmax (μg/mL) | 5.1 (4.3–6.5) | 5.0 (4.2–5.9) | 1.06 (0.85–1.34) | 0.67 |
| NFV Cl/F (L/h) | 54.9 (40.4–68.9) | 37.4 (28.7–43.7) | 1.54 (1.12–2.11) | 0.04 |
p-value for Wilcoxon rank-sum test; IQR = interquartile range, BID = twice daily, AUC0–12 = area under concentration vs time curve (0 to 12 hours post-dose), Cl/F = molar clearance, Cmin= minimum concentration, Cmax= maximum concentration, CI = confidence interval.
Table 3:
M8, M8/NFV and NFV+M8 PK Comparison of 3rd Trimester vs. Postpartum (N=16) – Table 3 describes the pharmacokinetics of M8, M8/NFV and NFV+M8 PK Comparison during the 3rd trimester, compared to postpartum.
|
Parameter |
3rd trimester: median (IQR) (n = 18) 1875mg BID |
Postpartum: median (IQR) (n=16) 1250mg BID |
Geometric mean ratio 3rd trimester/ postpartum (90% CI) |
P-value* |
|---|---|---|---|---|
| M8 AUC0–12
μg-h/mL [μM-h] |
3.9 (2.7–7.4) [6.7 (4.7–12.6)] |
8.6 (6.5–11.6) [14.8 (11.2–19.8)] |
0.53 (0.38–0.75) | 0.03 |
| M8 Cmin (μg/mL) | 0.11 (0.05–0.15) | 0.14 (0.04–0.21) | 0.78 (0.40–1.26) | 0.24 |
| M8 Cmax (μg/mL) | 0.66 (0.48–1.12) | 1.20 (1.07–1.71) | 0.54 (0.40–0.73) | 0.005 |
| M8/NFV AUC ratio | 0.11 (0.08–0.22) | 0.30 (0.17–0.35) | 0.51 (0.42–0.63) | 0.001 |
| NFV+M8 AUC0–12, μM-h |
71.6 (54.3–93.1) | 73.3 (66.3–91.7) | 0.93 (0.68–1.26) | 0.60 |
p-value for Wilcoxon rank-sum test; IQR = interquartile range, BID = twice daily, AUC0–12 = area under concentration vs time curve (0 to 12 hours post-dose), Cl/F = molar clearance, Cmin= minimum concentration, Cmax= maximum concentration, CI = confidence interval.
Figure 1 –
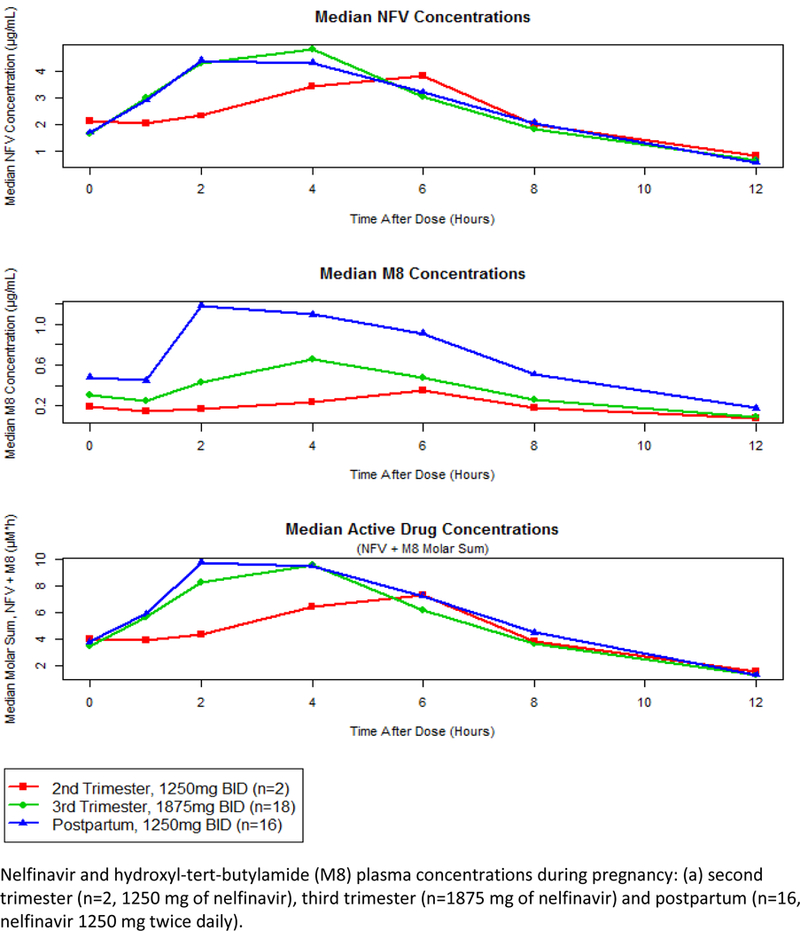
NFV and M8 curves, including the median AUC plasma concentration across the 2nd, 3rd trimester and postpartum.
Figure 2 –
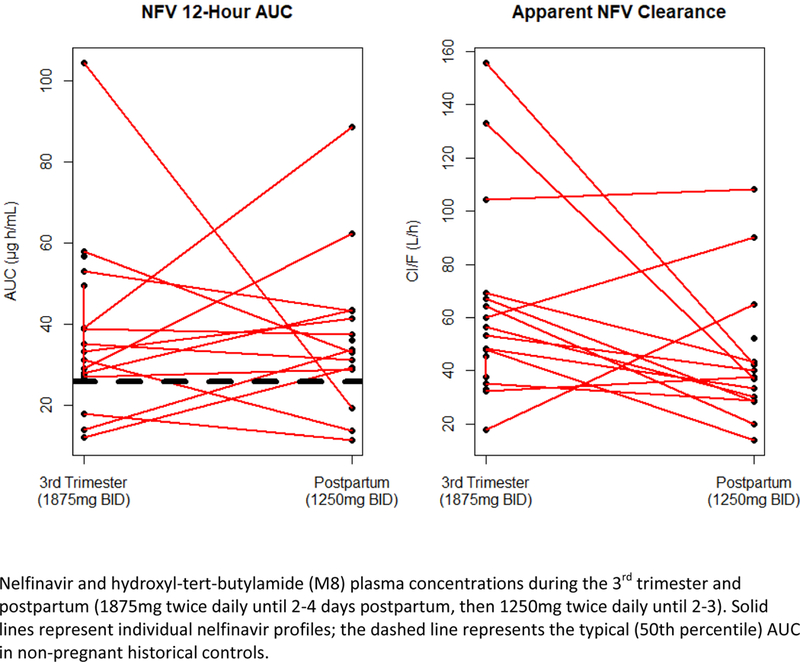
Nelfinavir area under the plasma concentration–time (AUCs) and Cl/F curves.
Umbilical cord blood nelfinavir concentrations were measured in 15 patients. NFV was detected in 10 out of 15 cord plasma samples, and in 13 out of 15 maternal plasma samples. Median umbilical cord/maternal ratio for nelfinavir in subjects with detectable maternal concentrations was 0.19. M8 was detectable in 10 maternal plasma samples but only in 3 umbilical cord samples.
All the 18 women enrolled in the cohort were on concomitant antiretrovirals in addition to nelfinavir: 16 participants were also on lamivudine/zidovudine, 1 participant was also on lamivudine/zidovudine/nevirapine, and 1 participant was also on abacavir/lamivudine/zidovudine. Of the 18 patients enrolled in the nelfinavir arm of P1026s, 5 (27.7%) experienced one or more grade 3–4 adverse events. However, none were determined to be treatment-related. No congenital anomalies identified by prenatal ultrasound or physical examination at the time of birth were determined to be treatment-related.
DISCUSSION:
Pregnancy impacts several drug metabolizing enzymes and drug exposure.20 Prior pharmacokinetic data from the IMPAACT P1026s protocol show that there are decreases in exposure with standard doses of CYP3A4 metabolized antiretrovirals. 21–23 These decreases in exposure can often be overcome with increased doses during pregnancy, as has been shown for lopinavir and atazanavir 23, which may be clinically important in protease-inhibitor-experienced pregnant women. 23–25 An exception is darunavir, where darunavir AUC and Cmax were substantially decreased in pregnancy with standard dosing, but increasing the dose from 600mg to 800mg daily had no effect on darunavir plasma concentration.26 This subsequently led to the practice of not recommending increased twice-daily darunavir dose during pregnancy for prevention of mother to child transmission of HIV. The decrease in darunavir exposure during pregnancy was not associated with an observed increase in mother-to-child transmission of HIV.27
If increased 3A4 activity was the only effect of pregnancy on nelfinavir metabolism, then nelfivavir exposure would go down. However, nelfinavir is also metabolized by another enzyme - CYP2C19. Activity of CYP2C19 is decreased in pregnancy, and etravirine, a second generation non-nucleotide reverse transcriptase inhibitor, whose predominant route of elimination involves CYP2C19 metabolism, is one of the rare drugs whose exposure is increased during pregnancy.28 If only CYP2C19 were affected by pregnancy, then nelfinavir exposure would go up, while M8 exposure and M8/nelfinavir ratio would be decreased. Therefore, examining the effect of nelfinavir and M8 exposures during pregnancy and postpartum provides an opportunity to compare the effect of pregnancy on these 2 enzyme systems. Our data show that during pregnancy, M8 AUC and the M8/nelfinavir AUC ratio are decreased, likely due to decreased CYP2C19 activity during pregnancy. However, since nelfinavir plasma exposure is also reduced, the effect of pregnancy on CYP3A4 overwhelms its effect on CYP2C19. Hence, reduction in exposure of nelfinavir with standard dosing during pregnancy can be overcome by increasing the dose.
Due to its highly variable drug exposure and rapid metabolism, dose escalation trials of nelfinavir have been done in pregnancy.19,29,30 The rationale for these studies is that therapeutic drug monitoring trials of nelfinavir plasma concentrations with appropriate adjustments for low drug exposure, resulted in improved outcomes in the non-pregnant population treated with nelfinavir.31,32 Hence, increasing the dose of nelfinavir during pregnancy was postulated to likely increase bioavailability in the maternal and fetal plasma for prevention of mother to child transmission of HIV. IMPAACT 102619 previously showed that 1250 mg twice daily dosing decreased NFV exposure by 31% and M8 by 75% during the third trimester of pregnancy versus postpartum, with only 56% of subjects meeting the AUC (area under the concentration versus time curve) target during the 3rd trimester.19 Other studies of increased dosing, like the PACTG 053 trial30 showed that NFV crossed the placenta poorly, and drug exposure was inadequate in most pregnant women receiving 750 mg TID but was much improved with 1250 mg twice daily.
Our study has several strengths. To our knowledge, this is the first study to report the pharmacokinetics of nelfinavir during pregnancy by varying the dosages during the various trimesters, with the highest dose at 1875mg twice daily between 30 weeks of gestation until the 3rd week postpartum in pregnancy. The participants in our study were followed longitudinally over time, and the collection of clinical findings related to nelfinavir exposure occurred at regular time intervals, so recall error or bias, systematic bias and confounding by genetic, sociodemographic and other individual characteristics were minimized. Any random measurement error that arose from the study would tend to diminish apparent effect size, causing estimates to be conservative. There was a high rate of follow up for mothers and neonates. The collection of samples followed a strict protocol with observed dosing to minimize errors due to sample collection.
This study had its limitations. First, the study cohort included a small number of women, reducing the precision of pharmacokinetic parameters because of greater influence of inter-individual variability. Second, the population studied within this network is mainly black or Hispanic, with only a limited number of non-Hispanic white patients included, so that limitations of generalizability may exist. Third, it is not known at what point NFV pharmacokinetics reverted to values observed in the pre-pregnant state. Ideally, prospective pharmacokinetic studies in women prior to, during and after pregnancy will be needed to resolve the exact timing of return to pre-pregnant levels. Fourth, the association between increased nelfinavir dosing and genetic resistance to HIV virus was not assessed in this study. Genotypic resistance was detected in 50% of women with detectable HIV RNA for whom samples were available for testing in a prior nelfinavir study in pregnant women.33 Fifth, this study was not designed to identify the precise pharmacokinetic mechanism(s) associated with reduced nelfinavir or M8 during pregnancy. Increased nelfinavir protein binding, volume of distribution and/or clearance are likely additional reasons for lower exposures of nelfinavir during the 3rd trimester compared to the postpartum period. Sixth, we did not assess the effect of co-administration of nelfinavir with other medications in pregnancy. In prior pharmacokinetic studies, nelfinavir was shown to interact with a myriad of drugs metabolized by the cytochrome P-450 group of enzymes. 34–36 Another limitation is the fact that we did not collect genotyping data; therefore, there is no information on possible protease inhibitor resistance.
In conclusion, our findings confirm that nelfinavir dose should be increased during late pregnancy, and increased dose nelfinavir dosing may still have some benefit to HIV positive individuals living in countries where novel protease inhibitors are currently unavailable, or in individuals who are intolerant to ritonavir-boosted HIV medications.
Supplementary Material
Study sites where the study was implemented.
ACKNOWLEDGEMENTS:
We would like to thank all the women who participated in the nelfinavir arm of the P1026s protocol, the sites that participated in this study and all the members of the P1026s protocol team.
Financial disclosures: Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest: All the authors had no other conflict of interest.
DATA SHARING:
If interested in our data, please contact David Shapiro (shapiro@sdac.harvard.edu) or Jiajia Wang (jwang@sdac.harvard.edu)
REFERENCES:
- 1.Timmermans S, Tempelman C, Godfried MH, et al. Nelfinavir and nevirapine side effects during pregnancy. AIDS. 2005;19(8):795–799. [DOI] [PubMed] [Google Scholar]
- 2.van Heeswijk RP, Khaliq Y, Gallicano KD, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin Pharmacol Ther. 2004;76(6):588–597. [DOI] [PubMed] [Google Scholar]
- 3.Hirt D, Treluyer JM, Jullien V, et al. Pregnancy-related effects on nelfinavir-M8 pharmacokinetics: a population study with 133 women. Antimicrob Agents Chemother. 2006;50(6):2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Department of Health and Human Services, November 2017. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed June 26th, 2018. [Google Scholar]
- 5.DHHS Panel on Antiretroviral Therapy Guidelines for Adults and Adolescents – Notice on Nelfinavir FDA-Pfizer Letter, September 13, 2007. Available at https://aidsinfo.nih.gov/contentfiles/AdultNFVNotice1.pdf. Assessed June 28th, 2018.
- 6.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. November, 2017. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed June 12th, 2018.
- 7.McIntyre J Use of antiretrovirals during pregnancy and breastfeeding in low-income and middle-income countries. Curr Opin HIV AIDS. 2010;5(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White AB, Mirjahangir JF, Horvath H, Anglemyer A, Read JS. Antiretroviral interventions for preventing breast milk transmission of HIV. Cochrane Database Syst Rev. 2014(10):Cd011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato A, Asano T, Okubo K, Isono M, Asano T. Nelfinavir and Ritonavir Kill Bladder Cancer Cells Synergistically by Inducing Endoplasmic Reticulum Stress. Oncol Res. 2018;26(2):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelfinavir Koltai T. and other protease inhibitors in cancer: mechanisms involved in anticancer activity. F1000Res. 2015;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gills JJ, Lopiccolo J, Tsurutani J, et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13(17):5183–5194. [DOI] [PubMed] [Google Scholar]
- 12.Hirani VN, Raucy JL, Lasker JM. Conversion of the HIV protease inhibitor nelfinavir to a bioactive metabolite by human liver CYP2C19. Drug Metab Dispos. 2004;32(12):1462–1467. [DOI] [PubMed] [Google Scholar]
- 13.Ke AB, Nallani SC, Zhao P, Rostami-Hodjegan A, Unadkat JD. Expansion of a PBPK model to predict disposition in pregnant women of drugs cleared via multiple CYP enzymes, including CYP2B6, CYP2C9 and CYP2C19. Br J Clin Pharmacol. 2014;77(3):554–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee Opinion No. 687: Approaches to Limit Intervention During Labor and Birth. Obstet Gynecol. 2017;129(2):e20–e28. [DOI] [PubMed] [Google Scholar]
- 15.Ke AB, Nallani SC, Zhao P, Rostami-Hodjegan A, Isoherranen N, Unadkat JD. A physiologically based pharmacokinetic model to predict disposition of CYP2D6 and CYP1A2 metabolized drugs in pregnant women. Drug Metab Dispos. 2013;41(4):801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke AB, Nallani SC, Zhao P, Rostami-Hodjegan A, Unadkat JD. A PBPK Model to Predict Disposition of CYP3A-Metabolized Drugs in Pregnant Women: Verification and Discerning the Site of CYP3A Induction. CPT: pharmacometrics & systems pharmacology. 2012;1:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang KE, Wu E, Patick AK, et al. Circulating metabolites of the human immunodeficiency virus protease inhibitor nelfinavir in humans: structural identification, levels in plasma, and antiviral activities. Antimicrob Agents Chemother. 2001;45(4):1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–639. [DOI] [PubMed] [Google Scholar]
- 19.Read JS, Best BM, Stek AM, et al. Pharmacokinetics of new 625 mg nelfinavir formulation during pregnancy and postpartum. HIV Med. 2008;9(10):875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong H Altered drug metabolism during pregnancy: hormonal regulation of drug-metabolizing enzymes. Expert Opin Drug Metab Toxicol. 2010;6(6):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54(4):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20(15):1931–1939. [DOI] [PubMed] [Google Scholar]
- 23.Kreitchmann R, Best BM, Wang J, et al. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquir Immune Defic Syndr. 2013;63(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirochnick M, Best BM, Stek AM, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56(5):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stek A, Best BM, Wang J, et al. Pharmacokinetics of Once Versus Twice Daily Darunavir in Pregnant HIV-Infected Women. J Acquir Immune Defic Syndr. 2015;70(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colbers A, Molto J, Ivanovic J, et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother. 2015;70(2):534–542. [DOI] [PubMed] [Google Scholar]
- 28.Mulligan N, Schalkwijk S, Best BM, et al. Etravirine Pharmacokinetics in HIV-Infected Pregnant Women. Front Pharmacol. 2016;7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang A, Valluri SR, O’Sullivan MJ, et al. Safety and pharmacokinetics of nelfinavir during the second and third trimesters of pregnancy and postpartum. HIV Clin Trials. 2012;13(1):46–59. [DOI] [PubMed] [Google Scholar]
- 30.Bryson YJ, Mirochnick M, Stek A, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008;9(2):115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burger D, Hugen P, Reiss P, et al. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS. 2003;17(8):1157–1165. [DOI] [PubMed] [Google Scholar]
- 32.Burger DM, Hugen PW, Aarnoutse RE, et al. Treatment failure of nelfinavir-containing triple therapy can largely be explained by low nelfinavir plasma concentrations. Ther Drug Monit. 2003;25(1):73–80. [DOI] [PubMed] [Google Scholar]
- 33.Baroncelli S, Villani P, Floridia M, et al. Trough concentrations of lopinavir, nelfinavir, and nevirapine with standard dosing in human immunodeficiency virus-infected pregnant women receiving 3-drug combination regimens. Ther Drug Monit. 2008;30(5):604–610. [DOI] [PubMed] [Google Scholar]
- 34.Pfister M, Labbe L, Lu JF, et al. Effect of coadministration of nelfinavir, indinavir, and saquinavir on the pharmacokinetics of amprenavir. Clin Pharmacol Ther. 2002;72(2):133–141. [DOI] [PubMed] [Google Scholar]
- 35.Shibata N, Gao W, Okamoto H, et al. Drug interactions between HIV protease inhibitors based on physiologically-based pharmacokinetic model. J Pharm Sci. 2002;91(3):680–689. [DOI] [PubMed] [Google Scholar]
- 36.Stocker H, Herzmann C, Breske A, et al. Saquinavir, nelfinavir and M8 pharmacokinetics following combined saquinavir, ritonavir and nelfinavir administration. J Antimicrob Chemother. 2007;59(3):560–564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study sites where the study was implemented.


