Abstract
MicroRNAs (miRNAs) are RNA molecules at about 22 nucleotide in length that are non-coding, which regulate gene expression in the post-transcriptional level by performing degradation or blocks translation of the target mRNA. It is known that they play roles in mechanisms such as metabolic regulation, embryogenesis, organogenesis, differentiation and growth control by providing post-transcriptional regulation of gene expression. With these properties, miRNAs play important roles in the regulation of biological processes such as proliferation, differentiation, apoptosis, drug resistance mechanisms in eukaryotic cells. In addition, there are miRNAs that can be used for cancer therapy. Tumor cells and tumor microenvironment have different miRNA expression profiles. Some miRNAs are known to play a role in the onset and progression of the tumor. miRNAs with oncogenic or tumor suppressive activity specific to different cancer types are still being investigated. This review summarizes the role of miRNAs in tumorigenesis, therapeutic strategies in human cancer and current studies.
Keywords: Cancer therapy, miRNA mimic, miRNA antagonists, miRNA sponges, miRNA masking
Introduction
MiRNAs have gained rapid diagnostic and therapeutic value by providing unique expression profiles and high stability in biological samples that have multiple targets or are involved in many cancer-related pathways. In general, the therapeutic side of miRNAs is provided by inhibiting oncogenic miRNAs or by regenerating tumor suppressor miRNAs. The first miRNA synthesized by RNA polymerase II is about 700 bases in length (Bartel 2004). These miRNAs, called pri-miRNAs, are transcribed in the nucleus, and then Drosha is a member of the RNAase III, cleaves this pri-miRNA from both ends with complex endonuclease activity (Denli et al. 2004). As a result of this process, pri-miRNA is called pre-miRNA and its length is about 70–100 bases. Pre-miRNAs are transferred from the nucleus to the cytoplasm by a carrier protein called “exportin-5” (Bartel 2004; Cullen 2004; Gregory et al. 2004; Lund et al. 2004).
The pre-miRNAs transferred to the cytoplasm are linked to the “Dicer” (RNAse III member) molecule. Dicer cleaves pre-miRNA’s stem-loop to yield shorter, double-stranded miRNAs (duplex miRNAs). These miRNAs are 21–23 nucleotides long with two unpaired 3′ nucleotides at each end. But only one of the strands is bound to RISC (RNA-stimulated silencing complex) (Lund et al. 2004; Pillai et al. 2007).
The RISC-miRNA complex matches the transcript in the 3′ UTR region of the target mRNA, so that the translation of the transcripts is repressed through different mechanisms. With this repression event, oncomiRs play roles such as inhibition of metastasis, induction of metastasis, drug resistance mechanisms (Chen et al. 2007; Shenouda and Alahari 2009; Shi et al. 2010). However, some miRNAs have been shown to work as tumor suppressor in breast cancer (Esquela-Kerscher and Slack 2006), lung cancer (Zhang et al. 2013a, b), colorectal cancer (Liu and Chen 2010) and ovarian cancer (Kinose et al. 2014), while others have been shown to have oncogenic properties with profiling studies.
One of the most attractive features of miRNAs as therapeutic agents is their ability to target multiple molecules. This advantage is highly effective in regulating different biological cell processes related to normal tissue and tumor tissue. Moreover, miRNAs have many roles in cancer progression, such as proliferation, apoptosis, cell cycle arrest (Park et al. 2011; Wang et al. 2014; Qin et al. 2016; Cui et al. 2018), migration, invasion, metastasis (Zhang et al. 2012; Mohammadi-Yeganeh et al. 2016; Lei et al. 2017), cytokine secretion (Yu et al. 2013; Mignacca et al. 2016; Muhammad et al. 2016), T cell differentiation (Jeker and Bluestone 2013; Kroesen et al. 2015; Tao et al. 2018), drug resistance (Li et al. 2015; Yang et al. 2015a; Zhao et al. 2017; Chen et al. 2018b), and chemo-sensitivity (Giunti et al. 2015; Li et al. 2017a; Chen et al. 2018b) by activating molecular target. This knowledges suggests that miRNAs can be used as adjunct vehicles for cancer progression (Shenouda and Alahari 2009; Babashah and Soleimani 2011; Garofalo and Croce 2013; Cheng et al. 2014) (Table 1).
Table 1.
Expression levels and functions of miRNAs in different types of cancers
| Cancer type | miRNAs | Expression | Putative function | References |
|---|---|---|---|---|
| Breast cancer | miR-340 | Down | Down-regulates the metastatic capability | (Mohammadi-Yeganeh et al. 2016) |
| miR-145 | Down | Inhibits cell proliferation, migration and invasion | (Kim et al. 2011; Mar-Aguilar et al. 2013; Zheng et al. 2015) | |
| miR-125b | Down | Suppresses proliferation and invasion | (O’Day and Lal 2010; Mar-Aguilar et al. 2013) | |
| miR-21 | Up | Stimulates cell proliferation and migration, and chemotherapy resistance | (Wang et al. 2011; Yan et al. 2011; Paik et al. 2013; Zhang et al. 2016; Haghnavaz et al. 2018) | |
| miR-155 | Up | Downregulation of FOXO3a and induces chemo-resistance in tumor cell | (Jang et al. 2017; Migita et al. 2017) | |
| miR-9 | Up | Regulates E-cadherin and cancer metastasis | (Ma et al. 2010b; Jang et al. 2017) | |
| Lung cancer | miR-145 | Down | Inhibits cell growth and metastasis | (Mataki et al. 2016; Chen et al. 2018a; Li et al. 2018; Skjefstad et al. 2018) |
| Let-7 family | Down | Represses cell proliferation and regulates the cell cycle | (An et al. 2015; Castro et al. 2017) | |
| miR-340 | Down | Negative regulator in tumorigenesis and cancer progression | (Fernandez et al. 2014) | |
| miR-221/222 | Up | Stimulates cell proliferation and migration | (Barger and Nana-Sinkam 2015) | |
| miR-21 | Up | Suppresses apoptosis and induces cell growth | (Xie et al. 2010; Yu et al. 2010; Li et al. 2014a; Barger and Nana-Sinkam 2015) | |
| miR-20a | Up | Promotes cell viability and motility | (Inamura and Ishikawa 2016; Wei and Ran 2018) | |
| miR-141 | Up | Plays role in epithelial-mesenchymal transition (EMT) | (Mei et al. 2014; Barger and Nana-Sinkam 2015) | |
| Prostate cancer | miR-199a | Down | Suppresses proliferation, invasion and chemotherapy resistance | (Qu et al. 2017; Chen et al. 2018b) |
| miR-31 | Down | Inhibits cell-cycle regulators | (Bhatnagar et al. 2010; Lin et al. 2013; Li and Mahato 2014) | |
| miR-145 | Down | Inhibits invasion, migration and arrests cell cycle | (Sachdeva et al. 2009; Avgeris et al. 2013) | |
| miR-15a/16-1 | Down | Induction of apoptosis, modulate cytokine secretion | (Li and Mahato 2014; Jin et al. 2018; Tao et al. 2018; Zidan et al. 2018) | |
| miR-148a | Up | Promotes PCa growth | (Dybos et al. 2018) | |
| miR-221/222 | Up | Regulates proliferation, apoptosis, and invasion | (Kneitz et al. 2014; Sun et al. 2014; Wang et al. 2015) | |
| Ovarian cancer | miR-133b | Down | Increases sensitivity to chemotherapy drugs | (Chen et al. 2015; Liu and Li 2015; Yang et al. 2017) |
| miR-199a | Down | Regulates drug resistance and enhances cisplatin sensitivity | (Wang et al. 2013; Cui et al. 2018) | |
| miR-200 family | Up | Potential candidate biomarkers for non-invasive screening | (Sulaiman et al. 2016; Pendlebury et al. 2017) | |
| miR-141 | Up | Plays chemotherapy resistance role | (Mak et al. 2017; Weidle et al. 2018) | |
| miR-130a/130b | Up | Enhances drug resistance | (Zhang et al. 2013b; Zong et al. 2014; Li et al. 2015) | |
| Colon cancer | miR-15b | Down | Promotes cellular apoptosis and reverses the chemo-resistance | (González-Vallinas et al. 2014; Zhao et al. 2017) |
| miR-200c | Down | Induces cell apoptosis, inhibits invasion, and metastasis | (Chen et al. 2012, 2014a; Pan et al. 2017) | |
| miR-145 | Down | Reverses the development of colon cancer | (Xu et al. 2012; Yu et al. 2015; Li et al. 2016; Qin et al. 2016) | |
| miR-126 | Down | Inhibits colon cancer proliferation and invasion | (Li et al. 2013; Wang 2014; Yuan et al. 2016) | |
| miR-135 | Up | Increases invasion and metastasis | (Valeri et al. 2012) | |
| Bladder cancer | miR-29c | Down | Regulates cell growth and invasion | (Xu et al. 2013a; Fan et al. 2014; Zhao et al. 2015) |
| miR-133b | Down | Inhibits cell proliferation, migration and invasion | (Zhou et al. 2013; Chen et al. 2014b) | |
| miR-96 | Up | Promotes cell proliferation and invasion, suppresses apoptosis | (Guo et al. 2012; Wu et al. 2015; Xu et al. 2018) | |
| Osteo-carcinoma | miR-497 | Down | Inhibits cell proliferation, migration, and invasion | (Shao et al. 2015; Wang et al. 2016a; Gui et al. 2017) |
Therapeutic strategies
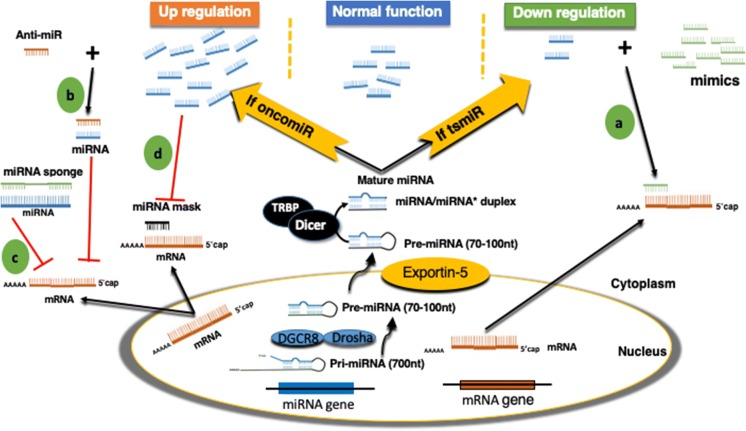
In generally, there are two basic strategies for therapeutic approaches to regulate miRNA expressions. These strategies involve the use of oligonucleotides or virus-based constructs to inhibit the expression of an oncogenic miRNA or to reactivate a tumor suppressor miRNA that has been lost in cancer. As shown in Fig. 1, there are four different mechanisms that are supposed to stop the onset and progression of the tumor. miRNA mimic, anti-miRNA oligonucleotides (anti-miRs), miRNA sponges, and miRNA masking treatment approaches have been described in detail.
Fig. 1.
Schematic diagram of miRNA biogenesis and the therapeutic strategies. miRNA-based molecular cancer therapy for oncogenic (oncomiR) and tumor suppressor miRNAs (tsmiRs). The cancer therapies include miRNA mimics (a), anti-miRNA oligonucleotides (anti-miRs) (b), miRNA sponges (c), and miRNA masking (d)
miRNA mimics approaches
MiRNA mimic technology (miR-Mimic) is an approach based on gene silencing. After transfection into cells, they act as mature endogenous miRNAs. miR-Mimics are chemically synthesized, double-stranded RNAs. The sequence at the 5′ end of these RNA fragments is designed to be partially complementary to the sequence at the 3′ UTR of the target gene (Ji et al. 2017). A lost or downregulated tumor suppressor miRNA (tsmiR) activity can be restored using miRNA mimics (Bader et al. 2011). This method has been studied in many types of cancer and successful progress has been recorded (Table 2).
Table 2.
Studies involving the use of miRNA mimics and the effects of these mimics on cancer development
| Mimic | Cancer type | Effect | References |
|---|---|---|---|
| Let-7 family | Breast | Inhibits growth and migration, and attenuates breast cancer cell metastasis | (Liu et al. 2015; Wang et al. 2016b) |
| Gastric | Reverses multidrug resistance | (Yang et al. 2015a) | |
| miR-1 | Gastric | Inhibition of cell proliferation, invasion, migration and promotion of apoptosis and cell cycle arrest targeting MET | (Han et al. 2015) |
| miR-21 | Breast | Stimulation of cellular proliferation targeting STAT3 | (Yu et al. 2016; Zhang et al. 2016) |
| Gastric | Stimulates cancer growth and invasion | (Li et al. 2014b) | |
| Endometrial | Contributes to cell proliferation | (Qin et al. 2012) | |
| miR-34a | Lung | Inhibits lung tumors in mice targeting Bcl-2 | (Trang et al. 2011; Kasinski and Slack 2012) |
| Prostate | Inhibits prostate cancer stem cells and metastasis | (Liu et al. 2011) | |
| Pancreatic | Inhibits pancreatic cancer growth in mice | (Pramanik et al. 2011) | |
| miR-125b | Breast | Targets erythropoietin (EPO) and its receptor (EPOR) | (Ferracin et al. 2013) |
| Retinoblastoma | Promotes tumor growth and suppresses apoptosis | (Bai et al. 2016) | |
| miR-143 | Gastric | Inhibits cell proliferation and invasion by targeting DNMT3A | (Zhang et al. 2017) |
| miR-145 | Lung | Inhibits migration and invasion by down-regulating FSCN1 | (Zhang and Lin 2015) |
| Colon | Reduces tumor proliferation and increased apoptosis targeting Pim-1 | (Ibrahim et al. 2011) | |
| Prostate | Modulates tumor sensitivity to radiation and reduces cell growth in the androgen-dependent AR positive cell lines | (Gong et al. 2015; Larne et al. 2015) | |
| miR-148a | Breast | Increases expression of the estrogen receptor-alpha targeting DNMT3A | (Xu et al. 2017) |
| Gastric | Enhances cisplatin cytotoxicity | (Li et al. 2017b) | |
| miR-155 | Ovarian | Promotes apoptosis | (Chen et al. 2016) |
| miR-181b/miR-217 | Prostate | Enhances chemo sensitivity of cell | (Lin et al. 2018) |
| miR-199a | Ovarian | Reverses cisplatin resistance in cells through the inhibition of mTOR targeting HIF1α | (Feng et al. 2017) |
| miR-203 | Cervical | Inhibits the proliferative and migratory capacities of cell | (Yin et al. 2016) |
| Endometrial | Decreases SOX4 gene expression and regulates gene expression and physiological processes including proliferation and cell migration | (Huang et al. 2014; Zierau et al. 2018) | |
| Glioblastoma | Inhibits the proliferation and invasion by directly targeting PLD2 | (Chen et al. 2014c) | |
| miR-221/222 | Prostate | Promotes growth and invasion | (Wang et al. 2015) |
| Hepatocellular Carcinoma | Promotes growth and invasion of cell by accompanies activation of NF-kB | (Liu et al. 2016) | |
| miR-340 | Lung | Suppresses cell proliferation and induces apoptosis | (Fernandez et al. 2014) |
| Hepatocellular Carcinoma | Inhibits the proliferation and invasion targeting JAK1 | (Yuan et al. 2017) | |
| Breast | Inhibits cancer progression | (Shi et al. 2017) | |
| miR-452 | Breast | Contributes to the docetaxel resistance and decreases apoptotic rate | (Hu et al. 2014) |
| miR-544a | Lung | Promotes the invasion of cancer cells | (Mo et al. 2014) |
| miR-892B | Breast | Activates NF-kB and promotes aggressiveness | (Jiang et al. 2016) |
Overexpression of miR-892b mediated by mimics in breast cancer cells attenuates the NF-κB signaling pathway. Jiang et al. reported that in vitro and in vivo tumor growth significantly reduced the induction of metastatic capacity and angiogenesis (Jiang et al. 2016). In non-small cell lung cancer (NSCLC), miR-340 expression was found to be inversely proportional to tumor prognosis. Mimics use of MiR-340 overexpression has been shown to suppress cell proliferation and induce apoptosis in NSCLC cells (Fernandez et al. 2014).
Down-regulation of miR-1 is present in gastric cancer cells. In a study by Han et al., MiR-1 mimic transfusion has been suggested to change this condition. It has been found that cell proliferation and migration are suppressed by Mimic regulation (Han et al. 2015). Wang and colleagues performed transfection of miR-221/222 mimics in prostate cancer cells. After transfection, it increased cell proliferation activity and inhibited pro-apoptotic effect by suppressing caspase-10 (Wang et al. 2015). The generation of viral vectors (adenoviral, lentiviral and retroviral vectors) that express specific miRNAs can increase the expression of miRNAs in tumor cells (Naidu et al. 2015). Although inhibition of tumor growth of miRNAs synthesized by viral vectors is another approach, miRNA mimics have the potential to be a more promising therapeutic approach since they lack vector-based toxicity.
miRNA antagonists (antagomiRs)
In the literature, it has been shown that miRNAs identified as oncogenes increase in cancer tissues. This leads to increased cell turnover and cell proliferation with increased expression of miRNAs. Inhibition of these miRNAs has become an important area for gene therapy. There are several different methods that are investigated to prevent the binding of oncogene miRNAs to their targets. Inhibiting these miRNAs has been a hope light in cancer therapies to transfect miRNA into cells or tissue to slow down and eliminate tumor growth.
Antagonistic oligonucleotides (antagomiRs, anti-miRs) affect miRNA-related pathways by binding and blocking oncomiR. The single-stranded anti-miRs are based on first-generation antisense oligonucleotides (ASO) designed to target mRNAs or modified by locked nucleic acids (LNAs). These synthetic small RNA molecules have a complementary sequence to inhibit the function of the miRNA by binding strongly (Rupaimoole and Slack 2017). mir-122, which is the first miRNA inhibited by anti-miR contain 3′conjugation cholesterol residues containing 2′-O-methylation of ribose residues (Krützfeldt et al. 2005). They involve the partial modification of phosphodiester bonds via phosphorothioate linkages by replacing one of the bridging oxygen atoms with sulfur (Garofalo and Croce 2013). For example, miR-21, is an oncomiR, known as an anti-apoptotic factor in breast cancer cells, blocks PTEN by activation of the PI3K pathway. Result of MiR-21 up regulation, Bcl2 regulation occurs and apoptosis is inhibited. Anti-miR-21 has been shown to affect breast cancer cells through apoptosis activation and decreased cellular proliferation (Yan et al. 2011). Transfusion of anti-miRNA oligonucleotides targeting MiR-21 has been shown to arrest cell growth in vitro conditions (Chan et al. 2005). Several recent studies on anti-miRNA oligonucleotides in different types of cancer have been shown in Table 3.
Table 3.
Studies involving the use of anti-miRNA and the effects of these anti-miRs on cancer development
| Anti-miRNA | Cancer type | Effect | References |
|---|---|---|---|
| miR-10b | Breast | Decreases metastasis targeting Hoxd10 | (Ma et al. 2010a; Yoo et al. 2015) |
| miR-21 | Breast | Enhances chemo-sensitivity and Inhibition of tumor growth | (Obad et al. 2011; Teng et al. 2013; Giunti et al. 2015) |
| Retinoblastoma | Inhibits malignant progression | (Ding et al. 2014) | |
| Colorectal | Inhibits cell growth and invasive behaviors | (Nedaeinia et al. 2016) | |
| Hepatocellular | Suppresses cell growth | (Wagenaar et al. 2015) | |
| mİR-96 | Lung | Exhibits a tumor-suppressor function targeting LMO7 | (Wu et al. 2017) |
| miR-133b | Ovary | Reduces ovarian cancer drug resistance targeting MDR1 | (Chen et al. 2015) |
| miR-155 | Breast | Inhibits cancer progression | (Babar et al. 2012) |
| Prostate | Promotes chemo-sensitivity and induces cell cycle arrest targeting ANX7 | (Cai et al. 2015; Li et al. 2017a) | |
| miR-182 | Ovary | Reduces ovarian cancer burden, invasion, and metastasis targeting BRCA1, FOXO3, HMGA2 | (Xu et al. 2014) |
| Breast | Deceases tumorigenicity of cell | (Chiang et al. 2013) | |
| miR-191 | Breast | Increases chemo-sensitivity | (Sharma et al. 2017) |
| miR-200 family | Endometrioid Carcinoma | Inhibits the growth of cancer cells | (Lee et al. 2011) |
| Colon | Decreases proliferation activity and increases apoptosis ability | (Fan et al. 2015) | |
| miR-203 | Breast | Suppresses ER-positive breast cancer proliferation and stemness | (Muhammad et al. 2016) |
| miR-221/222 | Hepatocellular Carcinoma | Inhibits growth and invasion | (Park et al. 2011; Liu et al. 2016) |
| Breast | Suppresses cell growth and invasion | (Obad et al. 2011) | |
| Liver tumorigenesis | Inhibits cancer progression | (Pineau et al. 2010) | |
| miR-494 | Breast | Inhibition of tumor growth and metastasis | (Liu et al. 2012) |
miRNA sponges
As an alternative to chemically modified antisense oligonucleotides, Ebert et al., have developed miRNA inhibitors that can be expressed in cells, such as transgenic RNAs. These competitive inhibitors, termed MiRNA sponges, are transcripts exerting from strong promoters containing multiple common binding sites to the target oncomiR (Ebert and Sharp 2010). When the vectors encoding these sponges are transiently transfected into the respective cells, it binds to the target oncomiR and prevents binding to the mRNA (de Melo Maia et al. 2015). Thus, the target mRNA remains free. This mechanism at least releases miRNA targets as well as chemically modified antisense oligonucleotides (Kluiver et al. 2012). Sponges contain two to seven consecutive nucleotides and are linked to the sequences of the respective miRNAs. They specifically inhibit miRNAs with a complementary heptameric sequence so that a single sponge can be used to inhibit members of an entire miRNA family (Ebert et al. 2007). Sponges run by RNA polymerase II (Pol II) contain a fluorescent reporter gene for the identification and classification of processed cells (van Rooij and Kauppinen 2014). In other words, miRNA sponges are composed of transgenic cells and block all other miRNAs of the same family. As indicated in Table 4, miRNA sponges are effectively used in cancers such as breast, lung, renal, melanoma. MiR-9 is an oncomiR that promotes cell migration and metastasis. Downregulation of E-cadherin originating from miR-9 contributes to up-regulation of the gene encoding VEGF (Vascular Endothelial Growth Factor). This is terminated by the activation of beta-catenin signaling. Thus, the tumor causes an increase in angiogenesis. The use of a miRNA sponge in highly malignant cells inhibits miR-9 inhibition and metastasis formation. Ma and colleagues reported using miR-9 sponges that their activity reduced miR-9 activity by 50% (Ma et al. 2010b). Mignacca et al. reported that miRNA sponges used against miR-19 and miR-155 inhibited the function of these miRNAs. This has been shown to increase the induction of p53 and SOCS1 (cytokine signaling-1) in human myeloma cells and mouse leukemia cells. It has been suggested that antagonism of miRNA activity may re-activate cytokine-induced tumor suppressor pathway activity in leukemic cells (Mignacca et al. 2016).
Table 4.
Studies involving the use of miRNA sponges and the effects of these sponges on cancer development
| Sponges | Cancer type | Effect | References |
|---|---|---|---|
| miR-9 | Breast cancer | Inhibits metastasis formation | (Ma et al. 2010b) |
| miR-10b | Breast cancer | Suppresses the colony formation and inhibits the migration and invasion of the cells | (Liang et al. 2016) |
| miR-19/miR-155 | Hematopoietic cancer | Increases ability of inhibiting cell growth and cell migration | (Mignacca et al. 2016) |
| miR-21 | Hepatocellular carcinoma | Suppresses cell proliferation by up-regulating MAP2K3 expression at both mRNA and protein levels | (Xu et al. 2013b) |
| Melanoma | Displays anti-cancer activities | (Liu et al. 2013) | |
| Non-small cell lung cancer | Reduces proliferation, migration, and invasion of A549 cells by upregulating PDCD4 expression | (Yang et al. 2015b) | |
| Renal cancer | Inhibited proliferation, migration and invasion of renal cancer cells | (Dey et al. 2012) | |
| miR-221 | Melanoma | Displays anti-cancer activities | (Liu et al. 2013) |
miRNA masking
The miRNA-masking (miR mask) developed by Choi et al., is another strategy based on antisense oligonucleotide technology (Choi et al. 2007). Unlike miRNA sponges, miR-masks completely complement the predicted miRNA binding sites in the 3′-UTR of a specific target mRNA and consist of antisense oligonucleotides modified with single chain 2′-O-methyl (Li et al. 2009). Thus, the miR-mask can prevent the miRNA from accessing the binding site on the target mRNA to disrupt the inhibitory function. By the miR-masking approach, miR-1 and miR-133, which complement the channel coding genes of the pacemaker such as HCN2 and HCN4, are blocked from inhibiting protein expression of these genes. In the miR-mask transfected rat model, heart rate acceleration was possible (Choi et al. 2007). Similar to endogenous miRNA, the effect of AMOs are sequence-specific, not gene-specific. For this reason, AMOs can cause adverse side effects and undesirable toxicity (Xiao et al. 2007). This may be a disadvantage for cancer treatment where multiple pathway targeting may be desirable, although unwanted or non-target factors may be significantly reduced with this approach. The unpredictability, deficiencies and disadvantages of the results of the miRNA masking approach make it far from a promising therapeutic approach. Therefore, there are no consistent studies conducted with this method in recent years.
MiRNA therapeutics in preclinical or clinical trials
Currently, adjuvant chemotherapy and radiation are frequently used in cancer patients except surgical resection. Recent advances provide hope for cancer treatment by regulating gene expression of non-coding RNAs. These developments aim to provide new therapeutic approaches for cancer treatment and to expand a new method to inhibit cancer by miRNA using recently developed LNA technology (Garzon et al. 2010; van Rooij and Kauppinen 2014). Recent innovations in miRNA applications have accelerated new product development. Global miRNA market size is expected to reach 626.27 million USD by 2025 (http://www.businesswire.com). So far, many clinical trials have been initiated using miRNA-based therapeutics. miRNA-based drugs, mainly managed by four RNA therapeutic companies are available. These companies include MiRagen Therapeutics, Regulus Therapeutics, and Mirna Therapeutics.
For example, MRX34, a mimic from miR-34 tumor suppressing liposome formulated lipid carrier NOV40, developed by Mirna Therapeutics, produced complete tumor regression in orthotopic mouse models of liver cancer, no immuno-stimulatory activity, or toxicity in normal tissues. MRX34 is the first miRNA-based therapy in a clinical trial for cancer treatment (Abba et al. 2017). Regulus Therapeutics is generally investigating the use of anti-miRs such as miR-122, miR-10b, miR-221, miR-21, miR-33 in the treatment of diseases such as fibrosis, hepatitis C virus (HCV) infection (Janssen et al. 2013), atherosclerosis and cancer (McLeod et al. 2011; Shah et al. 2016). MiRagen Therapeutics uses chemically modified structures of miRNAs such as miR-15/195, miR-155, miR-29, miR-92 in studies of metabolic and cardiovascular diseases (Querfeld et al. 2016). Finally, SPC3649 (miravirsen) compound, developed by Santaris Pharma, is a miR-122 inhibitor and clinical trials have been performed (Christopher et al. 2016). All these findings showed that miRNAs are now in the category of RNAi-based therapeutics. In particular, for the treatment of personalized cancer, specific miRNA mimic or antagonist sets may be designed for individuals based on miRNA expression profiles. In summary, expression profiling studies provide evidence of the role of miRNAs in cancer diagnosis and prognosis. Together with these new strategies, we hope that miR-1, miR-21, miR-10b, miR-141, miR-145, miR-155, miR-221, miR-340 and miR-494 will be promising candidates.
Conclusion
miRNA-based therapeutic approaches have been devised to provide a high mortality rate in cancer-related trials. These studies show that the regulation of expression of miRNAs can be controlled through many mechanisms. Given the advantages it has, it is possible that miRNAs can be used to increase the cell sensitivity of cancer drugs and to overcome drug resistance. In order for the method to be applied to be successful, it is very important that it can effectively reach cancerous cells. For this reason, different delivery strategies such as nanoparticle and liposome mediated delivery, especially for miRNA delivery, need to be optimized. miRNA-based therapies must come before some challenges before reaching clinical trials. As with other medicines used in cancer treatment, miRNAs have many molecular targets in both normal and cancer cells. Therefore, the efficiency and reliability of the miRNA needs to be further investigated.
Abbreviations
- miRNA
MicroRNA
- RISC
RNA-induced silencing complex
- oncomiR
Oncogenic miRNA
- tsmiR
Tumor suppressor miRNA
- UTR
Unstranslated region(s)
- anti-miR(s)
Anti-miRNA oligonucleotides
- NSCLC
Non-small-cell lung cancer
- antagomiR(s)
miRNA antagonists
- PTEN
Phosphatase and tensin homolog
- VEGF
Vascular epidermal growth factor
- SOCS1
Suppressor of cytokine signaling 1
- AMOs
Anti-miRNA oligonucleotides
- HCN1
Hyperpolarization-activated cyclic nucleotide-gated potassium channel 1
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abba ML, Patil N, Leupold JH, et al. MicroRNAs as novel targets and tools in cancer therapy. Cancer Lett. 2017;387:84–94. doi: 10.1016/j.canlet.2016.03.043. [DOI] [PubMed] [Google Scholar]
- An Z, Ren J, Yang G, et al. MicroRNA let-7: regulation, single nucleotide polymorphism, and therapy in lung cancer. J Cancer Res Ther. 2015;11:1. doi: 10.4103/0973-1482.163830. [DOI] [PubMed] [Google Scholar]
- Avgeris M, Stravodimos K, Fragoulis EG, Scorilas A. The loss of the tumour-suppressor miR-145 results in the shorter disease-free survival of prostate cancer patients. Br J Cancer. 2013;108:2573. doi: 10.1038/bjc.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babar IA, Cheng CJ, Booth CJ, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci. 2012;109:E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babashah S, Soleimani M. The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. Eur J Cancer. 2011;47:1127–1137. doi: 10.1016/j.ejca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Bader AG, Brown D, Stoudemire J, Lammers P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011;18:1121–1126. doi: 10.1038/gt.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Tian B, Li A, et al. MicroRNA-125b promotes tumor growth and suppresses apoptosis by targeting DRAM2 in retinoblastoma. Eye. 2016;30:1630–1638. doi: 10.1038/eye.2016.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger JF, Nana-Sinkam SP. MicroRNA as tools and therapeutics in lung cancer. Respir Med. 2015;109:803–812. doi: 10.1016/j.rmed.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar N, Li X, Padi SKR, et al. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1:e105. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai ZK, Chen Q, Chen YB, et al. microRNA-155 promotes the proliferation of prostate cancer cells by targeting annexin 7. Mol Med Rep. 2015;11:533–538. doi: 10.3892/mmr.2014.2744. [DOI] [PubMed] [Google Scholar]
- Castro D, Moreira M, Gouveia AM, et al. MicroRNAs in lung cancer. Oncotarget. 2017;8:81679–81685. doi: 10.18632/oncotarget.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- Chen ML, Sen Liang L, Wang XK. MiR-200c inhibits invasion and migration in human colon cancer cells SW480/620 by targeting ZEB1. Clin Exp Metastasis. 2012;29:457–469. doi: 10.1007/s10585-012-9463-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang W, Zhang Y, et al. The roles of miR-200c in colon cancer and associated molecular mechanisms. Tumor Biol. 2014;35:6475–6483. doi: 10.1007/s13277-014-1860-x. [DOI] [PubMed] [Google Scholar]
- Chen XN, Wang KF, Xu ZQ, et al. MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer Cell Int. 2014;14:70. doi: 10.1186/s12935-014-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li D, Cheng Q, et al. MicroRNA-203 inhibits the proliferation and invasion of U251 glioblastoma cells by directly targeting PLD2. Mol Med Rep. 2014;9:503–508. doi: 10.3892/mmr.2013.1814. [DOI] [PubMed] [Google Scholar]
- Chen S, Jiao JW, Sun KX, et al. MicroRNA-133b targets glutathione S-transferase π expression to increase ovarian cancer cell sensitivity to chemotherapy drugs. Drug Des Devel Ther. 2015;9:5225–5235. doi: 10.2147/DDDT.S87526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Huang L, Hao C, et al. MicroRNA-155 promotes apoptosis in SKOV3, A2780, and primary cultured ovarian cancer cells. Tumor Biol. 2016;37:9289–9299. doi: 10.1007/s13277-016-4804-9. [DOI] [PubMed] [Google Scholar]
- Chen GM, Zheng AJ, Cai J, et al. microRNA-145-3p inhibits non-small cell lung cancer cell migration and invasion by targeting PDK1 via the mTOR signaling pathway. J Cell Biochem. 2018;119:885–895. doi: 10.1002/jcb.26252. [DOI] [PubMed] [Google Scholar]
- Chen L, Cao H, Feng Y. MiR-199a suppresses prostate cancer paclitaxel resistance by targeting YES1. World J Urol. 2018;36:357–365. doi: 10.1007/s00345-017-2143-0. [DOI] [PubMed] [Google Scholar]
- Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2014;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CH, Hou MF, Hung WC. Up-regulation of miR-182 by β-catenin in breast cancer increases tumorigenicity and invasiveness by targeting the matrix metalloproteinase inhibitor RECK. Biochim Biophys Acta Gen Subj. 2013;1830:3067–3076. doi: 10.1016/j.bbagen.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of nodal agonist and antagonist by miR-430. Science. 2007;318:271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- Christopher A, Kaur R, Kaur G, et al. MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7:68. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wu F, Tian D, et al. MiR-199a-3p enhances cisplatin sensitivity of ovarian cancer cells by targeting ITGB8. Oncol Rep. 2018;39:1649–1657. doi: 10.3892/or.2018.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell. 2004;16:861–865. doi: 10.1016/j.molcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- de Melo Maia B, Ling H, Monroig P, et al. Design of a miRNA sponge for the miR-17 miRNA family as a therapeutic strategy against vulvar carcinoma. Mol Cell Probes. 2015;29:420–426. doi: 10.1016/j.mcp.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Denli AM, Tops BBJ, Plasterk RH, et al. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- Dey N, Das F, Ghosh-Choudhury N, et al. MicroRNA-21 governs TORC1 activation in renal cancer cell proliferation and invasion. PLoS ONE. 2012;6:e37366. doi: 10.1371/journal.pone.0037366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wu M, Liu J, et al. Seed-targeting anti-miR-21 inhibiting malignant progression of retinoblastoma and analysis of their phosphorylation signaling pathways. Exp Eye Res. 2014;122:1–8. doi: 10.1016/j.exer.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Dybos SA, Flatberg A, Halgunset J, et al. Increased levels of serum miR-148a-3p are associated with prostate cancer. APMIS. 2018;126:722–731. doi: 10.1111/apm.12880. [DOI] [PubMed] [Google Scholar]
- Ebert MS, Sharp PA. MicroRNA sponges: progress and possibilities. RNA. 2010;16:2043–2050. doi: 10.1261/rna.2414110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Fan Y, Song X, Du H, et al. Down-regulation of miR-29c in human bladder cancer and the inhibition of proliferation in T24 cell via PI3 K-AKT pathway. Med Oncol. 2014;31:65. doi: 10.1007/s12032-014-0065-x. [DOI] [PubMed] [Google Scholar]
- Fan L, Li M, Wang S, et al. Targeted inhibition of microRNA-200c on expression of AP-2α to enhance the proliferation of colon cancer cells in vitro. Cancer Res Clin. 2015;27:222–227. [Google Scholar]
- Feng X, Liu N, Deng S, et al. Mir-199a modulates cisplatin resistance in ovarian cancer by targeting Hif1α. Onco Targets Ther. 2017;10:5899–5906. doi: 10.2147/OTT.S145833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez S, Risolino M, Mandia N, et al. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene. 2014;34:3240. doi: 10.1038/onc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracin M, Bassi C, Pedriali M, et al. MiR-125b targets erythropoietin and its receptor and their expression correlates with metastatic potential and ERBB2/HER2 expression. Mol Cancer. 2013;12:130. doi: 10.1186/1476-4598-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M, Croce CM. MicroRNAs as therapeutic targets in chemoresistance. Drug Resist Updat. 2013;16:47–59. doi: 10.1016/j.drup.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunti L, Da Ros M, Vinci S, et al. Anti-miR21 oligonucleotide enhances chemosensitivity of T98G cell line to doxorubicin by inducing apoptosis. Am J Cancer Res. 2015;5:231–242. [PMC free article] [PubMed] [Google Scholar]
- Gong P, Zhang T, He D, Hsieh J-T. MicroRNA-145 modulates tumor sensitivity to radiation in prostate cancer. Radiat Res. 2015;184:630–638. doi: 10.1667/RR14185.1. [DOI] [PubMed] [Google Scholar]
- González-Vallinas M, Molina S, Vicente G, et al. Expression of MicroRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of rosemary diterpenes in colon and pancreatic cancer. PLoS ONE. 2014;9:e98556. doi: 10.1371/journal.pone.0098556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RI, Yan K-P, Amuthan G, et al. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Gui ZL, Wu TL, Zhao GC, et al. MicroRNA-497 suppress osteosarcoma by targeting MAPK/Erk pathway. Bratislava Med J. 2017;118:449–452. doi: 10.4149/BLL_2017_087. [DOI] [PubMed] [Google Scholar]
- Guo Y, Liu H, Zhang H, et al. miR-96 regulates FOXO1-mediated cell apoptosis in bladder cancer. Oncol Lett. 2012;4:561–565. doi: 10.3892/ol.2012.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghnavaz N, Asghari F, Elieh Ali Komi D, et al. HER2 positivity may confer resistance to therapy with paclitaxel in breast cancer cell lines. Artif Cells Nanomed Biotechnol. 2018;46:518–523. doi: 10.1080/21691401.2017.1326927. [DOI] [PubMed] [Google Scholar]
- Han C, Zhou Y, An Q, et al. MicroRNA-1 (miR-1) inhibits gastric cancer cell proliferation and migration by targeting MET. Tumor Biol. 2015;36:6715–6723. doi: 10.1007/s13277-015-3358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Chen WX, Zhong SL, et al. MicroRNA-452 contributes to the docetaxel resistance of breast cancer cells. Tumor Biol. 2014;35:6327–6334. doi: 10.1007/s13277-014-1834-z. [DOI] [PubMed] [Google Scholar]
- Huang YW, Kuo CT, Chen JH, et al. Hypermethylation of miR-203 in endometrial carcinomas. Gynecol Oncol. 2014;133:340–345. doi: 10.1016/j.ygyno.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AF, Weirauch U, Thomas M, et al. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71:5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- Inamura K, Ishikawa Y. MicroRNA in lung cancer: novel biomarkers and potential tools for treatment. J Clin Med. 2016;5:36. doi: 10.3390/jcm5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Kim HJ, Gwak JM, et al. Prognostic value of microRNA-9 and microRNA-155 expression in triple-negative breast cancer. Hum Pathol. 2017;68:69–78. doi: 10.1016/j.humpath.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Janssen HLA, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting MicroRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- Jeker LT, Bluestone JA. MicroRNA regulation of T-cell differentiation and function. Immunol Rev. 2013;253:65–81. doi: 10.1111/imr.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Sun B, Su C. Targeting microRNAs in cancer gene therapy. Genes (Basel) 2017;8:21. doi: 10.3390/genes8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yu L, Zhang X, et al. MiR-892b silencing activates NF-kB and promotes aggressiveness in breast cancer. Cancer Res. 2016;76:1101–1111. doi: 10.1158/0008-5472.CAN-15-1770. [DOI] [PubMed] [Google Scholar]
- Jin W, Chen F, Wang K, et al. miR-15a/miR-16 cluster inhibits invasion of prostate cancer cells by suppressing TGF-β signaling pathway. Biomed Pharmacother. 2018;104:637–644. doi: 10.1016/j.biopha.2018.05.041. [DOI] [PubMed] [Google Scholar]
- Kasinski AL, Slack FJ. MiRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 2012;72:5576–5587. doi: 10.1158/0008-5472.CAN-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Oh JS, Shin JY, et al. Development of microRNA-145 for therapeutic application in breast cancer. J Control Release. 2011;155:427–434. doi: 10.1016/j.jconrel.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Kinose Y, Sawada K, Nakamura K, Kimura T. The role of microRNAs in ovarian cancer. Biomed Res Int. 2014;2014:249393. doi: 10.1155/2014/249393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluiver J, Gibcus JH, Hettinga C, et al. Rapid generation of microRNA sponges for microRNA inhibition. PLoS ONE. 2012;7:e29275. doi: 10.1371/journal.pone.0029275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneitz B, Krebs M, Kalogirou C, et al. Survival in patients with high-risk prostate cancer is predicted by mir-221, which regulates proliferation, apoptosis, and invasion of prostate cancer cells by inhibiting IRF2 and SOCS3. Cancer Res. 2014;74:2591–2603. doi: 10.1158/0008-5472.CAN-13-1606. [DOI] [PubMed] [Google Scholar]
- Kroesen BJ, Teteloshvili N, Smigielska-Czepiel K, et al. Immuno-miRs: critical regulators of T-cell development, function and ageing. Immunology. 2015;144:1–10. doi: 10.1111/imm.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krützfeldt J, Rajewsky N, Braich R, et al. Silencing of microRNAs in vivo with “antagomirs”. Nature. 2005;438:685. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- Larne O, Hagman Z, Lilja H, et al. miR-145 suppress the androgen receptor in prostate cancer cells and correlates to prostate cancer prognosis. Carcinogenesis. 2015;36:858–866. doi: 10.1093/carcin/bgv063. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park YA, Choi JJ, et al. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol Oncol. 2011;120:56–62. doi: 10.1016/j.ygyno.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Lei C, Du F, Sun L, et al. MIR-143 & MIR-145 inhibit gastric cancer cell migration & metastasis by suppressing MYO6. Cell Death Dis. 2017;8:e3101. doi: 10.1038/cddis.2017.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Mahato RI. MicroRNAs and drug resistance in prostate cancers. Mol Pharm. 2014;11:2539–2552. doi: 10.1021/mp500099g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Feng Y, Coukos G, Zhang L. Therapeutic microRNA strategies in human cancer. AAPS J. 2009;11:747–757. doi: 10.1208/s12248-009-9145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Li X, Huang S, et al. MiR-126 inhibits colon cancer proliferation and invasion through targeting IRSI, SLC7A5 and TOMI gene. J Cent South Univ Med Sci. 2013;38:809–817. doi: 10.3969/j.issn.1672-7347.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Li B, Ren S, Li X, et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer. 2014;83:146–153. doi: 10.1016/j.lungcan.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou L, Li Y, et al. MicroRNA-21 stimulates gastric cancer growth and invasion by inhibiting the tumor suppressor effects of programmed cell death protein 4 and phosphatase and tensin homolog. J BUON. 2014;19:228–236. [PubMed] [Google Scholar]
- Li N, Yang L, Wang H, et al. MiR-130a and MiR-374a function as novel regulators of cisplatin resistance in human ovarian cancer A2780 cells. PLoS ONE. 2015;10:e0128886. doi: 10.1371/journal.pone.0128886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xu N, Li YQ, et al. Inhibition of SW620 human colon cancer cells by upregulating miRNA-145 basic study. World J Gastroenterol. 2016;22:2771–2778. doi: 10.3748/wjg.v22.i9.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Jin X, Meng H, et al. Morin promotes prostate cancer cells chemosensitivity to paclitaxel through miR-155/GATA3 axis. Oncotarget. 2017;8:47849. doi: 10.18632/oncotarget.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Wang W, Li Z, et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017;410:212–227. doi: 10.1016/j.canlet.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ding CM, Li YX, et al. MicroRNA-145 inhibits migration and induces apoptosis in human non-small cell lung cancer cells through regulation of the EGFR/PI3K/AKT signaling pathway. Oncol Rep. 2018;40:2944–2954. doi: 10.3892/or.2018.6666. [DOI] [PubMed] [Google Scholar]
- Liang A-L, Zhang T-T, Zhou N, et al. miRNA-10b sponge: an anti-breast cancer study in vitro. Oncol Rep. 2016;35:1950–1958. doi: 10.3892/or.2016.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PC, Chiu YL, Banerjee S, et al. Epigenetic repression of miR-31 disrupts androgen receptor homeostasis and contributes to prostate cancer progression. Cancer Res. 2013;73:1–13. doi: 10.1158/0008-5472.CAN-12-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HM, Nikolic I, Yang J, et al. MicroRNAs as potential therapeutics to enhance chemosensitivity in advanced prostate cancer. Sci Rep. 2018;8:7820. doi: 10.1038/s41598-018-26050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Chen H. The role of microRNAs in colorectal cancer. J Genet Genom. 2010;37:347–358. doi: 10.1016/S1673-8527(09)60053-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Li G. MicroRNA-133b inhibits proliferation and invasion of ovarian cancer cells through Akt and Erk1/2 inactivation by targeting epidermal growth factor receptor. Int J Clin Exp Pathol. 2015;8:10605–10614. [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lai L, Chen Q, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cui H, Wang W, et al. Construction of circular miRNA sponges targeting miR-21 or miR-221 and demonstration of their excellent anticancer effects on malignant melanoma cells. Int J Biochem Cell Biol. 2013;45:2643–2650. doi: 10.1016/j.biocel.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang C, Li T, et al. Let-7a inhibits growth and migration of breast cancer cells by targeting HMGA1. Int J Oncol. 2015;46:2526–2534. doi: 10.3892/ijo.2015.2949. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang C, Jiao X, et al. miR-221 promotes growth and invasion of hepatocellular carcinoma cells by constitutive activation of NFκB. Am J Transl Res. 2016;8:4764–4777. [PMC free article] [PubMed] [Google Scholar]
- Lund E, Güttinger S, Calado A, et al. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- Ma L, Reinhardt F, Pan E, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28:341. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, et al. MiR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak CSL, Yung MMH, Hui LMN, et al. MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol Cancer. 2017;16:11. doi: 10.1186/s12943-017-0582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar-Aguilar F, Mendoza-Ramírez JA, Malagón-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. 2013;34:163–169. doi: 10.3233/DMA-120957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataki H, Seki N, Mizuno K, et al. Dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget. 2016;7:72084–72098. doi: 10.18632/oncotarget.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BW, Hayman ML, Purcell AL, et al. The “real world” utility of miRNA patents: lessons learned from expressed sequence tags. Nat Biotechnol. 2011;29:129. doi: 10.1038/nbt.1765. [DOI] [PubMed] [Google Scholar]
- Mei Z, He Y, Feng J, et al. MicroRNA-141 promotes the proliferation of non-small cell lung cancer cells by regulating expression of PHLPP1 and PHLPP2. FEBS Lett. 2014;588:3055–3061. doi: 10.1016/j.febslet.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Migita K, Iwanaga N, Izumi Y, et al. TNF-α-induced miR-155 regulates IL-6 signaling in rheumatoid synovial fibroblasts. BMC Res Notes. 2017;10:403. doi: 10.1186/s13104-017-2715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignacca L, Saint-Germain E, Benoit A, et al. Sponges against miR-19 and miR-155 reactivate the p53-Socs1 axis in hematopoietic cancers. Cytokine. 2016;82:80–86. doi: 10.1016/j.cyto.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Mo X, Zhang F, Liang H, et al. miR-544a promotes the invasion of lung cancer cells by targeting cadherina 1 in vitro. Onco Targets Ther. 2014;7:895–900. doi: 10.2147/OTT.S61695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi-Yeganeh S, Paryan M, Arefian E, et al. MicroRNA-340 inhibits the migration, invasion, and metastasis of breast cancer cells by targeting Wnt pathway. Tumor Biol. 2016;37:8993–9000. doi: 10.1007/s13277-015-4513-9. [DOI] [PubMed] [Google Scholar]
- Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7:58595. doi: 10.18632/oncotarget.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu S, Magee P, Garofalo M. MiRNA-based therapeutic intervention of cancer. J Hematol Oncol. 2015;8:68. doi: 10.1186/s13045-015-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedaeinia R, Sharifi M, Avan A, et al. Locked nucleic acid anti-MIR-21 inhibits cell growth and invasive behaviors of a colorectal adenocarcinoma cell line: LNA-anti-MIR as a novel approach. Cancer Gene Ther. 2016;23:246–253. doi: 10.1038/cgt.2016.25. [DOI] [PubMed] [Google Scholar]
- O’Day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res. 2010;12:201. doi: 10.1186/bcr2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obad S, Dos Santos CO, Petri A, et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet. 2011;43:371. doi: 10.1038/ng.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik WH, Kim HR, Park JK, et al. Chemosensitivity induced by down-regulation of MicroRNA-21 in gemcitabine-resistant pancreatic cancer cells by indole-3-carbinol. Anticancer Res. 2013;33:1473–1482. [PubMed] [Google Scholar]
- Pan Q, Meng L, Ye J, et al. Transcriptional repression of miR-200 family members by Nanog in colon cancer cells induces epithelial–mesenchymal transition (EMT) Cancer Lett. 2017;392:26–38. doi: 10.1016/j.canlet.2017.01.039. [DOI] [PubMed] [Google Scholar]
- Park JK, Kogure T, Nuovo GJ, et al. miR-221 silencing blocks hepatocellular carcinoma and promotes survival. Cancer Res. 2011;71:7608–7616. doi: 10.1158/0008-5472.CAN-11-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendlebury A, Hannan NJ, Binder N, et al. The circulating microRNA-200 family in whole blood are potential biomarkers for high-grade serous epithelial ovarian cancer. Biomed Rep. 2017;6:319–322. doi: 10.3892/br.2017.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Pineau P, Volinia S, McJunkin K, et al. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci. 2010;107:264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramanik D, Campbell NR, Karikari C, et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yan L, Zhao X, et al. microRNA-21 overexpression contributes to cell proliferation by targeting PTEN in endometrioid endometrial cancer. Oncol Lett. 2012;4:1290–1296. doi: 10.3892/ol.2012.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W-W, Zhang R, Chen R-A, et al. MicroRNA-145 induces cell cycle arrest in G1 phase by directly targeting KLF5 in colon cancer. Int J Clin Exp Pathol. 2016;9:5197–5209. [Google Scholar]
- Qu F, Zheng J, Gan W, et al. MiR-199a-3p suppresses proliferation and invasion of prostate cancer cells by targeting Smad1. Oncotarget. 2017;8:52465. doi: 10.18632/oncotarget.17191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfeld C, Pacheco T, Foss FM, et al. Preliminary results of a phase 1 trial evaluating MRG-106, a synthetic microRNA antagonist (LNA antimiR) of microRNA-155, in patients with CTCL. Blood. 2016;128:1829. [Google Scholar]
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Zhu S, Wu F, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MY, Ferrajoli A, Sood AK, et al. microRNA therapeutics in cancer—an emerging concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XJ, Miao MH, Xue J, et al. The down-regulation of microRNA-497 contributes to cell growth and cisplatin resistance through PI3K/Akt pathway in osteosarcoma. Cell Physiol Biochem. 2015;36:2051–2062. doi: 10.1159/000430172. [DOI] [PubMed] [Google Scholar]
- Sharma S, Rajendran V, Kulshreshtha R, Ghosh PC. Enhanced efficacy of anti-miR-191 delivery through stearylamine liposome formulation for the treatment of breast cancer cells. Int J Pharm. 2017;530:387–400. doi: 10.1016/j.ijpharm.2017.07.079. [DOI] [PubMed] [Google Scholar]
- Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer Metastasis Rev. 2009;28:369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- Shi M, Liu D, Duan H, et al. Metastasis-related miRNAs, active players in breast cancer invasion, and metastasis. Cancer Metastasis Rev. 2010;29:785–799. doi: 10.1007/s10555-010-9265-9. [DOI] [PubMed] [Google Scholar]
- Shi Z, Li Y, Qian X, et al. MiR-340 inhibits triple-negative breast cancer progression by reversing EZH2 mediated miRNAs dysregulated expressions. J Cancer. 2017;8:3037–3048. doi: 10.7150/jca.19315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjefstad K, Johannessen C, Grindstad T, et al. A gender specific improved survival related to stromal miR-143 and miR-145 expression in non-small cell lung cancer. Sci Rep. 2018;8:8549. doi: 10.1038/s41598-018-26864-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman SA, Ab Mutalib NS, Jamal R. MiR-200c regulation of metastases in ovarian cancer: potential role in epithelial and mesenchymal transition. Front Pharmacol. 2016;7:271. doi: 10.3389/fphar.2016.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Wang X, He HH, et al. MiR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogene. 2014;33:2790–2800. doi: 10.1038/onc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Xu S, Ruan H, et al. MiR-195/-16 family enhances radiotherapy via T cell activation in the tumor microenvironment by blocking the PD-L1 immune checkpoint. Cell Physiol Biochem. 2018;48:801–814. doi: 10.1159/000491909. [DOI] [PubMed] [Google Scholar]
- Teng Y, Manavalan TT, Hu C, et al. Endocrine disruptors fludioxonil and fenhexamid stimulate miR-21 expression in breast cancer cells. Toxicol Sci. 2013;131:71–83. doi: 10.1093/toxsci/kfs290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang P, Wiggins JF, Daige CL, et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri N, Gasparini R, Nuovo G, et al. Anti-miR-135b in colon cancer treatment. J Clin Oncol. 2012;30:457. [Google Scholar]
- van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar TR, Zabludoff S, Ahn S-M, et al. Anti-miR-21 suppresses hepatocellular carcinoma growth via broad transcriptional network deregulation. Mol Cancer Res. 2015;13:1009–1021. doi: 10.1158/1541-7786.MCR-14-0703. [DOI] [PubMed] [Google Scholar]
- Wang XY. MiR-126 inhibits colon cancer proliferation and invasion by targeting CXCR4/Rho A signaling pathway. J Dig Dis. 2014;15:171. [Google Scholar]
- Wang ZX, Bin LuB, Wang H, et al. MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res. 2011;42:281–290. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ting Z, Li Y, et al. microRNA-199a is able to reverse cisplatin resistance in human ovarian cancer cells through the inhibition of mammalian target of rapamycin. Oncol Lett. 2013;6:789–794. doi: 10.3892/ol.2013.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gao W, Hu F, et al. MicroRNA-874 inhibits cell proliferation and induces apoptosis in human breast cancer by targeting CDK9. FEBS Lett. 2014;588:4527–4535. doi: 10.1016/j.febslet.2014.09.035. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu C, Li C, et al. Effects of microRNA-221/222 on cell proliferation and apoptosis in prostate cancer cells. Gene. 2015;572:252–258. doi: 10.1016/j.gene.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Wang L, Gao H, Gong N, Gong M. Downregulation of microRNA-497 is associated with upregulation of synuclein γ in patients with osteosarcoma. Exp Ther Med. 2016;12:3761–3766. doi: 10.3892/etm.2016.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang YX, Zhang DZ, et al. Let-7a mimic attenuates CCL18 induced breast cancer cell metastasis through Lin 28 pathway. Biomed Pharmacother. 2016;78:301–307. doi: 10.1016/j.biopha.2016.01.028. [DOI] [PubMed] [Google Scholar]
- Wei L, Ran F. MicroRNA-20a promotes proliferation and invasion by directly targeting early growth response 2 in non-small cell lung carcinoma. Oncol Lett. 2018;15:271–277. doi: 10.3892/ol.2017.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidle UH, Birzele F, Kollmorgen G, Nopora A. Potential microRNA-related targets for therapeutic intervention with ovarian cancer metastasis. Cancer Genom Proteom. 2018;15:1–15. doi: 10.21873/cgp.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liu K, Wang Y, et al. Upregulation of microRNA-96 and its oncogenic functions by targeting CDKN1A in bladder cancer. Cancer Cell Int. 2015;15:107. doi: 10.1186/s12935-015-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhou J, Mei S, et al. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J Cell Mol Med. 2017;21:1228–1236. doi: 10.1111/jcmm.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Yang B, Lin H, et al. Novel approaches for gene-specific interference via manipulating actions of microRNAs: examination on the pacemaker channel genes HCN2 and HCN4. J Cell Physiol. 2007;212:285–292. doi: 10.1002/jcp.21062. [DOI] [PubMed] [Google Scholar]
- Xie Y, Todd NW, Liu Z, et al. Altered miRNA expression in sputum for diagnosis of non-small cell lung cancer. Lung Cancer. 2010;67:170–176. doi: 10.1016/j.lungcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Liu L-Z, Qian X, et al. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Zhang Q, Cheng W, et al. Effect of miR-29b-1* and miR-29c knockdown on cell growth of the bladder cancer cell line T24. J Int Med Res. 2013;41:1803–1810. doi: 10.1177/0300060513505266. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhang Y, Wei J, et al. MicroRNA-21 promotes hepatocellular carcinoma HepG2 cell proliferation through repression of mitogen-activated protein kinase-kinase 3. BMC Cancer. 2013;13:469. doi: 10.1186/1471-2407-13-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ayub B, Liu Z, et al. Anti-miR182 reduces ovarian cancer burden, invasion, and metastasis: an in vivo study in orthotopic xenografts of nude mice. Mol Cancer Ther. 2014;13:1729–1739. doi: 10.1158/1535-7163.MCT-13-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Chao L, Wang J, Sun Y. miRNA-148a regulates the expression of the estrogen receptor through DNMT1-mediated DNA methylation in breast cancer cells. Oncol Lett. 2017;14:4736–4740. doi: 10.3892/ol.2017.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Du XW, Hu JB, et al. Anticancer effect of miR-96 inhibitor in bladder cancer cell lines. Oncol Lett. 2018;15:3814–3819. doi: 10.3892/ol.2018.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LX, Wu QN, Zhang Y, et al. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011;13:R2. doi: 10.1186/bcr2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cai H, Liang Y, et al. Inhibition of c-Myc by let-7b mimic reverses mutidrug resistance in gastric cancer cells. Oncol Rep. 2015;33:1723–1730. doi: 10.3892/or.2015.3757. [DOI] [PubMed] [Google Scholar]
- Yang Y, Meng H, Peng Q, et al. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22:23. doi: 10.1038/cgt.2014.66. [DOI] [PubMed] [Google Scholar]
- Yang L, Hou J, Cui X-H, et al. MiR-133b regulates the expression of CTGF in epithelial-mesenchymal transition of ovarian cancer. Eur Rev Med Pharmacol Sci. 2017;21:5602–5609. doi: 10.26355/eurrev_201712_14001. [DOI] [PubMed] [Google Scholar]
- Yin XZ, Zhao DM, Zhang GX, Liu L. Effect of miRNA-203 on cervical cancer cells and its underlying mechanism. Genet Mol Res. 2016 doi: 10.4238/gmr.15038680. [DOI] [PubMed] [Google Scholar]
- Yoo B, Kavishwar A, Ross A, et al. Combining miR-10b-targeted nanotherapy with low-dose doxorubicin elicits durable regressions of metastatic breast cancer. Cancer Res. 2015;75:4407–4415. doi: 10.1158/0008-5472.CAN-15-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Todd NW, Xing L, et al. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int J Cancer. 2010;127:2870–2878. doi: 10.1002/ijc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CC, Tsai LL, Wang ML, et al. MiR145 targets the SOX9/ADAM17 axis to inhibit tumor-initiating cells and IL-6-mediated paracrine effects in head and neck cancer. Cancer Res. 2013;73:3425–3440. doi: 10.1158/0008-5472.CAN-12-3840. [DOI] [PubMed] [Google Scholar]
- Yu Y, Nangia-Makker P, Farhana L, et al. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol Cancer. 2015;14:98. doi: 10.1186/s12943-015-0372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Li R, Shi W, et al. Silencing of MicroRNA-21 confers the sensitivity to tamoxifen and fulvestrant by enhancing autophagic cell death through inhibition of the PI3K-AKT-mTOR pathway in breast cancer cells. Biomed Pharmacother. 2016;77:37–44. doi: 10.1016/j.biopha.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Yuan W, Guo Y-Q, Li X-Y, et al. MicroRNA-126 inhibits colon cancer cell proliferation and invasion by targeting the chemokine (C-X-C motif) receptor 4 and Ras homolog gene family, member A, signaling pathway. Oncotarget. 2016;7:60230. doi: 10.18632/oncotarget.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ji H, Xiao F, et al. MicroRNA-340 inhibits the proliferation and invasion of hepatocellular carcinoma cells by targeting JAK1. Biochem Biophys Res Commun. 2017;483:578–584. doi: 10.1016/j.bbrc.2016.12.102. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lin Q. MicroRNA-145 inhibits migration and invasion by down-regulating FSCN1 in lung cancer. Int J Clin Exp Med. 2015;8:8794–8802. [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pu J, Qi T, et al. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene. 2012;33:387. doi: 10.1038/onc.2012.574. [DOI] [PubMed] [Google Scholar]
- Zhang W-C, Liu J, Xu X, Wang G. The role of microRNAs in lung cancer progression. Med Oncol. 2013;30:675. doi: 10.1007/s12032-013-0675-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang L, Zhao Y, Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim Biophys Sin (Shanghai) 2013;45:995–1001. doi: 10.1093/abbs/gmt113. [DOI] [PubMed] [Google Scholar]
- Zhang C, Liu K, Li T, et al. MiR-21: a gene of dual regulation in breast cancer. Int J Oncol. 2016;48:161–172. doi: 10.3892/ijo.2015.3232. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Feng Y, Liu P, Yang J. MiR-143 inhibits cell proliferation and invasion by targeting DNMT3A in gastric cancer. Tumor Biol. 2017;39:1–8. doi: 10.1177/1010428317711312. [DOI] [PubMed] [Google Scholar]
- Zhao X, Li J, Huang S, et al. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am J Transl Res. 2015;7:1382–1389. [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zhao Q, Zhang C, et al. MiR-15b-5p resensitizes colon cancer cells to 5-fluorouracil by promoting apoptosis via the NF-κB/XIAP axis. Sci Rep. 2017;7:4194. doi: 10.1038/s41598-017-04172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Sun X, Li Y, Zuo W. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol. 2015;37:8189–8196. doi: 10.1007/s13277-015-4722-2. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wu D, Tao J, et al. MicroRNA-133 inhibits cell proliferation, migration and invasion by targeting epidermal growth factor receptor and its downstream effector proteins in bladder cancer. Scand J Urol. 2013;47:423–432. doi: 10.3109/00365599.2012.748821. [DOI] [PubMed] [Google Scholar]
- Zidan HE, Abdul-Maksoud RS, Elsayed WSH, Desoky EAM. Diagnostic and prognostic value of serum miR-15a and miR-16-1 expression among egyptian patients with prostate cancer. IUBMB Life. 2018;70:437–444. doi: 10.1002/iub.1733. [DOI] [PubMed] [Google Scholar]
- Zierau O, Helle J, Schadyew S, et al. Role of miR-203 in estrogen receptor-mediated signaling in the rat uterus and endometrial carcinoma. J Cell Biochem. 2018;119:5359–5372. doi: 10.1002/jcb.26675. [DOI] [PubMed] [Google Scholar]
- Zong C, Wang J, Shi TM. MicroRNA 130b enhances drug resistance in human ovarian cancer cells. Tumor Biol. 2014;35:12151–12156. doi: 10.1007/s13277-014-2520-x. [DOI] [PubMed] [Google Scholar]