Abstract
Aims:
It has been recently suggested that follistatin (FST) and its homologous protein, follistatin-like 3 (FSTL3) may be a therapeutic target against type 2 diabetes due to their glucose-regulatory effects in rodents.
Materials and Methods:
We investigated this hypothesis in humans by studying i) the physiology of a possible glycemia–follistatin feedback loop i.e. whether glucose but not lipid intake (oral or intravenous) can regulate circulating FST and FSTL3 in healthy humans(n=32), ii) whether the levels of follistatins change in response to various types of bariatric operation in morbidly obese individuals with or without type 2 diabetes (n=41), and whether such changes are associated prospectively with improvement of glucose homeostasis/insulin sensitivity.
Results:
In healthy individuals, circulating FST decreases after intravenous or oral glucose intake compared to controls, indicating the presence of a negative feedback mechanism. In morbid obesity, insulin resistance, glycemia, circulating FST and FSTL3 are all reduced (by 22–33%) after RYGB and sleeve gastrectomy. Importantly, the changes in circulating FST three months after bariatric surgery are associated prospectively with the changes in glucose, insulin, HOMA-IR and HbA1c observed six months postoperatively both in subjects with and without type 2 diabetes.
Conclusions:
Our findings provide evidence for an important role of FST on glucose homeostasis in healthy individuals as well as in severe obesity accompanied by insulin resistance and type 2 diabetes. Our data extend recent results from animal studies to humans and support the need for further evaluation of FST inactivation strategies for targeting hyperglycemia and insulin resistance.
Introduction
Circulating levels of both follistatins (follistatin [FST] and follistatin-like 3 [FSTL3]) are significantly affected by energy status1–4. Follistatins participate in a wide spectrum of physiological procedures, ranging from reproductive function3 to muscle and liver metabolism5–7 as well as to glucose and lipid homeostasis1,5,8–13. It has been recently reported, that knockdown of FST in hyperglycemic mice improves glucose tolerance by increasing white adipose tissue insulin sensitivity and by reducing hepatic glucose output14. In addition, circulating FST concentrations were elevated in patients with type 2 diabetes (T2D)9 and decrease concomitantly with HbA1c in a limited number of obese individuals with T2D six months after Roux-en-Y gastric bypass (RYGB)14. These findings indicate that, if these data are extended to and confirmed in humans, FST may be an attractive therapeutic target for the treatment of T2D14. FSTL3 is a protein highly homologous to FST that may also participate in the regulation of glucose and lipid homeostasis. Knockout of FSTL3 leads to reduced visceral fat, decreased insulin resistance, increased islet number and size, and improved glucose tolerance in mice11. FST and FSTL3 are antagonists of activins and of myostatin, thus promoting muscle differentiation and growth15. Increased activin bioavailability, due to reduced FST or FSTL3 levels has been associated with improved hepatic insulin sensitivity but also with increased hepatic triglyceride accumulation in mice16.
Given the emerging role of FST and FSTL3 in metabolic diseases, we aimed, by utilizing three interventional human studies, to investigate: i) the potential existence and physiology of an endocrine loop linking increasing levels of blood glucose and/or free fatty acids with follistatins, i.e. whether oral or intravenous glucose or lipid intake can regulate the circulating levels of FST and FSTL3 in healthy humans (n=32)14, ii) the pathophysiological significance of this loop, i.e. whether the levels of FST and FSTL3 change after various types of bariatric operation in morbidly obese individuals with or without T2D (n=41), including the previously studied type of RYGB and, if yes, to study iii) the predictive value of FST and FSTL3 postoperatively, i.e. whether these changes are associated prospectively with improvement of glucose homeostasis and insulin sensitivity, i.e. with fasting glucose, insulin, HOMA-IR and HbA1c.
Materials and Methods
Study 1 – Healthy population
32 subjects were recruited for the first study. 26 of them were recruited at Beth Israel Deaconess Medical Center (BIDMC) and 6 of them were recruited by the First Department of Propaedeutic Medicine, Laiko General Hospital. The protocol has been previously described17. Inclusion and exclusion criteria are presented in supplemental table S1. After a 10-hour overnight fast, subjects were randomized in the following groups: a) Control group (n=9), who received intravenously 0,9% NaCl at 0,83 ml/kg/hr rate for 360 min (6 hours) and drank 300 ml of water at time 0 and at 3 hours to control for any effects of gastric distension. b) Oral lipid group (n=6), who received orally 1.25 grams/kg soybean oil at 0 and at 3 hours, followed by 200 ml of water and intravenously 0,9% NaCl at 0,83 ml/kg/hr rate for 6 hours, c) Oral glucose group (n=6), who received orally 1,25 grams/kg glucose with 200 ml water at time 0 and at 3 hours, as well as intravenously 0,9% NaCl at 0,83 ml/kg/hr rate for 6 hours. d) Low rate intravenous lipid group, who received Intralipid/Liposyn II 20% at 0.35 ml/kg/hr rate with 0.9% NaCl at 0.48 ml/kg/hr rate and drank 300 ml of water at time 0 and at 3 hours, e) High rate intravenous lipid group, who received Intralipid/Liposyn II 20% at 0.83 ml/kg/hr rate and also drank 300 ml of water at 0 and after 3 hours, f) Intravenous glucose group, receiving intravenously 10% glucose at a rate of 3.6 ml/kg/hr, who also drank 300 ml of water at 0 and after 3 hours (This was the highest rate that it could be administrated peripherally in order to almost match the dose of glucose administrated orally, i.e. 2,16 gram/kg total intravenous glucose intake over 6 hours vs 2,5 gram/kg total oral glucose intake over 6 hours). All participants received also heparin 800 IU/hr with a starting bolus dose of 1000 IU. The same participants were in the oral glucose group (c) and in the intravenous glucose group (f) and there was at least one week pause between the two interventions. Blood was collected before the start of each procedure (0 min), every 30 min in the first two hours and then every 60 min for up to 6 hours from the start. Collected blood was centrifuged immediately, and serum was stored in tubes in −80 degrees Celsius.
Study 2 – First bariatric study
A total of fourteen morbidly obese subjects (age 53.2 ± 8.9 years, BMI 50.2 ± 10.6 kg/m2, males/ females= 8/6, DM2/ non-DM2: 8/6) who underwent a bariatric surgery were recruited to this prospective study after they were approved for bariatric surgery. Participants underwent either a laparoscopic adjustable gastric banding procedure (AGB, n=9) or a Roux-en-Y gastric bypass surgery (RYGB, n=5). Each patient chose the type of surgery he preferred. All patients were between 18–65 years old and fulfilled the NIH criteria for bariatric surgery. Exclusion criteria included cancer, type 1 diabetes, untreated major depression or psychosis, binge eating disorders, current drug and alcohol abuse, severe cardiac disease with prohibitive anesthetic risks, severe coagulopathy, inability to comply with nutritional requirements including life-long vitamin replacement and pregnancy. The study was approved by the Institutional Review Board of BIDMC and participants provided written, informed consent to participate. Subjects were examined at baseline prior to surgery and at 3 and 6 months post operatively in the BIDMC General Clinical Research Center (GCRC). Blood samples were obtained at each visit after an overnight fast.
Study 3 – Second bariatric study
The protocol of this study has been previously described18. From that study, serum was available from twenty seventh morbidly obese subjects (age 40.4 ± 8.2 years, BMI 49.7 ± 6.9 kg/m2, males/females= 4/23, DM2/non-DM2: 4/23) who had been approved and were recruited for bariatric surgery. They underwent either a vertical sleeve gastrectomy (VSG, n=16) or a RYGB (n=11). In all cases, the type of surgery performed was determined by patient preference after surgical consultation. Inclusion criteria were a BMI> 40 kg/m2, age between 18 and 65 years, and proven failure to lose weight through non-surgical interventions. Exclusion criteria included serious and life threatening comorbidities (renal, cardiac, liver failure, or malignancy), patients’ inability to adhere to postsurgical instructions, alcohol or other substance abuse, and concurrent psychiatric illness. The study was approved by the ethics committee of Laiko General Hospital and participants provided written, informed consent before inclusion. Subjects were examined at baseline prior to surgery and at 3, 6 and 12 months post operatively in the diabetes laboratory of the First Department of Propaedeutic Medicine, Laiko General Hospital. Body weight and height were measured in light clothing. Epicardial fat thickness was measured with an echocardiogram on the free wall of the right ventricle during end-systole and body composition with Bioelectrical Impendence Analysis (BIA 101, Akern, Italy). Blood samples were obtained at each visit after an overnight fast.
All three studies described above were in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. Written informed consent was obtained from all the participants
Biochemical measurements
FST and FSTL3 were measured with ELISA immunoassays from Ansh Laboratories (Webster, Tx, USA), Glucagon from R&D Systems (Minneapolis, MN, USA), free fatty acids from WAKO Diagnostics (Boston, MA, USA). Insulin and Glucose were measured with automatic analyzers (s. Appendix S1 for details).
HOMA-IR was assessed by the following formula: (fasting Glucose × fasting Insulin)/405 and HOMA-β was calculated by the following formula: (360 × Insulin)/ (Glucose/63) with insulin given in μlU/mL and glucose in mg/dl.
Statistical analysis
Statistical analysis was performed with SPSS v25.0 (SPSS, Inc., Chicago, IL) for Windows and with Graphpad prism 7 (GraphPad Software Inc., La Jolla, CA). An intention-to-treat analysis was performed with one-way ANOVA and post-hoc Tukey’s test or Kruskal-Wallis with post-hoc Dunn’s test, as well as spearman correlations and linear regression analysis. The analysis is described in detail in Appendix S1.
Results
Oral and intravenous glucose intake reduces circulating FST but not FSTL3 [Study 1-Healthy population]
Six subjects participated both in the oral and the IV glucose group and were gender-, age- and BMI matched with the subjects of the oral lipid group (age=35.0 [26.5, 43.3] years old, BMI= 27.1± 4.6 kg/m2, fasting glucose before oral glucose administration= 93.4 ± 12.7 mg/dl, before IV glucose administration= 92.2 ± 12.3, fasting insulin before oral glucose intake = 9.00 [5.14, 14.52], before IV glucose= 8.74 [6.77, 14.98]). The baseline characteristics and the glucose and insulin levels of the lipid and the control groups (groups a, b, d, e) have been previously described17. There were no significant differences in age (p=0.22), BMI (p=0.41), fasting glucose (p=0.10) and fasting insulin (p=0.12) between the six groups. Similarly, baseline FST and FSTL3 levels (before any intervention) were not different between groups (s. Table S2).
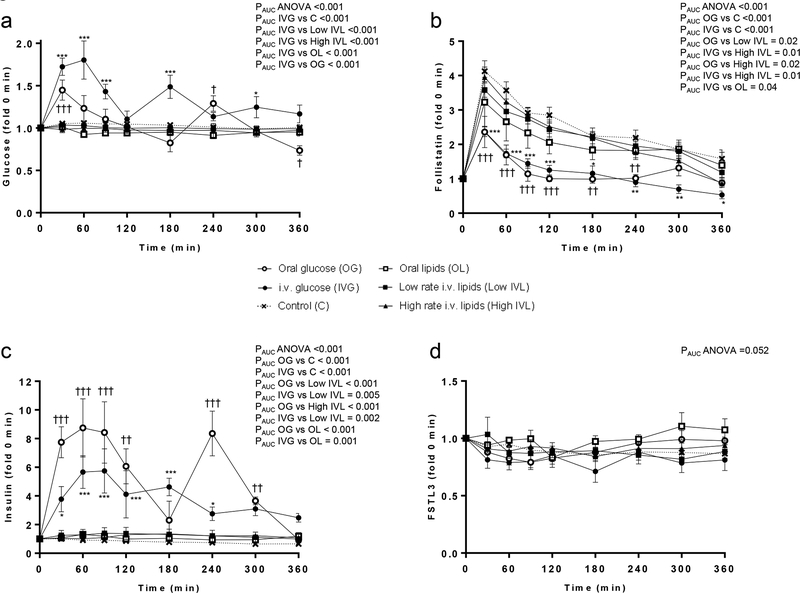
Blood glucose levels were increased profoundly after intravenous glucose infusion and, as expected, modestly after oral glucose intake (s. Figure 1a). This corresponds to higher postprandial insulin concentrations measured in the oral glucose group (s. Figure 1c). Neither glucose nor insulin concentrations changed after oral or intravenous lipid intake, in contrast to free fatty acids that were increased up to more than 5-fold in high rate IV lipid group and 2-fold in low rate IV lipid and oral lipid groups (s. supplemental table S2). Additionally, in the oral fat group an increase in glucagon/insulin ratio up to almost 10-fold was observed (s. figure S1)
Figure 1:
Circulating profile of glucose (a), FST (b), Insulin (c), and FSTL3 (d) after oral or intravenous glucose or lipid intake vs control.
PAUC ANOVA indicates the p value from one-way ANOVA of the area under the curve (AUC) between all groups; PAUC one group (e.g. IVG) vs PAUC another group (e.g. C), indicates the p value of the post-hoc Tukey’s test between the AUCs of the two groups; Tukey’s test for the specific timepoint between oral glucose group vs control: †, p<0.05; ††, p<0.01; ††† <0.001. Tukey’s test for the specific timepoint between intravenous glucose group vs control: *, p<0.05; **, p<0.01; ***, p<0.001.
FST levels increase in the first 30 minutes in all groups but when comparing between groups, concentration of FST is significantly lower compared to control from 30min up to 240min after oral glucose intake (that took place at 0min and at 180min), or from 30 min up to 360 min after continuous intravenous glucose infusion (s. Figure 1b). In contrast, concentration of FST does not change compared to the control group after oral or intravenous lipid intake. These findings suggest a negative feedback loop of glucose, but not of free fatty acids, affecting FST. In contrast, FSTL3 levels do not change after any intervention (Fig. 1d).
Circulating FST and FSTL3 are decreased on the third and up-to at least the twelfth month after RYGB and/or VSG [Study 2 and Study 3: Bariatric population]
In the first bariatric study [Study 2], subjects that had undergone an AGB had a ~13% BMI reduction 6 months after the intervention compared to a ~26% reduction in the RYGB group (s. Table 1). Body fat mass in both groups and fat percentage in the RYGB group were also decreased significantly (Fat mass [kg], AGB 0 months=54.6 ± 4.7, 6 months= 46.7 ± 9.7, p=0.03, RYGB 0 months= 63.5 ± 21.3, 6 months= 33.9 ± 23.1, p<0.001, / Fat percentage, AGB 0 months= 42.3 ± 7.5, 6 months= 39.5 ± 6.6, p=0.32, RYGB 0 months= 40.3 ± 9.9, 6 months= 27.0 ± 14.6, p= 0.02) HbA1c, glucose and HOMA-β did not change significantly in any of the groups. The RYGB group had a robust decrease in fasting insulin levels and a trend (p=0.07) to lower HOMA-IR after operation. Circulating FST was decreased ~33% at three months and FSTL3 ~24% at six months after operation only in the RYGB group and not in the AGB group.
Table 1:
BMI and hormonal changes after AGB or RYGB (Study 2 – First bariatric study)
| Months after bariatric operation | |||||
|---|---|---|---|---|---|
| Parameters | OP | 0 (a) | 3 (b) | 6 (c) | P-value |
| BMI | All | 50.2 ± 10.6b,c | 44.1 ± 8.2a | 41.4 ± 8.5a | <0.001 |
| (kg/m2) | AGB | 49.1 ± 10.5b,c | 43.3 ± 7.2a | 42.8 ± 7.8a | <0.001 |
| RYGB | 52.1 ± 11.8c | 46.3 ± 12.3 | 38.8 ± 9.9a | 0.002 | |
| Glucose | All | 88.0 (80.5, 112.5) | 93.0 (80.0, 129.0) | 87.5 (78.3, 113.8) | 0.64 |
| (mg/dl) | AGB | 88.0 (80.5, 110.5) | 99.0 (85.5, 129.0) | 102.0 (81.5, 126.5) | 0.31 |
| RYGB | 98.5 (77.3, 131.8) | 85.0 (77.0, 129.0) | 73.0 (72.0, 90.5) | 0.19 | |
| Insulin | All | 16.2 (10.8, 20.9) | 12.8 (7.9, 23.3) | 7.8 (3.7, 15.8) | 0.07 |
| (ulU/ml) | AGB | 15.1 (9.7, 19.8) | 9.5 (7.5, 23.4) | 7.9 (3.3, 17.9) | 0.42 |
| RYGB | 25.6 (12.2, 41.1)c | 16.2 (6.0, 21.3) | 4.8 (3.8, 14.5)a | 0.03 | |
| HOMA-IR | All | 2.58 (2.11, 5.13) | 2.87 (1.32, 5.22) | 1.64 (0.80, 4.98) | 0.20 |
| AGB | 2.76 (1.91, 4.93) | 2.35 (1.32, 5.72) | 1.75 (0.95. 5.81) | 0.73 | |
| RYGB | 2.58 (2.47, 11.52) | 2.99 (1.25, 3.68) | 0.84 (0.73, 3.29) | 0.07 | |
| HOMA-β | All | 218.8 (144.4, 367.4) | 111.3 (66.5, 216.8) | 110.3 (40.3, 193.0) | 0.11 |
| (%) | AGB | 188.1 (95.8, 342.6) | 117.4 (77.1, 212.4) | 47.4 (22.8, 167.7) | 0.22 |
| RYGB | 311.4 (203.9, 427.3) | 95.1 (34.6, 309.7) | 184.7 (110.3, 279.5) | 0.36 | |
| HbA1c | All | 6.00 (5.60,7.45) | 6.10 (5.65, 7.10) | 5.95 (5.43, 6.95) | 0.31 |
| (%) | AGB | 6.80 (5.60, 7.45) | 6.35 (5.95, 7.23) | 6.50 (5.60, 7.30 | 0.68 |
| RYGB | 5.95 (5.68, 7.20) | 5.80 (5.30, 6.45) | 5.50 (5.40, 6.50) | 0.13 | |
| Follistatin | All | 4.97 ± 1.03 | 4.25 ± 1.19 | 4.70 ± 1.09 | 0.07 |
| (ng/ml) | AGB | 4.94 ± 1.09 | 4.75 ± 1.22 | 4.92 ± 1.12 | 0.70 |
| RYGB | 5.03 ± 1.04b | 3.37 ± 0.33a | 4.19 ± 0.95 | 0.01 | |
| FSTL3 | All | 17.5 ± 6.1 | 16.1 ± 6.0 | 15.6 ± 5.4 | 0.14 |
| (ng/ml) | AGB | 16.9 ± 5.9 | 16.9 ± 6.8 | 16.5 ± 5.9 | 0.81 |
| RYGB | 18.6 ± 7.2 | 14.6 ± 4.2 | 14.1 ± 4.5 | 0.04 | |
Data are presented as mean ± SD if normally distributed and as median with first and third quartile if not normally distributed.
An intention-to treat analysis was performed. P-value corresponds to the p of paired one-way ANOVA.
By p-value<0.05, post-hoc Tukey’s test between groups were performed. Superscript letters (a,b,c) in the table indicate p<0.05 in post-hoc t-test between the subject group of the column in which the letters are written vs. the group indicated by the letter (a, b, or c). For example for BMI All at 0 months letters b,c indicate p<0.05 in post-hoc t-test for BMI when 0 months are compared with 3 months (b) or with 6 months (c).
In the second bariatric study [Study 3], the VSG group had a 32.5% and the RYGB group a 36% decrease in BMI at the 12 postoperative month (s. Table 2). Body fat mass as well as epicardial fat thickness were significantly reduced in both groups (Body fat mass [kg], VSG 0 months=50.5 ± 7.3, 12 months= 37.1 ± 8.7, p<0.001, RYGB 0 months= 51.0 ± 8.3, 12 months= 31.6 ± 8.9, p<0.001/ Body fat percentage [%], VSG 0 months= 37.3 ± 6.1, 12 months= 41.6 ± 8.1, p= 0.02, RYGB 0 months= 40.2 ± 8.2, 12 months= 38.5 ± 9.6, p= 0.80/ epicardial fat thickness [mm], VSG 0 months= 1.61 ± 0.14 , 12 months= 1.42 ± 0.10 , p<0.001, RYGB 0 months= 1.61 ± 0.12, 12 months= 1.41 ± 0.16, p= 0.03) Insulin, HOMA-IR and HOMA-β were significantly reduced both in VSG and in RYGB group. Circulating FST was decreased ~22% in the VSG and 28% in the RYGB group after one year. FSTL3 was decreased ~25% and only in the VSG group.
Table 2:
BMI and hormonal changes after VSG or RYGB (Study 3 – Second bariatric study)
| Months after bariatric surgery | ||||||
|---|---|---|---|---|---|---|
| 0 (a) | 3 (b) | 6 (c) | 12 (d) | P | ||
| BMI | All | 49.7 ± 6.9b,c,d | 41.2 ± 6.2a,c,d | 36.5 ± 5.7a,b,d | 32.9 ± 6.2a,b,c | <0.001 |
| (kg/m2) | VSG | 50.9 ± 7.5b,c,d | 42.9 ± 6.6a,c,d | 37.4 ± 6.2a,b,d | 34.4 ± 6.7a,b,c | <0.001 |
| RYGB | 48.0 ± 6.0b,c,d | 39.1 ± 5.3a,c,d | 35.3 ± 4.9a,b,d | 30.8 ± 5.2a,b,c | <0.001 | |
| Glucose | All | 100.5 (94.0, 112.5)b,c,d | 91.5 (87.0, 98.0)a | 90.5 (85.0, 98.8)a | 88.5 (84.8, 97.3)a | <0.001 |
| (mg/dl) | VSG | 108.0 (94.0, 123.0)c,d | 90.0 (86.5, 98.0) | 90.0 (85.0, 97.0)a | 89.0 (83.0, 98.0)a | <0.001 |
| RYGB | 99.0.(94.0, 103.0)b,c,d | 92.5 (88.3, 100.0)a | 91.0 (85.0, 101.0)a | 88.0 (85.0, 94.0)a | 0.40 | |
| Insulin | All | 23.1 (15.9, 28.1)b,c,d | 9.5 (7.9, 12.7)a | 8.7 (7.2, 12.9)a | 8.7 (7.2, 12.9)a | <0.001 |
| (ulU/ml) | VSG | 24.3 (19.0, 29.0)b,c,d | 8.2 (7.2, 14.1)a | 10.9 (7.2, 13.0)a | 10.9 (7.2, 13.0)a | 0.003 |
| RYGB | 22.3 (15.1, 26.0)b,c,d | 9.7 (8.4, 10.9)a | 8.3 (7.1,10.2)a | 8.3 (7.1, 10.2)a | <0.001 | |
| HOMA-IR | All | 5.76 ± 2.28 b,c,d | 2.35 ± 1.29a | 2.25 ± 1.13a | 2.22 ± 1.14a | <0.001 |
| VSG | 6.26 ± 2.52b,c,d | 2.36 ± 1.45a | 2.35 ± 1.09a | 2.33 ± 1.15a | <0.001 | |
| RYGB | 5.05 ± 1.78b,c,d | 2.33 ± 1.11a | 2.11 ± 1.22a | 2.06 ± 1.18a | <0.001 | |
| HOMA-β | All | 201.4 ± 61.2b,c,d | 116.5 ± 50.8a | 124.6 ± 56.6a | 132.3 ± 67.9a | <0.001 |
| (%) | VSG | 193.9 ± 67.8b,d | 99.7 ± 45.1a | 136.3 ± 63.2 | 137.5 ± 62.9a | 0.006 |
| RYGB | 214.7 ± 47.9b,c,d | 135.0 ± 52.3a | 109.7 ± 45.5a | 125.4 ± 77.4a | <0.001 | |
| Follistatin | All | 4.29 (3.28. 5.33)b,c,d | 3.76 (3.41, 4.25)a,d | 3.25 (2.80, 4.04)a | 3.22 (2.25, 3.44)a,b | <0.001 |
| (ng/ml) | VSG | 3.95 (3.25, 4.89)d | 3.73 (3.19, 4.24)d | 3.20 (2.82, 4.12) | 3.07 (2.25, 3.26)a,b | 0.003 |
| RYGB | 4.82 (3.57, 5.48)d | 3.85 (3.56, 4.33) | 3.30 (2.74, 4.01) | 3.44 (2.16, 4.33)a | 0.02 | |
| FSTL3 | All | 15.9 (13.9, 17.1)c | 14.3 (12.6, 17.4)c | 12.7 (10.8,15.2)a,b | 13.0 (10.8, 16.0) | <0.001 |
| (ng/ml) | VSG | 16.1 (14.7, 17.1)c,d | 14.2 (12.5, 17.3)c,d | 12.7 (11.2, 15.9)a,b | 12.1 (10.5, 14.6)a,b | <0.001 |
| RYGB | 14.2 (12.1, 19.1) | 14.4 (12.8, 18.0) | 12.1 (10.4, 15.1) | 13.9 (11.7, 20.2) | 0.25 | |
Data are presented as mean ± SD if normally distributed and as median with first and third quartile if not normally distributed.
An intention-to treat analysis was performed. P-overall corresponds to the p-value of paired one-way ANOVA.
By p-overall<0.05, post-hoc Tukey’s test between groups were performed. Superscript letters (a,b,c,d) indicate p<0.05 in post-hoc t-test between the subject group of the column in which the letters are written vs. the group indicated by the letter (a, b, c or d)
Changes in circulating FST three months after bariatric surgery are associated prospectively with changes in glucose homeostasis/insulin sensitivity six months after surgery
The populations of Study 2 (first bariatric) and Study 3 (second bariatric) consisted of subjects with T2D (n=8 for Study 2 and n=4 for Study 3), as well as without (n=6 for Study 2 and n=23 for Study 3). In a sub-analysis combining both studies for the first six months after operation. individuals with T2D demonstrated similar changes in FST and FSTL3 compared to individuals without T2D three and six months postoperatively. (FST: % change 3 months after intervention, T2D “No”= −15.2 [−26.4, 6.7] vs “Yes”= −13.0 [−27.4, 4.3], , p=0.73; % change 6 months after intervention, “No”= −17.2 [−30.8, 3.3] vs “Yes= −8.1 [−19.3, 2.5], p=0.29 , FSTL3: % change 3 months after intervention, T2D “No”= −8.5 [−21.3, −1.4] vs “Yes”= 1.2 [−19.3, 5.3], p=0.23; % change 6 months after intervention, “No”=: −14.9 [−24.3, 9.2], “Yes”=−10.0 [−23.5, 9.5], p=0.32).
The circulating concentrations of FST and FSTL3 correlated positively with BMI, body fat mass, insulin and HOMA-IR. Additionally, FST correlated strongly and positively with HbA1c and epicardial fat thickness. Interestingly, FSTL3 levels did not correlate with HbA1c, whereas they were negatively associated with epicardial fat thickness (s. Table S3).
The percentage change of FST three months after intervention was positively associated with the percentage changes of glucose, insulin, HOMA-IR, HbA1c as well as body fat (%) and fat mass but not epicardial fat thickness six months after intervention (Table 3). Of note, these predictive associations were stronger compared to the associations observed between FST and parameters of glucose homeostasis and adiposity at the same timepoint (s. Table 3 and Table S3), indicating that changes in FST may precedent the changes observed in glucose homeostasis and body fat mass. These positive associations were observed both in subjects with and without T2D (Table 3). The strongest association was for FST with HOMA-IR. In contrast, the changes of FSTL3 three months after operation were not associated with the changes of glucose, insulin, HOMA-IR or BMI, body fat (%) and mass after six months.
Table 3:
Spearman correlations of % changes of follistatin and FSTL3 with parameters of glucose homeostasis and linear regression of % changes of follistatin from 0 to 3 months with % changes of HOMA-IR from 0 to 6 months after operation (combined Study 2 - First bariatric study and Study 3 – Second bariatric study).
| % change 0–6 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % changes 0–3 months |
Glucose (mg/dl) |
Insulin (ulU/ml) |
HOMA-IR | HOMA-β (%) |
HbA1c‡ (%) |
BMI (kg/m2) |
Body Fat (%) | Body Fat (kg) | Eft‡
(mm) |
|
| Follistatin (ng/ml) | All | 0.502** | 0.447** | 0.583*** | −0.048 | 0.671* | 0.266 | 0.423* | 0.487** | 0.134 |
| Non-T2D | 0.548** | 0.515** | 0.603** | 0.030 | 0.543 | 0.267 | 0.668** | 0.681** | 0.392 | |
| T2D | 0.650† | 0.382 | 0.738* | −0.100 | 0.886* | 0.382 | −0.033 | 0.067 | n.a | |
| FSTL3 (ng/ml) | All | 0.138 | 0.089 | 0.089 | −0.160 | −0.070 | 0.153 | 0.332 | 0.336 | 0.376 |
| Non-T2D | 0.281 | 0.045 | 0.105 | −0.483* | 0.143 | 0.222 | 0.259 | 0.262 | 0.378 | |
| T2D | −0.067 | 0.248 | 0.143 | 0.300 | −0.143 | −0.027 | 0.600 | 0.533 | n.a | |
| Linear Regression† % change 0–6 for HOMA-IR |
R square | Unstandardized B | 95% CI B | p-value | ||||||
| % change 0–3 for FST | 0.381 | 0.638 | 0.31, 0.97 | <0.001 | ||||||
| Intercept | −54.61 | −62.69, −46.52 | <0.001 | |||||||
p<0.05
p<0.01
p<0.001;
p=0.058
linear regression model including subjects with a reduction in HOMA-IR after 6 months (n=28)
HbA1c measurements were available only in Study 2- First bariatric study, whereas Eft measurements were available only in Study 3- Second bariatric study.
Abbreviations: Eft, Epicardial fat thickness, n.a =not applicable due to low number of measurements
A linear regression analysis demonstrated that the % changes of FST after three months can best predict the % changes of HOMA-IR at 6 months in subjects with a reduction of HOMA-IR during this period (n=28) (s. table 3 and Figure S2 related to table 3). The r2 for this equation was 0.381 meaning that 38.1% of the variance in % changes of HOMA IR at six months was predictable from % changes of FST at 3 months. When subjects with an increase in HOMA-IR (n=4) the first six months were included, the model remained significant, though with an r2 of 0.24 due to higher heteroscedasticity and loss of linearity for HOMA-IR.
Similar associations (slightly weaker but significant) were observed when the absolute changes of circulating FST concentrations the first three months after intervention were compared with the absolute changes in parameters of glucose homeostasis and adiposity at six months (s. Table S4).
Discussion
It has been recently reported, that FST increase the blood glucose levels by promoting hepatic glucose output and insulin resistance in white adipose tissue in mice14. Here, we demonstrate that glucose itself is a potent down-regulator of FST, establishing an endocrine loop between FST and glucose in humans. Liver is considered a major source of circulating FST5,7. Hansen et al. have suggested that a high glucagon/insulin ratio results in elevated FST levels presumably through an increase in hepatic FST secretion5. This high ratio in that study was achieved after the infusion of glucagon and somatostatin and was aiming to mimic the glucose regulatory effects seen after energy demanding conditions, such as intensive exercise5. In our study, an increase in glucagon/insulin ratio up to almost 10-fold with stable glucose levels was observed in the oral fat group, but this was not associated with a stimulation of circulating FST levels, arguing against the regulation of FST by elevated glucagon/insulin ratio in conditions of energy excess. On the other hand, both oral intake and intravenous infusion of glucose were associated with lower circulating FST. Whether this reduction in FST is achieved directly through effects of the increased glucose or indirectly through effects of altered insulin levels on the synthesis-secretion sites of FST (i.e. primarily liver) cannot be definitely answered in the current experimental setting and should be probably addressed with more detail in future in both in vivo and in vitro studies. In contrast to FST, concentrations of FSTL3 did not change in any of the interventions. FLRG encoding FSTL3 is ubiquitously expressed but enriched in organs the function of which is less affected by acute changes in blood glucose or lipid levels such as gonads, lung, adrenal gland and heart muscle7, which might explain the lack of acute changes in circulating FSTL3 after glucose or lipid intake.
As recently reported, FST inactivation improves glucose tolerance in hyperglycemic mice and FST levels decrease in parallel with HbA1c in ten morbidly obese individuals with T2D after RYGB14. In our study, we observe a significant reduction of FST both after RYGB and VSG but not after AGB. FSTL3 levels are reduced after RYGB in the first study and after VSG in the second study, while similarly to FST they do not change after AGB. Generally, AGB does not affect gastric emptying time and is associated with slower weight loss and less profound metabolic benefit compared to the other bariatric operations19. RYGB and VSG are characterized by accelerated transport of nutrients into the distal intestine and rapid and robust improvement of hepatic insulin resistance and blood glucose levels post-operatively20. In our study, a significant decrease of FST is already achieved after three months indicating also a rapid change in its circulating profile, whereas the changes in FSTL3 are in the same direction but more modest. This is important, since FSTL3 demonstrates not only structural but also functional homology to FST. Thus, the lack of increase of FSTL3 in response to the robust reduction of FST argues against functional redundancy. Whether the accelerated gastric emptying after RYGB or VSG directly affects the circulating profile of FST or FSTL3 seems rather improbable, given that both FST and FLRG are not highly expressed in the gastrointestinal tract. Future studies should probably therefore focus on the mechanisms controlling hepatic FST synthesis or secretion and whether these are disrupted or over-activated in conditions of chronic energy excess, i.e. morbid obesity, insulin resistance and T2D.
In order to assess whether the reduction of FST precedes or follows the metabolic changes observed after bariatric surgery we investigated the association of early changes in FST (first three months post-operatively) with later changes in metabolic parameters (first six months post-operatively). Reduction in circulating FST in the first three months was associated with reductions in glucose, insulin, HOMA-IR, HbA1c, body fat (%) and body fat mass six months postoperatively. The associations related to parameters of glucose homeostasis were observed not only in diabetic but also in non-diabetic insulin resistant participants. This association implies a significant role for FST on glycemic control not only in T2D but also in prediabetes-insulin resistant state in humans, confirming and extending the importance of the recent findings in animals. Specifically, for each one percent decrease of circulating FST after three months, a 0.638 percent reduction in HOMA-IR after six month is expected. Additionally, FST levels were associated with parameters of adiposity, an observation which combined with the findings from animal studies further supports the presence of FST-adipose tissue crosstalk mechanisms in humans. Future studies should probably therefore investigate whether FST is involved in adipogenesis or vice versa whether high fat mass significantly contributes to the circulating concentrations of FST through high secretion of the hormone by adipocytes. In contrast, based on our findings circulating FSTL3 had no predictive value for the metabolic outcome of these patients arguing against an important role of this hormone on glucose homeostasis.
The current study has some limitations. First of all, the follow-up in the first bariatric study was shorter (6 months) compared to the second study (one year) not allowing us to perform a combined analysis for both studies for up to one year later or longer. Additionally, we did not measure HbA1c in the second bariatric study, although the majority of the subjects (23 out of 27) did not have T2D and thus no significant changes in HbA1c were expected. Moreover, it would have been informative to have measured FST and FSTL3 at earlier time points, i.e. in the first few days or weeks after operation to assess better the dynamic of the observed changes as well as to have liver biopsies to investigate the changes in hepatic FST expression after bariatric surgery. Finally, there is an unexpected acute increase in FST in the first minutes in all groups in Study 1. This finding may indicate possible effects of volume expansion or gastric distension on the circulating levels of FST and it should be further investigated in the future.
These findings may have some important clinical implications. FST binds and blocks the functions of TGF-β proteins7. Specifically, it binds to activin A and activin B, thus suppressing the stimulatory effects of these hormones on hypothalamic-pituitary-gonadal axis, and also binds to myostatin, inactivating its inhibitory effects on muscle growth and differentiation7,21. Both activins as well as myostatin are mediating their effects through binding to the activin-receptor II (actRII). Current efforts in on-going clinical trials are focusing on the evaluation of actRII-blockers for the treatment of obesity, insulin resistance and diabetes22, aiming to improve glucose homeostasis by promoting muscle growth, despite the lack of a profound muscle mass deficit in these conditions23. Our findings in combination with the results from animal studies support an alternative way to intervene in the follistatins-activins-myostatin system, i.e. through inactivation of FST aiming to improve glucose homeostasis by reducing insulin resistance in white adipose tissue and by decreasing hepatic glucose productions. Whether combining this latter approach with an actRII-blocker administration would provide even more significant results remains to be evaluated. Finally, all such approaches should be carefully assessed in terms of their impact not only on insulin sensitivity, glycemic control and body weight and composition but also in terms of muscle growth and reproductive function, especially in women.
In conclusion, our findings provide evidence in humans for an important role of FST on glucose homeostasis in healthy subjects as well as subjects with severe obesity, in insulin resistance and in T2D. These results extend recently published data from animal studies to humans and support the need for further evaluation of strategies for decreasing FST as a potential therapeutic approach for hyperglycemia and insulin resistance.
Supplementary Material
Figure S1: Changes in Glucagon/Insulin ratio from baseline (0 min) after oral or intravenous lipid intake (low or high rate) compared to control (0,9% NaCl).
PAUC ANOVA indicates the p value from one-way ANOVA of the area under the curve (AUC) between all groups; PAUC one group (e.g. IVG) vs PAUC another group (e.g. C), indicates the p value of the post-hoc Tukey’s test between the AUCs of the two groups; Tukey’s test for the specific timepoint between oral lipid group vs control: *, p<0.05; **, p<0.01; ***, p<0.001.
Figure S2: Scatterplot of linear regression analysis with linear equation line between % changes of FST from 0 to 3 months after operation (independent variable) vs % changes of HOMA-IR from 0 to 6 months after operation (dependent variable). r= Pearson correlation coefficient.
Acknowledgments
Funding
The current study was funded by NIH K24DK081913. NiP was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) –389891681 (PE 2431/2-1).
Footnotes
Clinical Trial Registration: Study 1 has the NCT01520454 number in ClinicalTrials.gov. Study 2 and Study 3 were performed in an earlier time-point, when registration was not obligatory. All studies were approved by the local institutional review boards and were in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice. Written informed consent was obtained from all the participants.
Declaration of Interests
CSM is advisor of Ansh Labs LLC
References
- 1.Hansen JS, Plomgaard P. Circulating follistatin in relation to energy metabolism. Molecular and cellular endocrinology. 2016;433:87–93. [DOI] [PubMed] [Google Scholar]
- 2.Moragianni VA, Aronis KN, Chamberland JP, Mantzoros CS. Short-term energy deprivation alters activin a and follistatin but not inhibin B levels of lean healthy women in a leptin-independent manner. The Journal of clinical endocrinology and metabolism. 2011;96(12):3750–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perakakis N, Upadhyay J, Ghaly W, et al. Regulation of the activins-follistatins-inhibins axis by energy status: Impact on reproductive function. Metabolism: clinical and experimental. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vamvini MT, Aronis KN, Chamberland JP, Mantzoros CS. Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. The Journal of clinical endocrinology and metabolism. 2011;96(11):3416–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen JS, Rutti S, Arous C, et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. The Journal of clinical endocrinology and metabolism. 2016;101(2):550–560. [DOI] [PubMed] [Google Scholar]
- 6.Polyzos SA, Kountouras J, Anastasilakis AD, Triantafyllou G, Mantzoros CS. Activin A and follistatin in patients with nonalcoholic fatty liver disease. Metabolism: clinical and experimental. 2016;65(10):1550–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makanji Y, Zhu J, Mishra R, et al. Inhibin at 90: from discovery to clinical application, a historical review. Endocrine reviews. 2014;35(5):747–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt C, Hansen RH, Hansen JB, et al. Over-expression of Follistatin-like 3 attenuates fat accumulation and improves insulin sensitivity in mice. Metabolism: clinical and experimental. 2015;64(2):283–295. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J, Rinnov A, Krogh-Madsen R, et al. Plasma follistatin is elevated in patients with type 2 diabetes: relationship to hyperglycemia, hyperinsulinemia, and systemic low-grade inflammation. Diabetes/metabolism research and reviews. 2013;29(6):463–472. [DOI] [PubMed] [Google Scholar]
- 10.Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. The Journal of clinical endocrinology and metabolism. 2016;101(7):2816–2825. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A, Sidis Y, Mahan A, et al. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva RN, Bueno PG, Avo LR, Nonaka KO, Selistre-Araujo HS, Leal AM. Effect of physical training on liver expression of activin A and follistatin in a nonalcoholic fatty liver disease model in rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2014;47(9):746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perakakis N, Mougios V, Fatouros I, et al. Physiology of activins/follistatins: associations with metabolic and anthropometric variables and response to exercise. The Journal of clinical endocrinology and metabolism. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tao R, Wang C, Stohr O, et al. Inactivating hepatic follistatin alleviates hyperglycemia. Nature medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98(16):9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungerleider NA, Bonomi LM, Brown ML, Schneyer AL. Increased activin bioavailability enhances hepatic insulin sensitivity while inducing hepatic steatosis in male mice. Endocrinology. 2013;154(6):2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vamvini MT, Hamnvik OP, Sahin-Efe A, et al. Differential Effects of Oral and Intravenous Lipid Administration on Key Molecules Related to Energy Homeostasis. The Journal of clinical endocrinology and metabolism. 2016;101(5):1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokkinos A, Alexiadou K, Liaskos C, et al. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obesity surgery. 2013;23(1):31–38. [DOI] [PubMed] [Google Scholar]
- 19.Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149(12):1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holst JJ, Madsbad S, Bojsen-Moller KN, et al. Mechanisms in bariatric surgery: Gut hormones, diabetes resolution, and weight loss. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2018;14(5):708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45(10):2333–2347. [DOI] [PubMed] [Google Scholar]
- 22.Garito T, Roubenoff R, Hompesch M, et al. Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals. Diabetes Obes Metab. 2018;20(1):94–102. [DOI] [PubMed] [Google Scholar]
- 23.Murton AJ, Marimuthu K, Mallinson JE, et al. Obesity Appears to Be Associated With Altered Muscle Protein Synthetic and Breakdown Responses to Increased Nutrient Delivery in Older Men, but Not Reduced Muscle Mass or Contractile Function. Diabetes. 2015;64(9):3160–3171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Changes in Glucagon/Insulin ratio from baseline (0 min) after oral or intravenous lipid intake (low or high rate) compared to control (0,9% NaCl).
PAUC ANOVA indicates the p value from one-way ANOVA of the area under the curve (AUC) between all groups; PAUC one group (e.g. IVG) vs PAUC another group (e.g. C), indicates the p value of the post-hoc Tukey’s test between the AUCs of the two groups; Tukey’s test for the specific timepoint between oral lipid group vs control: *, p<0.05; **, p<0.01; ***, p<0.001.
Figure S2: Scatterplot of linear regression analysis with linear equation line between % changes of FST from 0 to 3 months after operation (independent variable) vs % changes of HOMA-IR from 0 to 6 months after operation (dependent variable). r= Pearson correlation coefficient.