Abstract
Store-operated Ca2+ entry (SOCE) pathway plays important roles in many cellular processes, which is largely studied by using fluorescent Ca2+ indicator, Fura-2. Extracellular Mn2+ is able to cross the plasma membrane through SOCE and quenches the fluorescence signals from Fura-2. Thus, the fluorescence quenching rate by Mn2+ composes a convenient assay to monitor the extent of SOCE. This chapter describes an experimental method of Mn2+ quenching assay for both cultured esophageal epithelial and skeletal muscle cells. It also explains how to perform a quantitative analysis of graded SOCE.
Keywords: Store-operated Ca2+ entry, Mn2+ quenching, Fura-2-AM, Ratiometric dye, Isosbestic point, Esophageal squamous cell carcinoma, Skeletal muscle myotube
1. Introduction
Store-operated Ca2+ entry (SOCE) is important for many cellular processes, including proliferation, migration, gene transcription, and muscle contraction [1, 2]. In this pathway, intracellular Ca2+ release either through IP3 receptor or ryanodine receptor stimulated by a variety of agonists results in reduction of Ca2+ concentration in the lumen of endoplasmic or sarcoplasmic reticulum (ER/SR); the depleted Ca2+ stores in turn activate the SOCE channel to allow extracellular Ca2+ across the plasma membrane, which eventually refills the empty ER/SR Ca2+ stores [3]. A large body of knowledge about the functional properties of SOCE has been gained by fluorescence-based intracellular Ca2+ measurements using Fura-2, a ratiometric Ca2+ indicator [4]. To gain cell membrane permeability, Fura-2 is conjugated to acetoxymethyl ester (AM), and it remains in this nonfluorescent form [5]. After entering cells, Fura-2-AM is cleaved by intracellular esterase and converted into its fluorescent form. With emission wavelength at 510 nm, Fura-2, upon Ca2+ binding, presents not only a dramatic increase in fluorescence intensity but also a blueshift of excitation wavelength. Thus, the ratio of fluorescence with excitation wavelengths at 340 nm for a peak of Ca2+-bound form and 380 nm for a Ca2+-unbound form is commonly recorded to directly monitor the dynamics of intracellular Ca2+. This ratiometric readout avoids some experimental caveats, such as uneven loading, leakage, and photobleaching of fluorescent dye, and the issues that occur when measuring Ca2+ in the cells with uneven thickness of cell membranes or walls.
To further isolate the activity of unidirectional SOCE from intracellular Ca2+ release and Ca2+ extrusion on the simple intracellular Ca2+ measurement using Fura-2, Mn2+ quenching assay is employed [4]. Mn2+ is known to be able to permeate into cells via SOCE, while it circumvents the surface membrane extrusion mechanisms and translocation into ER by Ca2+ pumps. Since Mn2+ exhibits a much higher affinity to Fura-2 than Ca2+, Mn2+ influx leads to quenching of Fura-2 fluorescent signal. As the quenching rate of fluorescence is proportional to the amounts of Mn2+ that enter the cells through SOCE, Mn2+ quenching assay represents a convenient and selective method to determine the activity of SOCE. This assay can analyze both cell populations and single cells using a cuvette-based spectrofluorometer or a microscope-based system, respectively. This assay is also suitable for high-throughput screening.
2. Materials
Prepare all solutions using ultrapure water, which reaches a resistivity of 18.2 MΩ·cm at 25 °C (e.g., prepared by Milli-Q Water Purification System, EMD Millipore, MA), and analytical grade reagents. Prepare and store all reagents at room temperature unless indicated otherwise.
2.1. Solutions
-
10 × balanced salt solution (BSS) stock: 1400 mM NaCl, 28 mM KCl, 20 mM MgCl2, 120 mM glucose, 100 mM HEPES, pH 7.2.
Add about 300 mL water to a 1 L glass beaker, followed by 40.9 g NaCl, 1.05 g KCl, 2.04 g MgCl2, 10.8 g glucose, and 11.9 g HEPES (see Note 1). Mix well and adjust pH with NaOH to pH 7.2. Make up to 500 mL with water. Filter the solution with 0.22 μm sterile vacuum filtration system (500 mL Steritop bottle top filter unit, EMD Millipore, MA). Store at +4 °C.
1 M CaCl2 solution: Weigh 7.35 g CaCl2 and dissolve in water up to 50 mL.
0.5 M EGTA solution: Weigh 9.5 g EGTA into 30 mL water and stir with a magnetic stir bar. Add drop by drop of 1 M NaOH just enough to dissolve EGTA completely. Add water to a final volume of 50 mL (see Note 2).
Thapsigargin (TG): 5 mM in DMSO. Store at −20 °C protected from light.
Fura-2-AM: Dissolve into DMSO as 10 mM stock. Store at −20 °C protected from light (see Note 3).
2-Aminoethoxydiphenylborane (2-APB): 100 mM in DMSO. Store at +4 °C under desiccating conditions.
0.5 M MnCl2 solution.
20 mM N-benzyl-p-toluene sulfonamide (BTS) stock in DMSO.
2.2. Cell Culture
RPMI 1640/Ham’s F-12 mixed (1:1) medium containing 5% fetal bovine serum and 1% penicillin/streptomycin. Store at +4 °C.
DMEM complete medium containing 10% fetal bovine serum, 10% horse serum, and 1% penicillin/streptomycin. Store at +4 °C.
DMEM differentiation medium containing 2.5% horse serum and 1% penicillin/streptomycin. Store at +4 °C.
Glass bottom dishes (Cat.# P35G-1.5–14-C, MatTek, MA). The central part is No. 1.5 cover glass.
2.3. Equipment
Ratio imaging spectrofluorometer (e.g., Photon Technology International, NJ): xenon arc lamp, computer-controlled high-speed random access monochromator, cuvette-based emission monochromator, inverted fluorescence microscope (e.g., Nikon Eclipse TE200; Nikon, Japan), S Fluor 40×/1.30 oil objective (compatible for efficient UV transmission), and CCD camera (e.g., CoolSnap ES; Roper Scientific, GA).
3. Methods
3.1. Cell Culture
All cells are grown in glass-bottom dishes.
KYSE-150, a human esophageal squamous cell carcinoma (ECSS) cell line, is cultured in 5% CO2 atmosphere at 37 °C in RPMI 1640/Ham’s F-12 mixed medium.
C2C12, a mouse myogenic cell line, is cultured in 5% CO2 atmosphere at 37 °C in DMEM complete medium.
After C2C12 cells reach confluent, the culture medium is changed to DMEM differentiation medium. The C2C12 myo-blasts will fuse and differentiate into myotubes after 4 days.
3.2. Solutions and Dye Loading
- Prepare the following solutions freshly just before the experiment:
- BSS-EGTA: Take out 5 mL 10× BSS stock solution in tissue culture hood, and fill up to 50 mL with water. Add 5 μL 0.5 M EGTA to generate 0.5 mM final concentration of EGTA.
- BSS-Ca: Take out 5 mL 10× BSS stock solution in tissue culture hood, and fill up to 50 mL with water. Add 10 μL 1 M CaCl2 to generate 2 mM final concentration of CaCl2.
- BSS-EGTA-TG: Take out 5 mL BSS-EGTA solution, and add 1 μL TG to generate 10 μM final concentration of TG.
- BSS-Ca-2APB: Take out 10 mL BSS-Ca solution, and add 10 μL 2-APB to generate 100 μM final concentration of 2-APB.
- BSS: Take out 5 mL 10× BSS stock solution in tissue culture hood, and fill up to 50 mL with water.
- BSS-Mn: Take out 10 mL BSS solution, and add 10 μL MnCl2 to generate 0.5 mM final concentration of MnCl2.
- BSS-Mn-2APB: Add 10 μL 2-APB into 10 mL BSS-Mn to generate 100 μM final concentration of 2-APB.
- Triton X-100: 0.1% in BSS-EGTA.
Remove the medium and rinse the cells with BSS-Ca once.
Add 1 mL BSS-Ca solution containing 5 μM Fura-2-AM into the dish, and wrap the whole dish with foil to protect from light.
Incubate the cells for 40 min at 37 °C in cell culture incubator (see Note 4).
Take out the dishes and leave them for an additional 15 min period at room temperature to complete ester hydrolysis. Wash the cells with BSS-Ca twice.
3.3. Intracellular Ca2+ Measurement
Mount the dish in which KYSE-150 cells are cultured on the stage of inverted microscope, which is connected to PTI spec-trofluorometer, with excitation wavelengths at 350 and 385 nm and emission at 510 nm (see Note 5).
Set up a gravity perfusion system with four different solutions: BSS-Ca2+, BSS-EGTA, BSS-EGTA-TG, and BSS-Ca-2APB. Computer-controlled automated perfusion systems can also be used.
Position the opening tip of the perfusion system at 10× magnification until the tip is right above the region of interest at a 45° angle.
Choose emission wavelength as 510 nm and excitation wavelength as 350 nm and 385 nm. After drawing regions of interest (ROIs) around individual cells, simultaneously record the fluorescence intensities at 510 nm with excitation wavelengths at 350 and 385 nm. Ratio (F350 nm/F385 nm) is displayed in the third window (see Note 6).
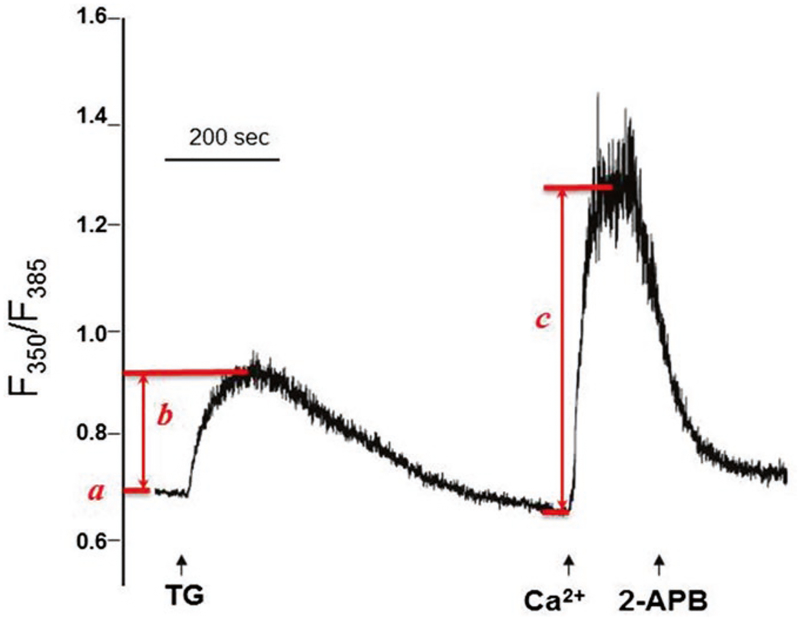
Change the perfusion solutions in the specified order: BSS-Ca2+ (30 s), BSS-EGTA (30 s), BSS-EGTA-TG (900 s), BSS-Ca (150 s), and BSS-Ca-2APB (300 s) (Fig. 1).
From the traces of ratio (F350 nm/F385 nm), several parameters regarding intracellular Ca2+ homeostasis can be determined: (a) resting intracellular Ca2+, (b) TG-releasable ER Ca2+ stores, and (c) SOCE (Fig. 1).
Fig. 1.
Intracellular Ca2+ measurement reveals SOCE in KYSE-150 cells. The initial ratio of F350/F385 represents resting intracellular Ca2+ (a). Addition of TG (indicated by first arrow) in BSS-EGTA bath solution induces passive Ca2+ release from ER. The TG-sensitive ER Ca2+ stores can be displayed as ΔF350/F385 (b). After ER Ca2+ stores are completely depleted (~900 s), addition of extracellular 2 mM Ca2+ (indicated by second arrow) activates a sustained intracellular Ca2+ elevation through SOCE, which can be represented by ΔF350/F385 (c). This SOCE can be blocked by SOCE inhibitor 2-APB (indicated by third arrow)
3.4. Mn2+ Quenching Recording and Analysis
Isosbestic point, a specific wavelength at which the total fluorescence of Fura-2 does not change along with Ca2+ concentration, should be determined first. Perform excitation wavelength scanning of Fura-2 in solutions containing various Ca2+ concentrations ranging from 0 to 39.8 μM to determine the Ca2+ concentration-independent wavelength as the isosbestic point. Generally, the isosbestic point is at 360 nm, but may be slightly different in different optical system (see Note 7).
Mount the dish with C2C12 myotubes on the stage of the microscope, and select the “excitation ratio” mode with excitation wavelengths at 360 nm and 385 nm and emission at 510 nm. Record the fluorescence intensity at 510 nm with excitation wavelengths at 360 and 385 nm. The ratio (F360 nm/F385 nm) can be displayed in separate windows, providing information regarding intracellular Ca2+ homeostasis: resting intra-cellular Ca2+, TG-releasable ER Ca2+ stores, and SOCE (see Note 8).
Pretreat the C2C12 myotubes with 20 μM BTS (a selective inhibitor of the ATPase activity of skeletal muscle myosin II that reversibly blocks gliding motility and suppresses force and twitch production in fast skeletal muscle) for 15 min in BSS-Ca solution.
Select ROIs for individual myotubes.
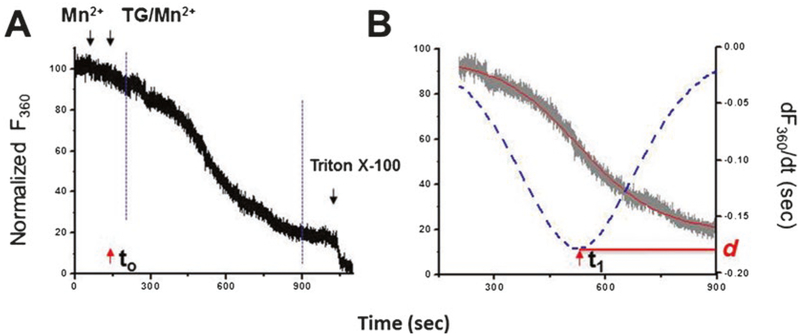
Perform the running protocol: BSS-Mn (30 s), BSS-TG/Mn (900 s), or BSS-TG/Mn-2APB (300 s) and Triton X-100 (Fig. 2a) (see Note 9).
The decay of Fura-2 fluorescence upon Mn2+ addition is expressed as percentage decrease in Fura-2 fluorescence per unit time (the initial fluorescence is set to be equal to 100%). The completely quenched fluorescence signal is established at the end of each experiment by lysing the cells with 0.1% Triton X-100 (as 0%).
F360 curve is fitted into sigmoidal curve using Origin Pro7.5 (OriginLab, MA). To quantify the graded activation of SOCE, the first-order derivative of changes in sigmoidal curve of F360, dF360/dt, is analyzed [6] (Fig. 2b). This analysis provides two useful kinetic parameters: SOCEmax (d), the peak slope of Mn2+ quenching, and Δτ, the duration from initiation of TG-induced Ca2+ release (t0) to the point where SOCEmax is reached (t1).
Fig. 2.
Mn2+ quenching assay reveals graded activation of SOCE in C2C12 myotubes. (a) The decay slope of Fura-2 fluorescence is measured at the Ca2+-independent excitation wavelength 360 nm of Fura-2 (F360). Addition of Mn2+ only (indicated by first black arrow) is recorded as baseline; the completely quenched fluorescence signal is established at the end of each experiment by lysing the cells with 0.1% Triton X-100 (as 0%). Simultaneous application of TG and Mn2+ (second black arrow, t0) results in a gradually accelerated decay of fluorescence until reaching a maximal quenching rate. The gradual developed Mn2+ quenching can be extracted for further analysis (between dashed blue lines). (b) The extracted trace of F360 can be fitted in a sigmoidal curve (red line). The activation of SOCE is expressed as percentage decrease in fluorescence per unit time (the initial value as 100%). To quantify the graded activation of SOCE, the first-order derivative of changes, dF360/dt, is determined (blue dotted curve). This analysis provides two useful kinetic parameters: SOCE (d), the peak slope of Mn2+ quenching, and Δτ, the duration from initiation of TG-induced Ca2+ release (t0) to the point where SOCEmax is reached (t1)
Acknowledgments
This work was supported by research grants from NIH R01 CA185055 and Pelotonia to ZP.
Notes
Put a magnetic stir bar into the beaker, and stir the water while putting in the salts and other reagents slowly, which can help dissolve the salts relatively easily at room temperature.
EGTA is only dissolvable in solutions with pH 8 and above. To avoid an overload of Na+ in the solution, it is necessary to add just enough NaOH adjusting pH. If the addition of water into the entirely dissolved EGTA causes small amounts of EGTA to precipitate in the solution, add one or two more drops of NaOH, and bring the final volume to 50 mL.
It is preferable to prepare aliquots of Fura-2-AM and store in a light-proof and air-tight package containing desiccant. Any opened, unused Fura-2-AM should be discarded after a week.
The concentrations of Fura-2-AM and incubation time vary for different cell types and tissues [7]. The optimal dye-loading condition should be determined by varying Fura-2-AM concentrations and incubation time prior to experiments. Unusually high concentrations of Fura-2-AM and prolonged incubation time at 37 °C may cause fluorescence to accumulate in intracellular vesicles. The optimal condition is homogenous fluorescence with good signal/noise ratio at lowest concentration of Fura-2-AM and shortest incubation time.
Although the excitation wavelengths for Ca2+-binding and Ca2+-free Fura-2 are 340 nm and 380 nm, respectively, the best ratio dynamic range of Fura-2 for Ca2+ measurement may occur at other wavelengths in a particular microscope system. Such shifts in wavelength are due to changes in the optical path with the addition of various optical components. Thus, the exact excitation wavelengths should be determined first for any particular microscope system. The excitation spectrum of Fura-2 salt can be scanned in solutions containing Ca2+ ranging from 0 to 39.8 μM (or higher concentration at which Fura-2 fluorescence is saturated). The two wavelengths with best ratio dynamic are chosen.
Consult the manufacture’s handbook for how to use the PTI Ratio Image program.
The isosbestic point for each system varies, too. Therefore, it is important to determine these wavelengths by spectrum scanning. Alternatively, Mn2+ quenching can be recorded by adjusted fluorescence at any two wavelengths in such a way that the final value is independent of Ca2+ concentration.
Since F360 nm is not the excitation peak for Ca2+-bound Fura-2, the ratio of F360/F385 is not as sensitive as F350/F385 to reflex the changes in intracellular Ca2+.
At the end of each experiment, Triton X-100 (0.1%) is employed to obtain the background reading. BSS-Mn-2APB solution is used instead of Mn2+ to examine the abolished fluorescence quenching, which indicates that the Mn2+ entry is carried out through SOCE pathway. For different cell types, it is also important to test the concentration of Mn2+ used in the quenching assay for a moderate quenching rate.
References
- 1.Pan Z, Ma J (2015) Open Sesame: treasure in store-operated calcium entry pathway for cancer therapy. Sci China Life Sci 58(1):48–53. 10.1007/s11427-014-4774-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Z, Brotto M, Ma J (2014) Store-operated Ca2+ entry in muscle physiology and diseases. BMB Rep 47(2):69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parekh AB, Putney JW Jr (2005) Store-operated calcium channels. Physiol Rev 85(2):757–810 [DOI] [PubMed] [Google Scholar]
- 4.Pan Z, Zhao X, Brotto M (2012) Fluorescence-based measurement of store-operated calcium entry in live cells: from cultured cancer cell to skeletal muscle fiber. J Vis Exp (60). 10.3791/3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poenie M, Tsien R (1986) Fura-2: a powerful new tool for measuring and imaging [Ca2+]i in single cells. Prog Clin Biol Res 210:53–56 [PubMed] [Google Scholar]
- 6.Zhao X, Weisleder N, Han X et al. (2006) Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J Biol Chem 281(44):33477–33486. 10.1074/jbc.M602306200 [DOI] [PubMed] [Google Scholar]
- 7.Malgaroli A, Milani D, Meldolesi J, Pozzan T (1987) Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol 105(5): 2145–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]