Abstract
The role of ANGPTL1 in cancer development is still little known, especially in colorectal cancer (CRC). We investigated the clinical significance of ANGPTL1 expression in CRC tissues and its potential role in the progression of epithelial to mesenchymal transition (EMT) in CRC cells, which has not been reported to our knowledge. ANGPTL1 expression in CRC tissues was much lower that than in paired adjacent normal tissues by IHC, WB and qRT-PCR assays. ANGPTL1 positive expression was negatively associated with tumor size (P = 0.034), T stage (P = 0.015), lymph nodes metastasis (P = 0.045) and TNM stage (P = 0.009) and poor prognosis of CRC patients (P = 0.003). In vitro, ANGPTL1 showed decreasing expression in CRC cell lines from primary tumor to ascites metastasis. Meanwhile, ANGPTL1 silencing enhanced EMT in HCT116 cells followed with the increase of Slug, Fibronectin and Vimentin, the decrease of E-cad, and the enhancement of EMT-like cell morphology and cell invasion and migration. Low ANGPTL1 expression is closely associated with multiple clinical significance and prognosis of CRC patients. ANGPTL1 inhibits EMT of CRC cells via inhibiting E-cad suppressor Slug expression.
Keywords: Angiopoietin-like protein 1, Epithelial to mesenchymal transition, Colorectal cancer, Slug
Introduction
From 2000 to 2011, colorectal cancer (CRC) takes up the third upward trend of age-standardized mortality rates in the population of China (Chen et al. 2016). In the United States, CRC remains the second leading cause of cancer death. More than 50% of CRC patients will develop liver metastases during their lifespan (Misiakos et al. 2011). Strong local invasion and distant metastasis is the most important contributor to the mortality of patients with colorectal cancer (Vu and Datta 2017). Epithelial to mesenchymal transition (EMT) contributes to this rapid and aggressive tumor progression. During EMT, CRC loses their epithelial characteristics and gains more invasive and migratory properties of mesenchymal cells, along with the loss of epithelial marker, gain of mesenchymal markers, and finally exerting a promoting effect on tumor invasion and metastasis (Bhangu et al. 2012). Thus, detecting EMT target genes is significant for inhibiting the malignant biology of CRC.
Angiopoietin-like protein 1 (ANGPTL1), a member of the angiopoietin-related protein family, participates in multiple biological processes, such as angiogenesis (Hato et al. 2008), hematopoietic stem cell expansion (Zhang et al. 2006), lipid metabolism (Oike et al. 2005; Kersten 2005) and inflammation (Tabata et al. 2009). The role of ANGPTL1 in cancer is still little known. Recently, growing evidence shows that ALTPL1 plays a significant role in the progression of various cancers, such as hepatocellular carcinoma (HCC) (Chen et al. 2016), malignant melanoma (Gardizi et al. 2012), lung (Kuo et al. 2013), prostate, kidney, thyroid, and urinary bladder cancers (Dhanabal et al. 2002). However, the clinical significance of ANGPTL1 expression in CRC tissues and its potential role in the progression of EMT in CRC cells has not been reported to our knowledge, which is investigated in the current study.
Materials and methods
Tissue samples
This study was approved by the institutional review board of the Fourth People’s Hospital of Shenyang and a consent form was signed by each participating patient. One hundred and twenty-nine formalin-fixed and paraffin-embedded CRC and corresponding normal tissues were obtained from CRC patients in Fourth People’s Hospital of Shenyang between 2011 and 2016. All data were confirmed by pathological diagnosis. The histologically normal tissues were at least 5 cm away from the cancer. Additionally, we randomly selected 18 cases of CRC fresh tissues for late Western blot (WB) and Real-time quantitative PCR (qRT-PCR) assays.
Cell lines and culture
CoLo205, SW620 and HCT116 cell lines of human CRC were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). These cell lines were maintained in the recommended growth media with 10% fetal calf serum (Hyclone, Logan, UT, USA).
Immunohistochemistry assays
Immunohistochemistry (IHC) was performed as described previously (Sheng et al. 2014). Briefly, 4-μm sections were deparaffinized with xylene three times, dehydrated three times in a gradient series of ethanol (100, 95, and 75%), and rinsed with PBS. Each section was covered with 0.3% peroxyacetic acid for 20 min to block endogenous peroxidase activity, subjected to high pressure for antigen retrieval (1 min), and cooled at room temperature for 2 h. Non-specific binding sites were blocked with 10% normal goat serum for half an hour. Sections were first incubated with rabbit polyclonal anti-ANGPTL1(Santa Cruz, CA, USA, sc-271841) overnight at 4 °C, and then rinsed twice with PBS. This was followed by incubation with a secondary antibody for 20 min at room temperature, and two more rinses with PBS. Slides were then treated with streptavidin–peroxidase reagent at room temperature for 15 min, and rinsed twice with PBS. The sections were visualized with 3,3′-diaminobenzidine (DAB) for 5 min, counterstained with haematoxylin, and mounted for microscopy. Staining intensity was scored as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). Extent of staining was scored as 0 (< 5%), 1 (5–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%) according to the percentages of the positive staining areas in relation to the whole carcinoma area. The sum of the intensity and extent score was used as the final staining scores (0–7). Tumors having a final staining score ≥ 2 were considered to be ANGPTL1 positive expression.
Western blot
WB was performed as described previously (Liu et al. 2015), whole-cell lysates were prepared from CRC cells and tissues. Samples were loaded onto 10% SDS-polyacrylamide gels, transferred to PVDF membranes (Millipore Corp, Bedford, MA, USA) and incubated with primary ANGPTL1 (Santa Cruz), Slug (Proteintech, Chicago, IL, USA, 12129-1-AP), Snail (Proteintech, 13099-1-AP), Twist (Abcam, Cambridge, UK, 25465-1-AP), E-cadherin (E-cad, Santa Cruz, sc-7870), Fibronectin (Proteintech, 66042-1-Ig) and Vimentin (Proteintech, 10366-1-AP), MMP9 (Proteintech, 10375-2-AP), N-cadherin (N-cad, Abcam, ab98952) and α-Smooth muscle actin (a-SMA, Proteintech, 55135-1-AP) antibodies overnight at 4 °C. Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Proteintech) for 1 h at room temperature. Immunoreactive protein bands were visualized with an ECL detection kit (Thermo Fisher Scientific Inc, Waltham, MA, USA). Each experiment was repeated three times.
Real-time quantitative PC
qRT-PCR was performed as described previously (Liu et al. 2015), ANGPTL1 mRNA from CRC cells and tissues was analyzed in a Light Cycler 2.0 with the Light Cycler kit (Applied Biosystems, Foster City, CA, USA). The conditions were as follows: 95 °C for 30 s and 45 cycles of 95oC for 5 s and 60 °C for 30 s. The primers were as follows: ALTPL1, 5′-GGGCAAGATGCAAGTACCAT-3′ (sense) and 5′-GACACATGGGTGTCTTGTCG-3′ (antisense); GADPH, 5′-CATGAGAAGTATGACAACAGCCT-3′ (sense) and 5′-AGTCCTTCCACGATACCAAAGT-3′ (antisense). Quality of the PCR products was monitored with post-PCR melt-curve analysis. The expression level was calculated using the 2−ΔΔCt method.
RNA interference
Three effective sequences for ANGPTL1 interference were: sense: 5′-GCAUUCGGUCAGUGGGAUUTT-3′; antisense: 5′-AAUCCCACUGACCGAAUGCTT-3′; sense: 5′-GGAGAUAGAUGUUCUGCAATT-3′; antisense: 5′-UUGCAGAACAUCUAUCUCCTT-3′ sense: 5′-CCAGGUUAUCCCAGAGAUUTT-3′; antisense: 5′-AAUCUCUGGGAUAACCUGGTT-3′. We just choose the first sequence used in vitro as a representative. The sequences for the siRNA control used were: sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3′. The above sequences were synthesized by GenePharma company (GenePharma Co, Ltd, Shanghai, China). siRNA transfections (20 μM) were mixed with Oligofectamine 3000 (Invitrogen, Carlsbad, CA, USA) for transfection as described by the manufacturer.
EMT construction
ANGPTL1siRNA and siRNAcontrol transfected HCT116 cells were cultured with growth media containing only 1% fetal calf serum (FBS) for four days. During this period, treated cells were repeatedly cultured with 1% FBS media twice. Observating the change of EMT-like cell morphology, the expression of EMT related markers and ability in cell invasion and migration were used for detecting the EMT formation.
Cell invasion assays
Cell invasion was assessed with modified Boyden chamber (BD Biosciences, Sparks, MD, USA) assays. Briefly, ANGPTL1siRNA and siRNAcontrol transfected HCT116 cells were seeded onto 8.0-µM pore size membrane inserts coated with matrigel (BD Biosciences) in 24 well plates with FBS-free growth medium. 10% FBS was added to the bottom wells as a chemoattractant. After 24 h, cells that did not migrate were removed from the top side of the inserts with a cotton swab. Cells that had migrated to the underside of the inserts were stained with Crystal Violet Hydrate (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. The migratory cells were counted in five random fields per insert under a microscope at 20 × magnification. Results were expressed as cells migrated per field.
Statistical analysis
Statistical analysis was performed using SPSS software 13.0 (SPSS, Chicago, IL, USA). The differential expression of ANGPTL1 in CRC tissues and paired adjacent normal tissues by IHC and its clinicopathological significance with CRC patients were analyzed by paired nonparametric test and Chi-squared, respectively. The Kaplan–Meier method was used to estimate survival, and differences were analyzed by the log-rank test. The differential expression of ANGPTL1 in CRC tissues and paired adjacent normal tissues by WB and qRT-PCR was analyzed by paired sample t test. WB, qRT-PCR and cell invasion and migration assays in vitro were expressed as means ± SE. The differences of parameters were compared through independent sample t test. P < 0.05 was considered to be statistically significant.
Results
The clinicopathological significance of ANGPTL1expression in CRC tissues
The location of ANGPTL1 in cytoplasm and nucleus in CRC and paired adjacent tissues was considered for scoring (Fig. 1). IHC showed that ANGPTL1 was positively expressed in 56 cases of 129 CRC samples, which was much lower than its expression in paired adjacent normal tissues (56/129, 43.4% vs 89/129, 68.2%, P < 0.01).
Fig. 1.
ANGPTL1 expression in one of 129 cases CRC and paired adjacent normal tissues by IHC. ANGPTL1 was highly expressed in normal pancreatic tissues with intensively brown staining. Low ANGPTL1 expression was shown in CRC tissues with weakly brown staining. The blue staining of cell nuclear was routinely stained by hematoxylin. The size bar was inserted into the bottom-right of figure with ×100 and ×200 magnification, respectively
Consistent with the IHC results, WB and qRT-PCR also showed that the protein and mRNA levels of ANGPTL1 in 18 cases of CRC tissues were much lower than that in paired adjacent normal tissues (t = 3.078, P = 0.007; t = 2.365, P = 0.030, respectively) (Fig. 2).
Fig. 2.
ANGPTL1 protein and mRNA levels in 18 cases of CRC and paired normal tissues. a ANGPTL1 protein expression in 9 cases (randomly selected) of CRC and paired normal tissues by WB. b ANGPTL1 relative mRNA expression in 18 cases of CRC and paired normal tissues by qRT-PCR (N/C ratio). C: CRC tissues; N: paired adjacent normal tissues. Bars indicate ± SE. *P < 0.05; **P < 0.01 compared with the control
Chi-squared showed that ANGPTL1 positive expression was negatively associated with tumor size (P = 0.034), T stage (P = 0.015), lymph nodes metastasis (P = 0.045) and TNM stage (P = 0.009), respectively, but had no relationship with age, gender, tumor location, differentiation and distance metastasis of CRC patients (P > 0.05) (Table 1).
Table 1.
The association of ANGPTL1 expression with clinical data
| Parameters | Cases | ANGPTL1 | χ2 | P | |
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Cases | 129 | 73 | 56 | ||
| Age (years) | |||||
| ≤ 65 | 80 | 43 | 37 | 0.691 | 0.406 |
| > 65 | 49 | 30 | 19 | ||
| Gender | |||||
| Female | 61 | 31 | 30 | 1.568 | 0.210 |
| Male | 68 | 42 | 26 | ||
| Tumor location | |||||
| Colon | 57 | 29 | 28 | 1.356 | 0.244 |
| Rectum | 72 | 44 | 28 | ||
| Tumor size (cm) | |||||
| < 5 | 60 | 28 | 32 | 4.496 | 0.034 |
| ≥ 5 | 69 | 45 | 24 | ||
| Differentiation | |||||
| Moderate and poor | 99 | 59 | 40 | 1.567 | 0.211 |
| Well | 30 | 14 | 16 | ||
| T stage | |||||
| T1 + T2 | 27 | 8 | 18 | 7.518 | 0.006 |
| T3 + T4 | 102 | 64 | 38 | ||
| Lymph nodes metastasis | |||||
| N0 (negative) | 84 | 41 | 43 | 5.593 | 0.015 |
| N1 (positive) | 45 | 32 | 13 | ||
| TNM stage | |||||
| I + II | 78 | 37 | 41 | 6.729 | 0.009 |
| III + IV | 51 | 36 | 15 | ||
| Distance metastasis | |||||
| M0 (negative) | 114 | 62 | 52 | 1.937 | 0.164 |
| M1 (positive) | 15 | 11 | 4 | ||
Additionally, CRC patients with ANGPTL1 positive expression (total average survival time 41.27 ± 0.88 months) had a significantly better overall survival compared with CRC patients with ANGPTL1 negative expression (total average survival time 32.5 ± 1.16 months) (log rank, χ2 = 9.118, P = 0.003) (Fig. 3).
Fig. 3.
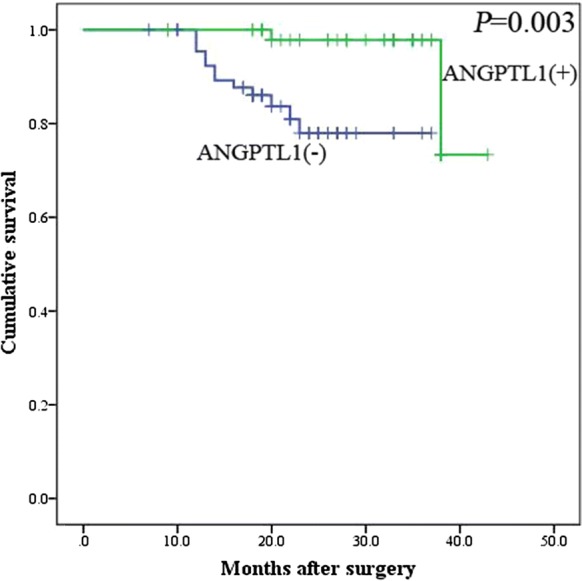
The relationship of ANGPTL1 with the survival of 129 postoperative CRC patients in Kaplan–Meier analysis. Positive (+) and negative (−) expression of ANGPTL1 was plotted against overall survival time
The expression and interference effect of ANGPTL1 in CRC cell lines
WB and qRT-PCR were used to detect the protein and mRNA levels of ANGPTL1 in 3 CRC cell lines derived from different sources (Fig. 4a, b). ANGPTL1 expression was high in HCT116 cells (derived from primary CRC tissues), moderate in SW620 cells (derived from lymph nodes metastasis) and low in CoLo205 cells (derived from ascites metastasis). This indicated that ANGPTL1 was negatively associated with the various metastases of CRC cells.
Fig. 4.
The expression and interference effect of ANGPTL1 in CRC cell lines. ANGPTL1 protein (a) and mRNA (b) expression in 3 CRC cell lines. ANGPTL1 protein (c) and mRNA (d) expression in siRNAcontrol and ANGPTL1siRNA transfected HCT116 cells (×200 magnification). Bars indicate means ± SE. *P < 0.05; **P < 0.01 compared with the control
HCT16 cells with high ANGPTL1 expression were used for ANGPTL1 interference. WB showed that ANGPTL1 protein expression in ANGPTL1siRNA group was significant lower that than in siRNAcontrol group, whatever transfected ANGPTL1siRNA for 2d or 4d (Fig. 4c). The same results were also observed in qRT-PCR assays (Fig. 4d). Culture duration after transfection (4d) is enough for detecting the function of ANGPTL1 silencing in EMT of CRC cells.
ANGPTL1 silencing enhanced EMT in CRC cells
HCT16 cells transfected with ANGPTL1siRNA and siRNAcontrol were culture with growth medium containing 1% FBS for 4d for late EMT detection. WB, cell morphology and cell invasion/migration were used to detect the effect of ANGPTL1 in EMT formation in CRC cells (Figs. 5, 6, 7).
Fig. 5.
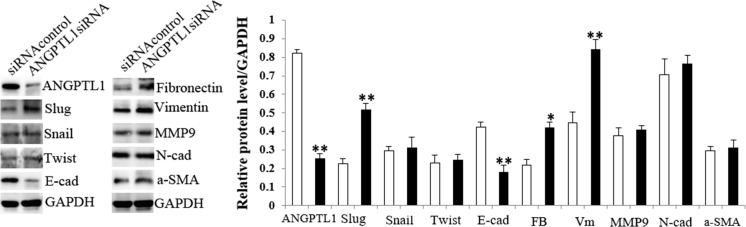
The protein expression of ANGPTL1, Slug, Snail, Twist, E-cad, Fibronectin, Vimentin, MMP9, N-cad and a-SMA in siRNAcontrol and ANGPTL1siRNA transfected HCT116 cells for 4d. The corresponding protein bands and statistic bar graph were shown in left and right side, respectively. White bars: siRNAcontrol group; Black bars: ANGPTL1siRNA group. E-cad E-cadherin, FB Fibronectin, VM Vimentin, N-cad N-cadherin, α-SMA Alpha-smooth muscle actin. Bars indicate ± SE. *P < 0.05; **P < 0.01 compared with the control
Fig. 6.
Cell morphology of siRNAcontrol and ANGPTL1siRNA transfected HCT116 cells after 4d
Fig. 7.
Cell invasion (a) and migration (b) in siRNAcontrol and ANGPTL1siRNA transfected HCT116 cells. Bars indicate ± SE. *P < 0.05; **P < 0.01 compared with the control (×200 magnification)
WB showed that ANGPTL1 silencing significantly inhibited E-cad expression, but increased Slug, Fibronectin and Vimentin protein levels. However, Snail, Twist, MMP9, N-cad and a-SMA expression showed no obvious differences between ANGPTL1siRNA and siRNAcontrol groups.
Cell morphology observation showed that ANGPTL1siRNA transfected HCT116 cells exhibited EMT-like cell morphology compared with siRNAcontrol group: most cells lost their epithelial characteristics, and presented a spindle-shaped and fibroblast-like morphology (Fig. 6).
Cell invasion and migration assays showed that cell invasion and migration were significantly enhanced in ANGPTL1siRNA group compared with siRNAcontrol group (Fig. 7).
All above results indicated that ANGPTL1 inhibited the initiation of EMT in CRC cells.
Discussion
Recently, increasing studies focus on the role of ANGPTL1 in the development of various cancers. For example, ANGPTL1 interacts with integrin α1β1 to suppress HCC angiogenesis and metastasis by inhibiting JAK2/STAT3 signaling (Yan et al. 2017). ANGPTL1 suppresses Slug to inhibit cell motility in lung and breast cancer cells (Kuo et al. 2013). A decreased ratio between serum levels of the antagonistic angiopoietins 1 and 2 indicates tumor progression of malignant melanoma (Gardizi et al. 2012). However, the clinical significance of ANGPTL1 expression in CRC tissues and its potential role in the progression of EMT in CRC cells has not been reported to our knowledge. Our studies first show that the decrease of ANGPTL1 expression in CRC tissues is closely associated with multiple clinical characters and poor prognosis in CRC patients. Meanwhile, ANGPTL1 silencing induced EMT in CRC cells via inhibiting the E-cad suppressor Slug.
ANGPTL1 was decreased in various cancers, such as malignant melanoma and HCC. Our study also showed that ANGPTL1 protein and mRNA expression were significant lower in CRC tissues than that in corresponding adjacent normal tissues. Meanwhile, the positive expression of ANGPTL1 was negatively associated with tumor size, T stage, lymph nodes metastasis, TNM stage and poor prognosis of CRC patients. The clinical significance of ANGPTL1 expression in cancers has only been reported in lung and liver cancers to our knowledge. ANGPTL1 was inversely correlated with stage, tumor status, lymph node status, invasion, and poor prognosis of lung cancer (Kuo et al. 2013), while high ANGPTL1 expression was negatively associated with tumor size, clinical stage, vascular invasion, and poor prognosis of patients with HCC (Yan et al. 2017). Taking together, ANGPTL1 acts as a tumor suppressor in most cancer tissues, which drove us to further investigate its function in vitro.
We next found that ANGPTL1 showed decreasing expression in CRC cell lines from primary tumor to ascites metastasis, which drove us to investigate its potential role in the cancer progression contributor EMT. ANGPTL1 silencing enhanced EMT in CRC cells followed with the increase of Slug, Fibronectin and Vimentin, the decrease of E-cad, and the enhancement of both EMT-like cell morphology and cell invasion/migration. However, Snail and Twist expression showed no significant difference between ANGPTL1siRNA and siRNAcontrol groups. Ectopic expression of ANGPTL1 also suppresses EMT by reducing the expression of the zinc-finger protein Slug but not Snail and Twist in lung cancer cells (Kuo et al. 2013), while ANGPTL1 significantly decreases EMT-driven sorafenib resistance, cancer stemness and tumor growth of HCC cells by repressing Slug expression but is not involved with Snail and Slug (Chen et al. 2016). However, Yan et al. (2017) showed that ANGPTL1 could downregulate both Snail and Slug in HCC cell lines. The subtle differences might be due to different cell types and microenvironment in various cancers. It is well known that the transcription factor Slug plays a significant role in the initiation of EMT (Shih and Yang 2011; Fenouille et al. 2012; Naber et al. 2013). Taking together, ANGPTL1 inhibits EMT of CRC cells via inhibiting E-cad suppressor Slug independent of Snail and Twist regulation.
In conclusion, we first found that low ANGPTL1 expression is closely associated with multiple clinical significance and prognosis of CRC patients. ANGPTL1 inhibits EMT of CRC cells via inhibiting Slug expression. However, the corresponding molecular mechanism is poorly understood. In addition, the 7 other members of ANGPTL (ANGPTL 2-8) also show various function in the development of cancers (Carbone et al. 2018). For example, ANGPTL2 is overexpressed in pancreatic cancer cells leading to EMT and, in turn, acquired resistance to anti-VEGF treatment (Carbone et al. 2011; Gaianigo et al. 2017). In HCC cells, ANGPTL3 inhibited cell proliferation and invasion through downregulation of p38MAPK and MMP-9 cascade’s activation (Yu et al. 2011). ANGPTL4 overexpression induces an elevation of adenylate energy charge by ANGPTL4 enhances EMT (Teo et al. 2017). The co-expression of ILT4 and ANGPTL5 was associated with low non-small cell lung cancer differentiation and lower overall survival rates (Wang et al. 2015). Up-regulation of ANGPTL6 by miRNA-128 contributes to glioma and glioblastoma multiforme (GBM) resulting in the proliferation of undifferentiated GBM cells (Cui et al. 2010). ANGPTL7 was initially described as potent target gene of the Wnt/β-catenin pathway (Adhikary et al. 2013). The cooperation and crosstalk between ANGPTL1 and other members in the development of CRC is still little known. All of above will be investigated in our future study.
Acknowledgements
We thank for the General Laboratory of the First Hospital of China Medical University for technical supports.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adhikary T, Brandt DT, Kaddatz K, Stockert J, Naruhn S, Meissner W, et al. Inverse PPARβ/δ agonists suppress oncogenic signaling to the ANGPTL4 gene and inhibit cancer cell invasion. Oncogene. 2013;32:5241–5252. doi: 10.1038/onc.2012.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangu A, Wood G, Mirnezami A, Darzi A, Tekkis P, Goldin R. Epithelial mesenchymal transition in colorectal cancer: seminal role in promoting disease progression and resistance to neoadjuvant therapy. Surg Oncol. 2012;21:316–323. doi: 10.1016/j.suronc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Carbone C, Moccia T, Zhu C, Paradiso G, Budillon A, Chiao PJ, et al. Anti-VEGF treatment–resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype. Clin Cancer Res. 2011;17:5822–5832. doi: 10.1158/1078-0432.CCR-11-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone C, Piro G, Merz V, Simionato F, Santoro R, Zecchetto C, et al. Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci. 2018;19:431. doi: 10.3390/ijms19020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST, Yen CJ, et al. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology. 2016;64:1637–1651. doi: 10.1002/hep.28773. [DOI] [PubMed] [Google Scholar]
- Cui JG, Zhao Y, Sethi P, Li YY, Mahta A, Culicchia F, et al. Micro-RNA-128 (miRNA-128) down-regulation in glioblastoma targets ARP5 (ANGPTL6), Bmi-1 and E2F-3a, key regulators of brain cell proliferation. J. Neuro Oncol. 2010;98:297–304. doi: 10.1007/s11060-009-0077-0. [DOI] [PubMed] [Google Scholar]
- Dhanabal M, Larochelle WJ, Jeffers M, Herrmann J, Rastelli L, McDonald WF, et al. Angioarrestin: an antiangiogenic protein with tumor-inhibiting properties. Cancer Res. 2002;62:3834–3841. [PubMed] [Google Scholar]
- Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS ONE. 2012;7:e40378. doi: 10.1371/journal.pone.0040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaianigo N, Melisi D, Carbone C (2017) EMT and treatment resistance in pancreatic cancer. Cancers (Basel) 9:pii:E122 [DOI] [PMC free article] [PubMed]
- Gardizi M, Kurschat C, Riese A, Hahn M, Krieg T, Mauch C, et al. A decreased ratio between serum levels of the antagonistic angiopoietins 1 and 2 indicates tumour progression of malignant melanoma. Arch Dermatol Res. 2012;304:397–400. doi: 10.1007/s00403-012-1228-2. [DOI] [PubMed] [Google Scholar]
- Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med. 2008;18:6–14. doi: 10.1016/j.tcm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Kersten S. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem Soc Trans. 2005;33:1059–1062. doi: 10.1042/BST0331059. [DOI] [PubMed] [Google Scholar]
- Kuo TC, Tan CT, Chang YW, Hong CC, Lee WJ, Chen MW, et al. Angiopoietin-like protein 1 suppresses SLUG to inhibit cancer cell motility. J Clin Invest. 2013;123:1082–1095. doi: 10.1172/JCI64044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sheng W, Dong M, Dong X, Dong Q, Li F. Gli1 promotes transforming growth factor-beta1- and epidermal growth factor-induced epithelial to mesenchymal transition in pancreatic cancer cells. Surgery. 2015;158:211–224. doi: 10.1016/j.surg.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Misiakos EP, Nikolaos P, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol. 2011;17:4067–4075. doi: 10.3748/wjg.v17.i36.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber HP, Drabsch Y, Snaar-Jagalska BE, ten Dijke P, van Laar T. Snail and Slug, key regulators of TGF-β-induced EMT, are sufficient for the induction of single-cell invasion. Biochem Biophys Res Commun. 2013;435:58–63. doi: 10.1016/j.bbrc.2013.04.037. [DOI] [PubMed] [Google Scholar]
- Oike Y, Akao M, Kubota Y, Suda T. Angiopoietin-like proteins: potential new targets for metabolic syndrome therapy. Trends Mol Med. 2005;11:473–479. doi: 10.1016/j.molmed.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Sheng W, Chen C, Dong M, Zhou J, Liu Q, Dong Q, Li F. Overexpression of calreticulin contributes to the development and progression of pancreatic cancer. J Cell Physiol. 2014;229:887–897. doi: 10.1002/jcp.24519. [DOI] [PubMed] [Google Scholar]
- Shih JY, Yang PC. The EMT regulator slug and lung carcinogenesis. Carcinogenesis. 2011;32:1299–1304. doi: 10.1093/carcin/bgr110. [DOI] [PubMed] [Google Scholar]
- Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10:178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Teo Z, Sng MK, Chan JSK, Lim MMK, Li Y, Li L, et al. Elevation of adenylate energy charge by angiopoietin-like 4 enhances epithelial-mesenchymal transition by inducing 14-3-3γ expression. Oncogene. 2017;36:6408–6419. doi: 10.1038/onc.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T, Datta PK (2017) Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel) 9:pii:E171 [DOI] [PMC free article] [PubMed]
- Wang L, Geng T, Guo X, Liu J, Zhang P, Yang D, et al. Co-expression of immunoglobulin-like transcript 4 and angiopoietin-like proteins in human non-small cell lung cancer. Mol Med Rep. 2015;11:2789–2796. doi: 10.3892/mmr.2014.3029. [DOI] [PubMed] [Google Scholar]
- Yan Q, Jiang L, Liu M, Yu D, Zhang Y, Li Y, et al. ANGPTL1 interacts with integrin α1β1 to suppress HCC angiogenesis and metastasis by inhibiting JAK2/STAT3 signaling. Cancer Res. 2017;77:5831–5845. doi: 10.1158/0008-5472.CAN-17-0579. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhang H, Li D, Xue H, Pan C, Zhao S, et al. Effects of angptl3 antisense oligodeoxynucleotides transfection on the cell growths and invasion of human hepatocellular carcinoma cells. Hepatogastroenterology. 2011;58:1742–1746. doi: 10.5754/hge10647. [DOI] [PubMed] [Google Scholar]
- Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, Lodish HF. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]