Abstract
A critical limitation for tissue engineering and autologous therapeutic applications of bone marrow derived EPCs is their low frequency, which is even lower in number and activity level in patients with cardiovascular risk factors and other diseases. New strategies for obtaining and reserving sufficient ready-to-use EPCs for clinical use have hit major obstacles, because effects of serial passage and cryopreservation on EPC phenotype and functions are still needed to be explored. The present study aims at investigating effects of a limited number of culture passages as well as cryopreservation on EPC phenotype and functions. We isolated EPCs from rat bone marrow and cultured them up to passage 12 (totaling achievements of 40 population doublings). The phenotype and functions of fresh cultured and post-cryopreserved EPCs at passages 7 and 12, respectively, were evaluated. EPCs at passage 12 maintained the morphological characteristics, marker phenotype, Dil-ac-LDL uptake and FITC-UEA-1 binding functions, enhanced EPCs proliferation, tube formation and migration, but decreased CD133 expression compared with EPCs at passage 7. Cryopreservation caused limited impairment in EPC phenotype and functions. In brief, our results demonstrated that a limited number of culture passages and cryopreservation did not change EPC phenotype and functions, and can be used for the development of robust strategies and quality control criterion for obtaining sufficient and high-quality ready-to-use EPCs for tissue engineering and therapeutic applications.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0234-4) contains supplementary material, which is available to authorized users.
Keywords: Endothelial progenitor cells, Passage, Cryopreservation, Bone marrow
Introduction
Since their discovery in 1997, accumulating findings have shown the great potential of Endothelial progenitor cells (EPCs) in vascular tissue engineering for possible clinical application in coronary artery disease and wound healing due to their abilities to proliferate, migrate to the site of ischemic injury, and participate in the process of vascular repair and angiogenesis (Asahara et al. 1997; Hristov et al. 2003; Tsai et al. 2009; Urbich and Dimmeler 2004). However, there are still several problems to be resolved in EPC-based therapy: (1) the low frequency of EPCs in bone marrow (BM) and peripheral blood (PB) (Kalka et al. 2000; Teraa et al. 2013). Notably the number and the activity level of EPCs seem to be inversely correlated with the severity and degree of cardiovascular risk factors and other disease (Teraa et al. 2013; Umemura et al. 2008; Vasa et al. 2001), which hampered the efficacy and availability of EPC-based therapies in patient groups who would gain the greatest benefit from new EPC-based clinical concepts; (2) every autologous delivery of EPCs inevitably involves a considerable time delay in treatment, due to the time needed for collection, identification, isolation, and then propagation of progenitors ex vivo (Leeper et al. 2010). These facts necessitate developing appropriate methods to obtain and reserve high quality and quantity of ready-to-use EPCs, which remains to be one of the major obstacles in EPC autologous therapeutic use (Kretlow et al. 2008; Lu et al. 2008; Teraa et al. 2013).
Preclinical and clinical studies have shown that bone marrow derived EPCs (BM-EPCs) hold extensive prospects for autologous EPC-based therapy because of their easy availability as well as few ethical concerns and low immunogenicity (Chen et al. 2017; Garbuzova-Davis et al. 2017; Hristov et al. 2003; Shintani et al. 2001; Yang et al. 2004). To benefit from the transplantation of EPCs, sufficient and ready-to-use autologous EPCs are required for injection into the ischemic area or for coating on the surface of vascular grafts (Kalka et al. 2000; Kaushal et al. 2001). Ex vivo expansion is an acceptable strategy for enriching EPCs (Kalka et al. 2000; Wu et al. 2012). However, the culture time of most studies was about a month (within 4 passages of culture) (Mieno et al. 2008; Wu et al. 2012; Yu et al. 2014), and insufficient for obtaining adequate EPCs for therapeutic applications, which can be roughly extrapolated from previous animal studies (Kalka et al. 2000). Extensive expansion for obtaining a large quantity of EPCs with a satisfying quality for personal autotransplantation and effects of serial passage on phenotype and functions of EPCs still need to be explored.
Cryopreservation could be a promising strategy for long term storage of a large quantity of EPCs allowing their immediate availability (Mieno et al. 2008; Wu et al. 2012). Many studies have stored EPCs by freezing bone marrow (BM), peripheral blood (PB), or umbilical cord blood (UCB) derived MNCs and yielded controversial results with respect to the effect of cryopreservation on EPC viability and functions. Some reports have indicated that cryopreservation of MNCs from BM, UCB, and PB impaired EPCs viability and functions. For example, the freezing and thawing process led to a decreased proliferation, EPC marker expression, potential to differentiate into ECs, and recovery of artery injury (Bogoslovsky et al. 2013; Lu et al. 2008; Papasavvas et al. 2012; Vanneaux et al. 2010). However, several studies have reported that EPCs have undergone cryopreservation without altered viability, proliferation and their endothelial functions (Lin et al. 2011; Mieno et al. 2008; Wu et al. 2012). The effectiveness of EPCs cryopreservation could be affected by many factors including cell type and size, composition of cells, cell density at freezing, and cooling rate (Karlsson 2002; von Bomhard et al. 2016; Wu et al. 2012). In our opinion, the low cryopreservation efficacy may be due to the low frequency of mature or immature EPCs in each BM- or UCB-MNCs unit which makes EPCs more difficult to recover from the severity of freezing and thawing. Thus freezing pure EPCs at high-density will favor the opportunity to minimize cryoinjury and ensure retention in phenotype and therapeutic characteristics of EPCs after cryopreservation. So far by our knowledge, few studies investigated effects of cryopreservation and thawing on serial passaged EPCs with high purity.
The present study aims at investigating effects of a limited number of culture passages and cryopreservation on EPC phenotype and functions. We isolated EPCs from rat bone marrow and cultured them up to passage 12. The morphology, proliferation, surface marker expression, and endothelial functions of fresh cultured and post-cryopreserved EPCs at passage 7 and 12 were evaluated. Our work will be helpful for providing robust strategies and quality control criteria for obtaining sufficient and ready-to-use EPCs during tissue engineering and therapeutic applications.
Materials and methods
Experiments involving Sprague–Dawley rats were carried out in strict accordance with guidelines for the Care and Use of Laboratory Animals of the Beijing Municipal Science & Technology Commission. The protocol was approved by the Ethics Review Committee for Animal Experimentation of the Peking University [SYXK (Beijing) 2006-0025]. Surgeries were performed under phenobarbital anesthesia, and all efforts were made to minimize suffering.
Cell culture
The 4-week-old male Sprague Dawley rats were purchased from Vital River Experimental Animal Center (Beijing, China), where the use of animal was approved by the local Ethics Committee. EPCs were isolated from the femur and tibia of Sprague Dawley rats by flushing marrow with M199 medium (Gibco, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS, Gibco). After flushing, the BM-derived cells were centrifuged in a 1.073 g/ml Percoll (Pharmacia, Uppsala, Sweden) density gradient at 550 g for 20 min (Hermle Z326, Wehingen, Germany). The enriched cells were collected from the interface, resuspended in M199 medium containing 10% FBS, and transferred into culture dishes. The BM-derived cells were cultured with M199 medium, containing 10% FBS, 50% Endothelial cell medium (ECM, Sciencell, Carlsbad, CA, USA), 100 U/ml penicillin (Sangon, Shanghai, China), 100 mg/ml streptomycin (Sangon), and 50 U/ml heparin (Ameresco, Solon, OH, USA) in cell incubator at 37 °C with 5% CO2 for 4 days. Then the medium was changed every 3 days.
Cryopreservation and thawing of EPCs
EPCs at passages 5 and 10 were used for cryopreservation. Separated EPCs were suspended in cold freezing solution containing 80% M199, 10% FBS, and 10% dimethyl sulphoxide (DMSO, Sangon), aliquoted into 2-ml cryotubes at a density of 1 × 106 cells/ml, placed in a Cryo 1 °C Freezing Container (Nalgene, Thermo Scientific, Waltham, MA, USA) and stored at − 80 °C freezer overnight. The cells were then transferred to liquid nitrogen for long-term storage.
The cryotubes were thawed very rapidly in a 37 °C water bath and the EPCs were immediately transferred to 10 ml thawing medium containing 90% M199 medium and 10% FBS. The EPCs were then centrifuged at 200 g for 2 min at 4 °C. The supernatant was removed and EPCs were resuspended with EPC medium described above. The cells were counted and assessed for viability by trypan blue dye exclusion. Then the resuscitated cells [passages 6 or 11, abbreviated as EPC (P6) or EPC (P11), respectively] were cultured with EPC medium. The post-cryopreserved EPCs (passages 7 or 12, abbreviated as C7 or C12, respectively) were used in EPC phenotype and function evaluation by comparing with fresh EPCs at passage 7 or 12 (abbreviated as F7 or F12, respectively).
The influence of cell density on resuscitation was evaluated by cryopreserving and thawing of EPCs at different cell densities of 1 × 106 cells/ml, 1.4 × 104 cells/ml and 5 × 102 cells/ml. We used the EPC density (1.4 × 104 cells/ml and 5 × 102 cells/ml) according to Peichev’s work who found 1.4 ± 0.5% or 1.4 × 104 EPCs in 1 × 106 cord blood derived stem cells (Peichev et al. 2000), and Kalka’s work who reported that 0.05% or 5 × 102 hEPCs can be isolated from 1 × 106 hPBMCs of human subjects (Kalka et al. 2000).
Proliferation assay
The cellular proliferation of EPCs was measured using a colorimetric assay based on the tetrazolium salt MTT [(3-(4, 5-dimethyldiazol-2-yl)-2, 5-diphenyl tetrazolium bromide; Sigma, St. Louis, MO, USA]. EPCs were plated into 96-well culture plates at a density of 1 × 104 cells/ml with 200 µl of culture medium per well and were cultured for various time periods. Then, 20 µl MTT solution (5 mg/ml) was added to each well and incubated at 37 °C for 4 h. The medium was removed and 200 µl of DMSO was added. The OD of samples was measured at 490 nm using a Varioskan Flash spectrophotometer (Thermo Scientific).
Fluorescent staining
Cells were fixed in 4% paraformaldehyde, then permeabilized with 0.1% Triton X-100 in PBS and blocked in 1% bovine serum albumin. Primary antibodies to rat CD34, CD31, CD133 (1:100, all from Biosen Biotech, Beijing, China), VE-Cadherin, VEGFR-2 (1:100, both from Boster, Wuhan, China), and von Willebrand Factor (vWF,1:100, ZSGB-BIO, Beijing, China) were incubated with the cells at 4 °C overnight. After washing with PBS, FITC-conjugated secondary antibodies (ZSGB-BIO, Beijing, China) at a dilution of 1:100 were added to the cells for 60 min. Nuclei were labeled with DAPI (Sigma). All fluorescent stainings were visualized and photos were taken under a Leica TCS SPE confocal microscope (Leica Microsystems GmbH, Wetzlar, Germany) using a 63 × oil immersion objective lens.
Flow cytometric analysis of EPC markers
The expression of EPC markers including CD34, CD31, CD133 and VEGFR-2 were determined by flow cytometry. Cells harvested after 7 and 12 passages were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS and then blocked in 1% bovine serum albumin. Primary antibodies to rat CD34, CD31, CD133 and VEGFR-2 were incubated with the cells at 4 °C overnight. Then cells were washed with PBS, FITC-conjugated secondary antibodies at a dilution of 1:100 were added to the cells for 60 min, and the cell markers were analyzed on a flow cytometer (Merck Millipore, Darmstadt, Germany).
Dil-ac-LDL uptake and FITC-UEA-1 binding of EPCs
EPCs were washed with PBS three times, and incubated in medium containing 20 μg/ml Dil-ac-LDL (Invitrogen, Carlsbad, CA, USA) for 4 h at 37 °C, 5% CO2. Cells were fixed with 4% paraformaldehyde, and incubated for 1 h with 10 μg/ml FITC-UEA-1 (Invitrogen) at room temperature. Nuclei were labeled with DAPI. Subsequently, incorporation of Dil-Ac-LDL and binding of FITC-UEA-1 were observed and photos were taken by a Leica TCS SPE confocal microscope using a 63 × oil immersion objective lens.
In vitro capillary tube formation assay in Matrigel
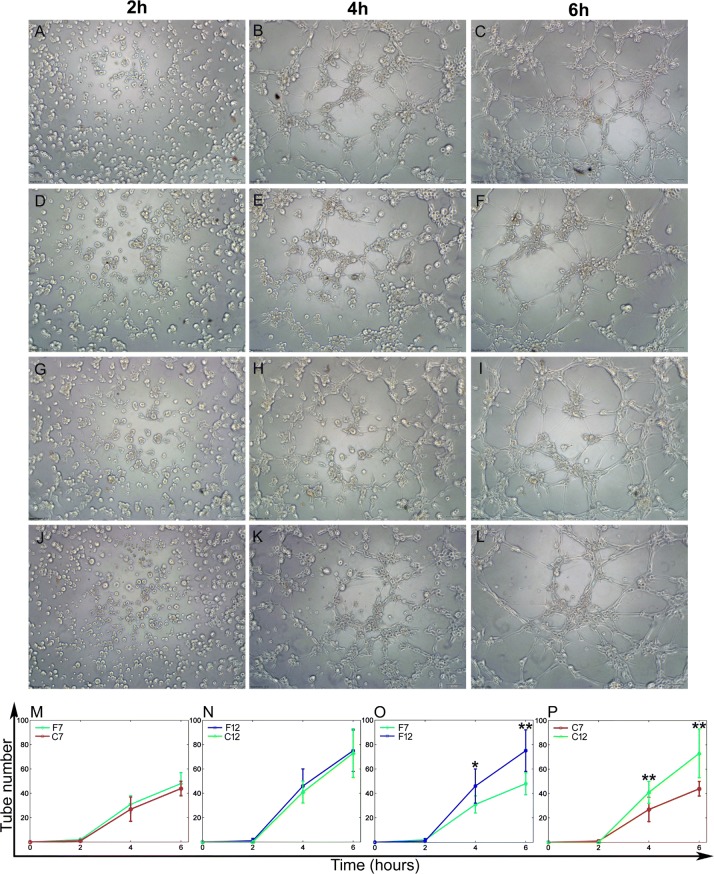
EPCs were seeded on Matrigel (50 µl/well, BD, Franklin Lakes, NJ, USA) coated 96-well plate at a cell density of 3.5 × 104 cells/well and incubated with EPC culture medium at 37 °C, 5% CO2. The in vitro capillary-like tube formation was observed and images were captured using an inverted phase contrast optical microscope (IX71, Olympus Inc., Tokyo, Japan) after EPCs were incubated for 2, 4 and 6 h, respectively. For each sample, at least 5 micrographs were taken at different positions. The ability of EPCs to form tube-like structures was assessed by counting the number of network circles in each image.
Migration assay
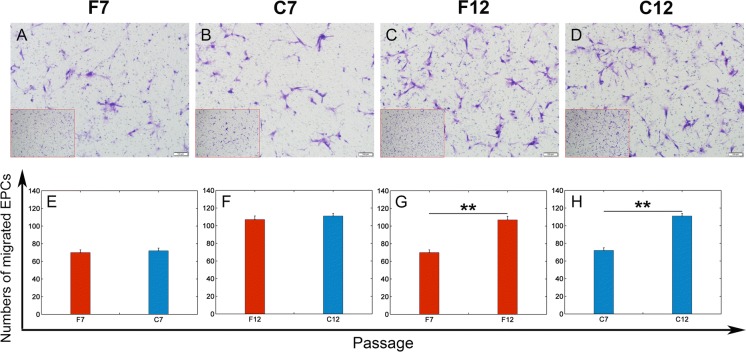
Fresh and post-cryopreserved EPCs at passages 7 and 12 were used to evaluate the migration ability. Briefly, EPCs were starved in M199 contained 0.5% FBS at 37 °C, 5% CO2 for 24 h. After starvation, EPCs were detached with 0.25% Trypsin and 1 mM EDTA in PBS (pH 7.4) and counted. EPCs at a density of 4 × 104 cells/well were seeded in the upper chamber of a modified Boyden chamber (Corning, Corning, NY, USA). A polycarbonate filter with 8-μm pore size was placed between the upper and lower chambers. The chamber was placed in a 24-well plate containing 0.8 ml of culture medium. After 24 h incubation at 37 °C and 5% CO2, the migrated cells on the lower side of the filter were washed with PBS, fixed in 4% paraformaldehyde and stained with 0.5% Crystal violet solution. The number of migrated cells was counted manually in 5 random microscopic fields.
Statistical analysis
Each experiment was repeated independently for at least 3 times. All data are expressed as mean ± SD (n = 3). Statistical analysis was performed using an unpaired two-tailed Student’s t-test. A value of p < 0.05 was considered statistically significant.
Results
Effects of passage number and cryopreservation on EPC morphology
The isolated EPCs formed blood island structure within 24 h of the primary culture (Fig. S1 A). Then the spindle-shaped and adherent cells sprouted from the edge of the blood island within 3 days (Fig. S1 B). After 7 days of the primary culture, the network structure appeared when a lager cell monolayer formed (Fig. S1 C). The isolated cells were positive for EPC markers including CD31, CD34, CD133, VEGFR-2, VE-Cadherin, and vWF (Fig. S1 D-I).
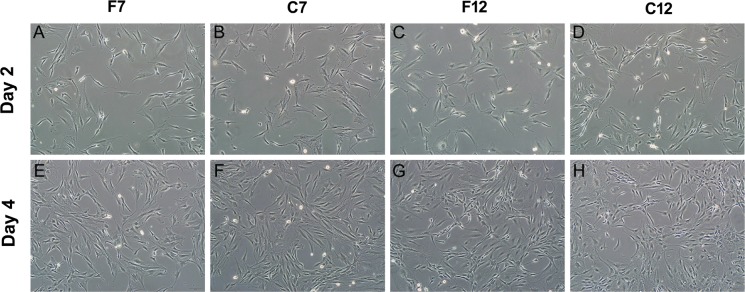
Most of EPCs at passages 0–12 maintained spindle-shaped morphology, except a very small amount of EPCs showed a more flat appearance (Fig. S2). Moreover, no significant changes in EPC morphology were found between them under our culture condition (Fig. 1, S1, S2). Notably, EPCs at passages 7 and 12 showed similar morphological characteristics such as spindle-shaped morphology (Fig. 1A, C, vs. Fig. S1 B) and network structures (Fig. 1E, G, vs. Fig. S1 C) in comparison with those seen in the primary culture. Furthermore, the post-cryopreserved EPCs of the seventh and twelfth passage exhibited morphological properties similar to those of fresh EPCs at the same passages (Fig. 1).
Fig. 1.
Morphologies of BM-EPCs after a limited number of passages and cryopreservation. The fresh EPCs were cultured to the seventh (F7) and twelfth (F12) passage respectively, A, C after 2 days of culture, E, G after 4 days of culture. Post-cryopreserved EPCs which were thawed at passage 6 and cultured to passage 7 (abbreviated as C7) (B, F), and thawed at passage 11 and culture to passage 12 (abbreviated as C12) (D, H). Scale bar: 100 μm
Effects of passage number and cryopreservation on EPC proliferation and viability
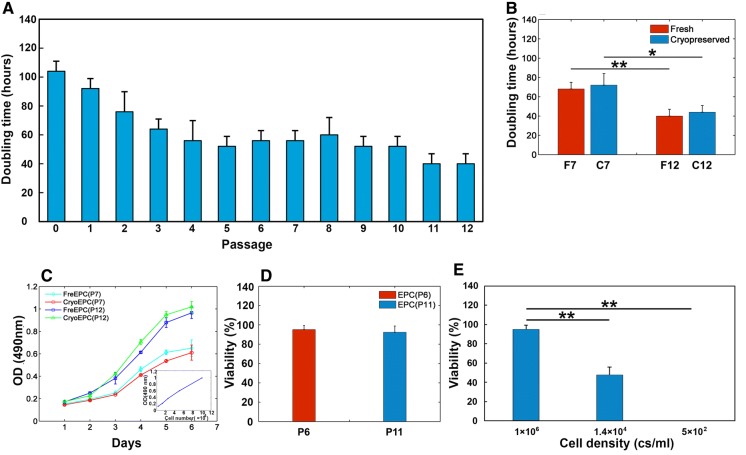
EPCs at each of the 12 passages were examined for changes in growth kinetics. Cells underwent 2-4 population doublings at each passage, totaling 40 population doublings by passage 12 (Fig. 2A). The doubling time of BM-derived EPCs at passages 0-3 decreased from 104.00 ± 6.93 to 64.00 ± 6.93 h, whereas it stayed stable at 54.85 ± 8.64 h from passage 4 to passage 10, and then decreased to 40.00 ± 6.93 h at passages 11 and 12. Consistently, growth and proliferation of EPCs at passage 12 were significantly higher than that at passage 7 according to the growth curves (Fig. 2B, p < 0.05). Furthermore, there were no significant difference in proliferation rate and doubling time between the fresh and the post-cryopreserved EPCs (Fig. 2B, C). In addition, there was no significant difference in the recovery rate of cryopreserved EPCs between the passage 6 and 11 [95.17 ± 4.13%, EPC (P6) vs. 92.33 ± 6.51%, EPC (P11), Fig. 2D], indicating that a limited number of passages did not affect viability of the resuscitated cells. However, there were significant differences in the recovery rate of EPCs cryopreserved at different cell density (Fig. 2E, p < 0.05). In brief, our experiments observed that a limit number of culture passages improved EPC proliferation, while cryopreservation did not impair proliferation and viability of EPCs.
Fig. 2.
Proliferation and recovery characteristics of EPCs after a limited number of passages and cryopreservation. A Average doubling time of EPCs at each passage. B Average doubling time of fresh and post-cryopreserved EPCs at passages 7 and 12. (Data in mean ± SD, n = 3), *significant difference between post-cryopreserved EPCs at passages 7 and 12 at p < 0.05, **significant difference between fresh EPCs at passages 7 and 12 at p < 0.01. C The growth curve of fresh and post-cryopreserved EPCs at passages 7 and 12. Results from the 7-day assessment of EPC proliferation by MTT assay. A representative standard curve was inserted in the lower right corner. D Average viability of cryopreserved EPCs immediately after thawing at passage 6 and passage 11 assessed by trypan blue dye exclusion. E Average viability of EPCs cryopreserved at different density immediately after thawing assessed by trypan blue dye exclusion (data are presented as mean ± SD, n = 3), **significant difference between 1 × 106 c/ml and 1.4 × 104 c/ml or 5 × 102 c/ml at p < 0.01
Effects of passage number and cryopreservation on EPC marker expression
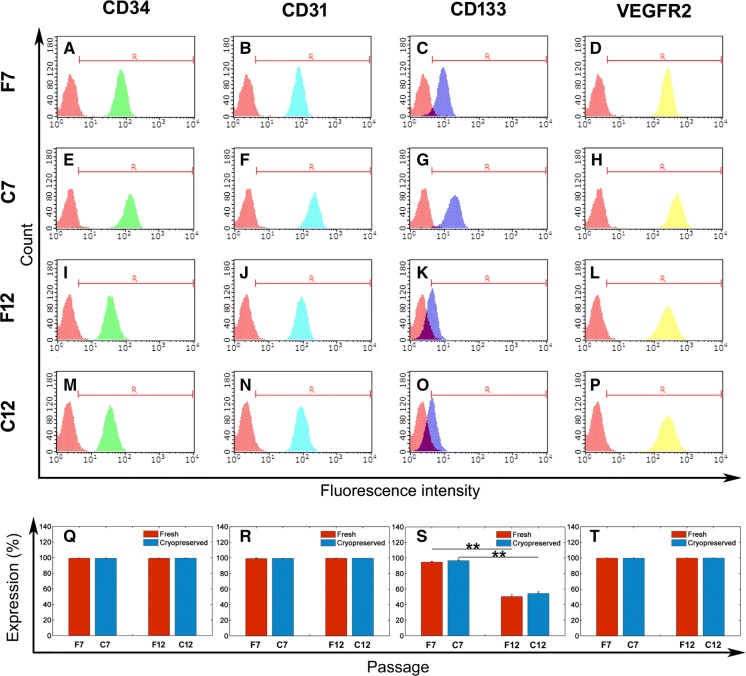
Flow cytometry analysis and immunofluorescence staining were performed to evaluate the immunophenotype of EPCs after a limited number of culture passages or cryopreservation. Fresh EPCs and cryopreserved EPCs at passage 7 and 12 were positive for CD133, CD34, CD31 and VEGFR-2 as shown in Fig. 3 and Fig. S3, while the rat aortic endothelial cells (RAECs) were negative for CD34 and CD133, and the bone marrow derived stroma cells (BMSCs) were negative for CD133, CD34, CD31 and VEGFR-2 (Fig. S4). The cells at passage 7 exhibited high positive ratios for CD133, CD34, CD31 and VEGFR-2, which indicated a high purity of EPCs at passage 7 (Fig. 3Q–T). No significant differences in the positive ratio for CD34, CD31 and VEGFR-2 were observed between EPCs at passages 7 and 12 (Fig. 3). The immunofluorescence staining of CD34, CD31 also showed no obvious changes between the fresh and post-cryopreserved groups at passages 7 and 12 (Fig. S3). However, more intensive fluorescent staining of the VEGFR-2 was observed at passage 12 when compared with those at passage 7 (Fig. S3 D, H, L, P), although the positive ratio for VEGFR-2 did not change (Fig. 3T). The positive ratio for CD 133 decreased from 95.72 ± 1.61% at passage 7 to 50.43 ± 2.25% at passage 12 (Fig. 3S), while the cryopreservation did not alter the expression of CD133 (Fig. 3C, G, K, O, S). Consistently, the fluorescent staining of CD133 at passage 12 was less intensive than those at passage 7 (Fig. S3 C, G, K, O).
Fig. 3.
Flow cytometry analysis of cell surface marker of EPCs after a limited number of passages and cryopreservation. A–P Representative results of flow cytometry analysis of CD34 (green), CD31 (light blue), CD133 (dark blue), and VEGFR-2 (yellow) on EPCs. Cells were incubated with antibodies to CD34, CD31, CD133, and VEGFR-2, and then labeled with FITC-conjugated secondary antibodies. The red peak: negative control. Similar results were obtained for at least three experiments. Percentage of cells positive to CD34 (Q), CD31 (R), CD133 (S), and VEGFR-2 (T) determined by comparison with corresponding negative control labeling (data are presented as mean ± SD, n = 3), **significant difference between passage 7 and 12 at p < 0.01. (Color figure online)
Effects of passage number and cryopreservation on Ac-LDL uptake and UEA-1 expression by EPC
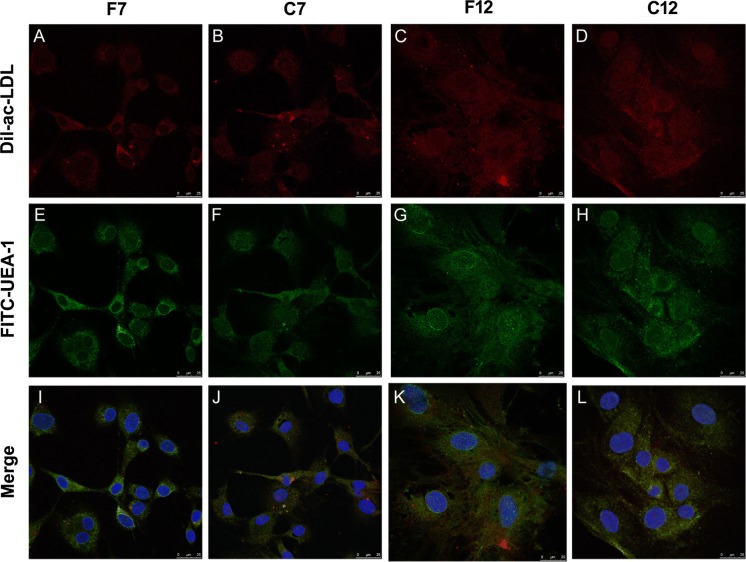
Incorporation of Dil-Ac-LDL and binding of FITC-UEA-1 was used to evaluate endothelial functions that EPCs have. Both Dil-ac-LDL (Fig. 4A–D, red) and FITC-UEA-1 (Fig. 4E–H, green) were detected in fresh EPCs and post-cryopreserved EPCs, but not detected in BMSCs (Fig. S5). Double positive EPCs (Fig. 4I–L, yellow) indicated endothelial functions that EPCs retained after a limited number of passage or cryopreservation. No obvious differences in the DiI-Ac-LDL uptake and UEA-1 binding potential between the fresh and post-cryopreserved groups at passages 7 and 12 were observed.
Fig. 4.
Dil-ac-LDL uptake and FITC-UEA-1 binding of EPCs after a limited numbers of passage and cryopreservation. EPCs were incubated with Dil-ac-LDL and FITC-UEA-1, respectively; dye uptake and lectin binding were then assessed by immunofluorescent microscopy. A–D Dil-ac-LDL (red); E–H FITC-UEA-1 (green); I–L double stained cells (yellow); nuclei were labeled with DAPI (blue). Scale bar: 25 μm. (Color figure online)
Effects of passage number and cryopreservation on EPC tube formation activity
The number of tube-like structures formed in Matrigel-based media was used to evaluate the angiogenesis capacity of the post-cryopreserved and the fresh EPCs at passages 7 and 12 (Fig. 5). The tube-like structures were found in both the fresh and the post-cryopreserved cells (Fig. 5A–L). EPCs at passage 12 showed better capacity for tube formation than EPCs at passage 7 (Fig. 5O, P, p < 0.05). However, the post-cryopreserved EPCs exhibited similar tube formation capacity of fresh EPCs (Fig. 5M, N).
Fig. 5.
Tube formation of EPCs after a limited number of passages and cryopreservation. A–L Representative phase-contrast microscopic images of the tube-like structures formed by fresh and post-cryopreserved EPCs at passages 7 and 12. EPCs were culture in matrigel for 2 h (A, D, G, J), 4 h (B, E, H, K), and 6 h (C, F, I, L). Scale bar: 100 μm. M–P The average numbers of tube-like structures formed by EPCs at different incubation times (data are presented at mean ± SD, n = 3), *significant difference between passages 7 and 12 at p < 0.05, **significant difference between passages 7 and 12 at p < 0.01
Effects of passage number and cryopreservation on EPC migration
The number of migrated cells on the lower side of the filter was used to evaluate the homing function that EPCs have (Fig. 6). The migration capacity of EPCs at passage 12 was much higher than that of EPCs at passage 7 (109 ± 2 cells at passage 12 vs. 71 ± 3 cells at passage 7, Fig. 6A–D, G, H, p < 0.05). The fresh EPCs showed no significant difference in migration capacity compared with the post-cryopreserved EPCs (Fig. 6A–D, E, F).
Fig. 6.
Migration activity of EPCs after a limited number of passages and cryopreservation. A–D Representative phase-contrast microscopic images of migrated cells which are located at the lower surface of the filter. Scale bar: 100 μm. A lower magnification image in the left lower corner shows a representative overview of each group. Scale bar: 200 μm. E–H Average numbers of migrated EPCs per microscopic field (data are presented as mean ± SD, n = 3), **significant difference between passages 7 and 12 at p < 0.01
Discussion
A critical limitation, so far, for tissue engineering and autologous therapeutic applications of BM-EPCs is their low frequency in bone marrow, which is even lower in number and activity level in patients with cardiovascular risk factors (Kuki et al. 2006; Papa et al. 2006; Tamarat et al. 2004; Tepper et al. 2002). Thus the success of using BM-EPCs for these applications will depend greatly on the ability of BM-EPCs to be extensively cultured and long-term stored while maintaining their phenotype and functions (Chong et al. 2016; Sukmawati and Tanaka 2015), which have not been well determined at present. This study found that EPCs after a limited number of passages in culture and cryopreservation maintained the morphological characteristics, were positive for CD31, CD34, VEGFR-2, Dil-ac-LDL uptake and FITC-UEA-1 binding functions, enhanced EPCs proliferation, tube formation and migration, but showed decreased CD133 expression compared with EPCs at passage 7. Our results may suggest a new strategy for obtaining sufficient ready-to-use BM-EPCs with higher quality and stability for tissue engineering and autologous EPC-based therapy.
EPCs show important differences in morphology, phenotype and behavior when they are under different isolation and culture conditions (Bai et al. 2012; Kim et al. 2016; Yang et al. 2011). For example, the so called early EPCs showed spindle-shaped morphology at the early stage of culture (within 2 weeks), while late EPC with cobblestone shape appeared 2–3 weeks later, where time was the main difference of the condition for EPC culture and isolation (Hur et al. 2004; Mukai et al. 2008). The present study showed that most of the EPCs maintained a spindle-shaped morphology at passages 0–12. Moreover, no significant changes of EPC morphology were found between them under our culture condition (Figs. 1, 2, S2). The morphological difference between our EPCs and late EPCs in some studies is probably due to the different isolation and culture methods used. A good example of this is that BM-MNCs in different culture media have different morphological phenotypes and proliferation rates (Jianguo et al. 2010; Yang et al. 2011). Yang et al found that BM-MNCs could be differentiated into spindle-shaped, early EPC-like cells in complete medium (M199 containing FBS, VEGF and bFGF), or differentiated into late EPC-like cells with cobblestone shape in EGM-2MV. Both of the two types of induced cells had LDL-uptake and lectin binding functions, as well as the potential of differentiation into ECs (Yang et al. 2011). Furthermore, most studies investigated morphological characteristics of EPCs within 4 passages (Hur et al. 2004; Jianguo et al. 2010; Mukai et al. 2008). Although the effect of isolation and culture condition on other stem cells was reported (Wall et al. 2007), to our knowledge, our study is the first one which reported the effects of serial passages on EPC morphological characteristics. Our data and other previous studies suggested that EPC functions should be considered as more important criteria than morphological characteristics for identifying the phase of EPCs.
Our data displayed increased growth and proliferation capabilities of EPCs at passage 12 in comparison with EPCs at passage 7. Moreover, totaling 40 population doublings were achieved till passage 12. EPCs in adult body are very scarce that about 12L of blood may be necessary to obtain adequate numbers of EPCs to treat critical limb ischemia in patients according to animal study (Kalka et al. 2000). Furthermore, the number and the activity level of EPCs are even lower in clinical patients with a variety of phenotypes such as aging, smoking, diabetes, hypertension, metabolic syndrome, and coronary artery disease, which constitute major limitations of primary EPC transplantation (Garolla et al. 2009; Teraa et al. 2013; Umemura et al. 2008; Vasa et al. 2001; Yin et al. 2015). To our knowledge, limited research compared the potential difference of nature of EPCs between rat and human till now. However, human BMSCs were reported to be less sensitive to plating density and to expand more slowly than rat BMSC (Javazon et al. 2001), which infers the importance of the in vitro purification and expansion of human EPCs. Although it is necessary to confirm our data with human BM-EPCs, our findings indicate that ex vivo expansion may be available to enhance growth and proliferation capabilities of EPCs and to overcome the primary scarcity of a viable and functional EPC population.
Cell markers including CD34, CD133, CD31, VEGFR-2, VE-Cadherin, and vWF are commonly considered markers for EPCs (Hristov et al. 2003). CD34 is an adhesion molecule typically considered a progenitor marker (Fadini et al. 2006). CD133 is another progenitor marker being used to identify more immature progenitor cells than CD34 alone (Fadini et al. 2006). CD31 and VEGFR-2 are harboring markers which play an important role in EPC homing (Lev et al. 2006). The bone marrow is a reservoir of progenitor cells, including hematopoietic progenitor cells (HPCs), stromal progenitor cells (SPCs), and EPCs (Pitchford et al. 2009). HPCs are known to be negative for VEGFR-2, VE-Cadherin, and vWF, while SPCs were negative for CD45 and CD34 (Pitchford et al. 2009). Cells obtained in this study showed typical marker phenotype of EPCs (positive for CD34, CD133, VEGFR-2, CD31, VE-Cadherin, and vWF), as well as clear marker expression differences from the HPCs and SPCs (Fig. S 1). Furthermore, the in vitro cultured EPCs at passage 7 in this study exhibited high positive ratio for EPC markers (Fig. 3), which demonstrated that ex vivo expansion facilitated high purity and quality of EPCs. In our opinion, a major reason for this is that culturing BM-MNCs with media containing the cytokine mixture for EPC, such as VEGF, bFGF, IGF, and EGF, appears to preferentially promote endothelial lineage differentiation. Previous works also found that cytokine composition of the culture media may influence in vitro mononuclear cell differentiation (Jianguo et al. 2010; Kalka et al. 2000; Yang et al. 2011). In this study, EPCs at passage 12 retained high positive ratio for CD34, CD 31, and VEGFR-2 similar to those at passage 7, while a part of cells lost CD133 expression (Fig. 3, Fig. S3). CD133 is reported to be gradually lost during the differentiation of EPCs into endothelial cells (Bai et al. 2012). In the present study, no significant differences in ac-LDL uptake and UEA-1 binding functions between EPCs at passages 7 and 12 were found, demonstrating that a limited number of passages causes little impairment in EPC functions.
In this study, EPCs at passage 12 showed higher level of immunofluorescence staining for VEGFR-2 than EPCs at passage 7. Although no significant change in positive ratio for VEGFR-2 was found during the limited serial passage, the number of EPCs with higher fluorescence intensity with respect to VEGFR-2 increased at passage 12 (data not shown). VEGF is considered the central factor influencing the differentiation of EPCs into mature endothelial cells and enhancing EPC function including homing, migration and endothelial tube formation through VEGFR-2 (Ohtani et al. 2004; Young et al. 2002; Yu et al. 2014). Thus increase of VEGFR-2 expression will contribute to enhancement of the efficiency and/or persistence of EPC engraftment (Kim et al. 2016; Ohtani et al. 2004). Our study found that the tube formation and migration capacity of EPCs at passage 12 is better than EPCs at passage 7. Kim’s study also found long culture time facilitated the tube formation and long-term survival potential of EPCs (Kim et al. 2016).
Unlike storing EPCs by freezing bone marrow or umbilical cord blood derived MNCs as done in previous studies which often resulted in poor viability and differentiation potential of EPCs after thawing (Lu et al. 2008; Vanneaux et al. 2010), we cryopreserved the purified EPCs, obtained by a limited passage culture, at high-density. Since the number of EPCs in each bone marrow or cord blood unit is so low (Kalka et al. 2000; Teraa et al. 2013), damage during freezing and thawing process may even further decrease the EPCs numbers and make them more difficult to derive. We found that EPCs with high density and purity exhibited higher tolerance to harsh environmental conditions arisen from freezing and thawing (Fig. 2E). Our result is supported by Wu et al. (2012) who froze the first passage EPCs derived from the BM-MNCs and obtained high EPCs viability, as well as good proliferation and migration capacities after thawing. Our data indicate that cryopreservation could be a promising strategy for long term storage in order to provide timely supply of sufficient amount of EPCs.
While our data suggested a novel strategy for obtaining sufficient high quality ready-to-use BM-EPCs, they are not without limitations. First, we used an in vitro model in the present work, further transplantation studies are necessary to convincingly demonstrate the suitability of our data for therapeutic applications. Second, our work is also necessary to be confirmed by using human BM-EPCs, because the in vitro expanded human BM-EPCs may not show the same profiles of proliferation and stability, although bone marrow derived stem cells of human and rat share many phenotypical and functional similarities(Chabannes et al. 2007; Javazon et al. 2001). In the future, we hope to conduct similar experiments with human BM-EPCs and animal models.
Conclusion
Our present data underlined that a limited number of culture passages facilitates obtaining sufficient EPCs with high purity, quality, and stability. Moreover, cryopreservation of purified high-density EPCs did not change phenotype and function of EPCs. Our results may be useful for the development of robust strategies and quality control criteria for obtaining sufficient and high-quality ready-to-use EPCs for tissue engineering and therapeutic applications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31771019, 11172031, 31470901). The national key research and development plan (No. 2016YFC1101101). International Joint Research Center of Aerospace Biotechnology and 344 Medical Engineering from Ministry of Science and Technology of China, 111 Project 345 (No. B13003).
Compliance with ethical standards
Conflict of interest
The authors have no additional conflict of interest to disclose.
Contributor Information
Xianghui Gong, Phone: 86-10-82339428, Email: xhgong@buaa.edu.cn.
Yubo Fan, Phone: 86-10-82339428, Email: yubofan@buaa.edu.cn.
References
- Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Bai CY, Hou L, Zhang M, Pu Y, Guan W, Ma Y. Characterization of vascular endothelial progenitor cells from chicken bone marrow. BMC Vet Res. 2012;8:54. doi: 10.1186/1746-6148-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoslovsky T, Wang D, Maric D, Scattergood-Keepper L, Spatz M, Auh S, Hallenbeck J. Cryopreservation and enumeration of human endothelial progenitor and endothelial cells for clinical trials. J Blood Disord Transfus. 2013;4:158. doi: 10.4172/2155-9864.1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabannes D, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- Chen C, Zheng S, Zhang X, Dai P, Gao Y, Nan L, Zhang Y. Transplantation of amniotic scaffold-seeded mesenchymal stem cells and/or endothelial progenitor cells from bone marrow to efficiently repair 3-cm circumferential urethral defect in model dogs. Tissue Eng Part A. 2017;24:47–56. doi: 10.1089/ten.tea.2016.0518. [DOI] [PubMed] [Google Scholar]
- Chong MSK, Ng WK, Chan JKY. Concise review: endothelial progenitor cells in regenerative medicine: applications and challenges. Stem Cell Transl Med. 2016;5:530–538. doi: 10.5966/sctm.2015-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A, Vigili de Kreutzenberg S. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37:2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Lin R, Borlongan CV. Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity toward blood-brain barrier repair in ischemic stroke rats. Stem Cells. 2017;35:1246–1258. doi: 10.1002/stem.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garolla A, et al. Reduced endothelial progenitor cell number and function in inflammatory bowel disease: a possible link to the pathogenesis. Am J Gastroenterol. 2009;104:2500–2507. doi: 10.1038/ajg.2009.332. [DOI] [PubMed] [Google Scholar]
- Hristov M, Erl W, Weber PC. Endothelial progenitor cells mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–1189. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- Hur J, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow sromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- Jianguo W, Tianhang L, Hong Z, Zhengmao L, Jianwei B, Xuchao X, Guoen F. Optimization of culture conditions for endothelial progenitor cells from porcine bone marrow in vitro. Cell Prolif. 2010;43:418–426. doi: 10.1111/j.1365-2184.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalka C, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.97.7.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson JOM. Cryopreservation: freezing and vitrification. Science. 2002;296:655–656. doi: 10.1126/science.296.5568.655d. [DOI] [PubMed] [Google Scholar]
- Kaushal S, et al. Functional small diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim S, Baek SH, Kwon SM. Pivotal cytoprotective mediators and promising therapeutic strategies for endothelial progenitor cell-based cardiovascular regeneration. Stem Cells Int. 2016;2016:1–14. doi: 10.1155/2016/8340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretlow JD, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuki S, Imanishi T, Kobayashi K, Matsuo Y, Obana M, Akasaka T. Hyperglycemia accelerated endothelial progenitor cell senescence via the activation of p38 mitogen-activated protein kinase. Circ J. 2006;70:1076–1081. doi: 10.1253/circj.70.1076. [DOI] [PubMed] [Google Scholar]
- Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev EI, et al. Potential role of activated platelets in homing of human endothelial progenitor cells to subendothelial matrix. Thromb Haemost. 2006;96:498–504. doi: 10.1160/TH06-05-0250. [DOI] [PubMed] [Google Scholar]
- Lin RZ, Dreyzin A, Aamodt K, Dudley AC, Melero-Martin JM. Functional endothelial progenitor cells from cryopreserved umbilical cord blood. Cell Transpl. 2011;20:515–522. doi: 10.3727/096368910X532729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XM, Proctor SJ, Dickinson AM. The effect of cryopreservation on umbilical cord blood endothelial progenitor cell differentiation. Cell Transpl. 2008;17:1423–1428. doi: 10.3727/096368908787648155. [DOI] [PubMed] [Google Scholar]
- Mieno S, Clements RT, Boodhwani M, Sodha NR, Ramlawi B, Bianchi C, Sellke FW. Characteristics and function of cryopreserved bone marrow-derived endothelial progenitor cells. Ann Thorac Surg. 2008;85:1361–1366. doi: 10.1016/j.athoracsur.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Mukai N, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008;314:430–440. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Ohtani K, et al. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- Papa ND, et al. Bone marrow endothelial progenitors are defective in systemic sclerosis. Arthritis Rheum. 2006;54:2605–2615. doi: 10.1002/art.22035. [DOI] [PubMed] [Google Scholar]
- Papasavvas E, et al. Increased CD34(+)/KDR(+) cells are not associated with carotid artery intima-media thickness progression in chronic HIV-positive subjects. Antivir Ther. 2012;17:557–563. doi: 10.3851/IMP2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichev M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34 + cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.CIR.103.6.897. [DOI] [PubMed] [Google Scholar]
- Sukmawati D, Tanaka R. Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine. Am J Transl Res. 2015;7:411–421. [PMC free article] [PubMed] [Google Scholar]
- Tamarat R, et al. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–466. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper OM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.CIR.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Teraa M, et al. Bone marrow alterations and lower endothelial progenitor cell numbers in critical limb ischemia patients. PLOS ONE. 2013;8:e55592. doi: 10.1371/journal.pone.0055592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009;49:502–510. doi: 10.1016/j.jvs.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, et al. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am J Hypertens. 2008;21:1203–1209. doi: 10.1038/ajh.2008.278. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Vanneaux V, et al. In vitro and in vivo analysis of endothelial progenitor cells from cryopreserved umbilical cord blood: are we ready for clinical application? Cell Transpl. 2010;19:1143–1155. doi: 10.3727/096368910X504487. [DOI] [PubMed] [Google Scholar]
- Vasa M, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- von Bomhard A, Elsässer A, Ritschl LM, Schwarz S, Rotter N. Cryopreservation of endothelial cells in various cryoprotective agents and media-vitrification versus slow freezing methods. PLoS ONE. 2016;11:e0149660. doi: 10.1371/journal.pone.0149660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall ME, Bernacki SH, Loboa EG. Effects of serial passaging on the adipogenic and osteogenic differentiation potential of adipose-derived human mesenchymal stem cells. Tissue Eng. 2007;13:1291–1298. doi: 10.1089/ten.2006.0275. [DOI] [PubMed] [Google Scholar]
- Wu J, et al. Optimization of cryopreservation procedures for porcine endothelial progenitor cells. J Biosci Bioeng. 2012;113:117–123. doi: 10.1016/j.jbiosc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang ZH, Li ZJ, Yang RC, Qian GQ, Han ZC. Enhancement of neovascularization with cord blood CD133 + cell-derived endothelial progenitor cell transplantation. Thromb Haemost. 2004;91:1202–1212. doi: 10.1160/TH03-06-0378. [DOI] [PubMed] [Google Scholar]
- Yang N, et al. The characteristics of endothelial progenitor cells derived from mononuclear cells of rat bone marrow in different culture conditions. Cytotechnology. 2011;63:217–226. doi: 10.1007/s10616-010-9329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Liu H, Wang F, Li L, Deng M, Huang L, Zhao X. Transplantation of cryopreserved human umbilical cord blood-derived endothelial progenitor cells induces recovery of carotid artery injury in nude rats. Stem Cell Res Ther. 2015;6:37. doi: 10.1186/s13287-015-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc Natl Acad Sci USA. 2002;99:11951–11956. doi: 10.1073/pnas.182215799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Chen W, Ren J, Zhang T, Yang K, Wu G, Liu H. VEGF-PKD1-HDAC7 signaling promotes endothelial progenitor cell migration and tube formation. Microvasc Res. 2014;91:66–72. doi: 10.1016/j.mvr.2013.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.