Abstract
Parkia speciosa Hassk is a plant found abundantly in the Southeast Asia region. Its seeds, with or without pods, have been used in traditional medicine locally to treat cardiovascular problems. The pathogenesis of cardiovascular diseases involves inflammation and oxidative stress. Based on this information, we sought to investigate the potential protective effects of Parkia speciosa empty pod extract (PSE) on inflammation in cardiomyocytes exposed to tumor necrosis factor-α (TNF-α). H9c2 cardiomyocytes were divided into four groups; negative control, TNF-α, PSE + TNF-α and quercetin + TNF-α. Groups 3 and 4 were pretreated with PSE ethyl acetate fraction of ethanol extract (500 µg/mL) or quercetin (1000 µM, positive control) for 1 h before inflammatory induction with TNF-α (12 ng/mL) for 24 h. TNF-α increased protein expression of nuclear factor kappa B cell (NFκB) p65, p38 mitogen-activated protein kinase (p38 MAPK), inducible nitric oxide synthase, cyclooxygenase-2 and vascular cell adhesion molecule-1 when compared to the negative control (p < 0.05). It also elevated iNOS activity, nitric oxide and reactive oxygen species levels. These increases were significantly reduced with PSE and quercetin pretreatments. The effects of PSE were comparable to that of quercetin. PSE exhibited anti-inflammatory properties against TNF-α-induced inflammation in H9c2 cardiomyocytes by modulating the NFκB and p38 MAPK pathways.
Keywords: TNF-α, Inflammation, Petai, NFκB, MAPK
Introduction
Parkia speciosa Hassk, locally known as petai papan, was reported to contain large amounts of antioxidants, like polyphenol (Ko et al. 2014). This plant is abundantly found in tropical regions, like the Philippines, Thailand, Indonesia and Malaysia (Samuel et al. 2010; Rozaq and Sofriani 2009). The empty pods of this plant have higher polyphenol content compared to its other parts (Kamisah et al. 2013). With this, Ko et al. (2014) reported the presence of gallic acid, ellagic acid, catechin, quercetin, vanillic acid and epicatechin in empty pods. Our previous study showed that quercetin was detected in the ethyl acetate fraction of the ethanol extract of Parkia speciosa empty pods (Mustafa et al. 2018). Its seeds, with or without pods, are used traditionally to help control hypertension (Lim 2012) and heart problems (Yullia 2008). Another study has previously demonstrated the cardioprotective effects of Parkia speciosa empty pods extract (PSE) supplementation in rats given Nω-nitro-l-arginine methyl ester (L-NAME), an inhibitor of nitric oxide synthase (NOS) (Kamisah et al. 2017).
Oxidative stress and inflammation play important roles in the pathogenesis of diseases, including heart disease (Siti et al. 2015). Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine that has been used in many in vitro studies to induce inflammation (Mustafa et al. 2018; Xia et al. 2014). It stimulates inflammatory markers, such as inducible nitric oxide synthase (iNOS), nitric oxide (NO), cyclooxygenase-2 (COX-2) and vascular cell adhesion moelcule-1 (VCAM-1), through nuclear factor kappa-B (NFκB) pathway activation (Karunaweera et al. 2015), as well as increased production of reactive oxygen species (ROS) in cardiomyocytes (Xia et al. 2014). The activation of NFκB p65 induces cardiomyocyte hypertrophy owing to increased phosphorylation of the p65 subunit (Sorriento et al. 2010). Elevated mRNA expression and activity of NFκB p65 were demonstrated in the left ventricles of spontaneously hypertensive rats (SHR) (Miguel-Carrasco et al. 2010). p38 mitogen-activated protein kinase (p38 MAPK) was also described to be involved in the inflammatory process by triggering the synthesis of inflammatory regulators, such as TNF-α and COX-2 (Huang et al. 2012). Elevated p38 MAPK activity augmented inflammatory, hypertrophic and fibrotic processes in patients with end-stage heart failure and ischemic heart disease (Denise Martin et al. 2012).
The antioxidant properties of Parkia speciosa have been reported in many publications, but accounts of its anti-inflammatory properties are still lacking. Based on this situation, the aim of the current work was to investigate the potential anti-inflammatory effect of the PSE within an in vitro model employing H9c2 cardiomyocytes. TNF-α was utilized to induce inflammation in the cells.
Materials and methods
Reagents and chemicals
All reagents and chemicals used for this experiment are as follows: H9c2 (ATCC, Manassas, VA, USA); Dulbecco’s Modified Eagle Medium (DMEM; ATCC); fetal bovine serum (FBS) (PAA Laboratories GmbH, Pasching, Austria); bovine serum albumin (BSA; Santa Cruz Biotechnology, Dallas, TX, USA); sodium chloride (NaCl; Sigma-Aldrich, St. Louis, MO, USA); sodium hydroxide (NaOH; Systerm, Selangor, Malaysia); disodium phosphate (Na2HPO4; Sigma-Aldrich); sodium bicarbonate (NaHCO3; Bendosen Laboratory Chemicals, Selangor, Malaysia); 2′, 7′ dichlorofluorescin diacetate (DCFH-DA; Sigma-Aldrich); quercetin (Sigma-Aldrich); recombinant human TNF-α (R & D Systems, Minneapolis, MN, USA), CellTiter 96 Aqueous One-Solution Proliferation Assay kit (Promega, Madison, WI, USA); iNOS assay kit (Elabscience, Wuhan, China); COX-2 antibody (Cell Signaling, Danvers, MA,USA); iNOS antibody (Abcam, Cambridge, UK); VCAM-1 antibody (Santa Cruz Biotechnology); NF-κB p65 (Santa Cruz Biotechnology); p38 MAPK antibody (Cell Signaling); β-actin antibody (Santa Cruz Biotechnology); anti-rabbit IgG-HRP (Cell Signaling); and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology). All reagents used for Western blot analysis were purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
Plant material
Parkia speciosa pods were purchased from a farm in Bidor, Perak (Malaysia) in December 2014. The plant was authentically identified by a botanist. Its voucher specimen (UKMB 40239) was deposited in the Herbarium of Universiti Kebangsaan Malaysia, Bangi, Selangor, Malaysia.
Preparation of PSE
The ethyl acetate fraction of ethanolic PSE was prepared according to the method described by Mustafa et al. (2018). Briefly, de-seeded pods were air-dried and ground before being immersed in absolute alcohol for 3 days and filtered. The filtration process was repeated three times before the resultant solution was evaporated with a rotary evaporator. Then, the residue of the powdered pods was immersed in hexane until the color of the solution turned white. This solvent was then evaporated. The residue was reimmersed in ethyl acetate and evaporated. The resultant ethyl acetate fraction was stored at 4 °C until use. The overall yield was 11.261%.
Cell-culture conditions
Commercial rat cardiomyocyte secondary cells, H9c2, were used in this study and suspended in DMEM supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin in an incubator humidified with 5% CO2 at 37 °C. Subculturing of H9c2 cells was carried out every 2–3 days when the cell population density reached 70–80% confluence. Cells were seeded at an appropriate density based on each experimental design.
Cell-viability assay
Cell viability of TNF-α and PSE in H9c2 cells was determined according to the protocol that accompanied the CellTiter 96 Aqueous One Solution Proliferation Assay kit. The cell-seeding density for the viability assay was 3 × 104 cells per well in a 96-well plate. Three experiments were conducted: (1) cells were treated with various concentrations of TNF-α (5–80 ng/mL) for 24 h; (2) cells were treated with different concentrations of PSE (31.25–1000 µg/mL) for 1 h; and (3) cells were pretreated with different concentrations of extract for 1 h, and then treated with the IC50 of TNF-α for 24 h. The absorbance was measured at 490 nm with a microplate reader (Bio-Rad).
Cell-culture treatment
H9c2 cardiomyocytes were seeded in a T-75 flask with density of 1.2 × 105 cell/mL. The cells were pretreated with PSE (500 µg/mL) or quercetin (1000 µM) (positive control) (Chen et al. 2013) for 1 h before exposure to TNF-α (12 ng/mL) together with actinomycin D (1 µg/mL) (Zhou-Stache et al. 2002) for 24 h. A group without any treatment was given vehicle (DMSO) only and acted as the negative control group. The concentration of quercetin in the PSE (500 µg/mL) was 2.17 μM.
Western blot analysis
The cell-seeding density for the Western blot analysis was 1.2 × 105 cell/mL in a T-75 flask. Briefly, equal amounts of protein (50 µg) of each sample lysate (Sambrook et al. 2001; Mahmood and Yang 2012) were separated on polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane. After blocking with phosphate-buffered saline (PBS) containing 1% casein for 1 h, the membrane was incubated with primary antibodies against iNOS (1:1000), COX-2 (1:1000), VCAM-1 (1:200,), NF-κB p65 (1:500), p38 MAPK (1:1000) and β-actin (1:1000) overnight at 4 °C. The membrane was subsequently incubated with secondary antibodies, specifically goat anti-rabbit IgG-HRP or goat anti-mouse IgG-HRP, at a 1:3000 dilution. Protein intensity was read with a C-DiGit Blot Scanner (LI-COR Biosciences, Lincoln, NE, USA) and analyzed via Image-J software. The sample relative intensity was calculated by dividing the percentage of each sample band by the percentage of the negative control sample band.
iNOS activity and NO assay
The cell-seeding density for iNOS activity and NO assays was 5 × 104 cell/mL in six-well plate. The secreted iNOS was measured in the cellular supernatant by means of a commercial iNOS activity assay kit. The absorbance was read with a microplate reader (Bio-Rad) and expressed in ng/mg protein. The NO assay in the supernatant of H9c2 cells was performed by measuring the accumulation of nitrite using Griess reagent and estimated against the sodium nitrite standard curve (Miranda et al. 2001).
Determination of intracellular ROS levels
Intracellular levels of ROS was determined using cell-permeant fluorescent probe, 2′,7′ dichlorodihydrofluorescein (DCFH-DA, Sigma Co.) (Yang et al. 2014). The cell-seeding density with the viability assay was 3 × 104 cell/mL in a 96-well plate. After treatment, cells were incubated with 50 µM DCFH-DA for 45 min at room temperature in the dark. Next, cells were washed twice with PBS and optical density was read at 485 nm excitation and 535 nm emission in a fluorescence plate reader (EnSpire Multimode Plate Reader, Perkin Elmer, Houston, USA,).
Statistical analysis
The experiments were conducted in triplicate and the data were expressed as mean ± standard error of mean (SEM). The Shapiro–Wilk test was applied to check for data normality. Data which were normally distributed were evaluated with one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Non-normally distributed data was statistically assessed via the Kruskal–Wallis test. p values < 0.05 were considered significant.
Results
H9c2 cell viability
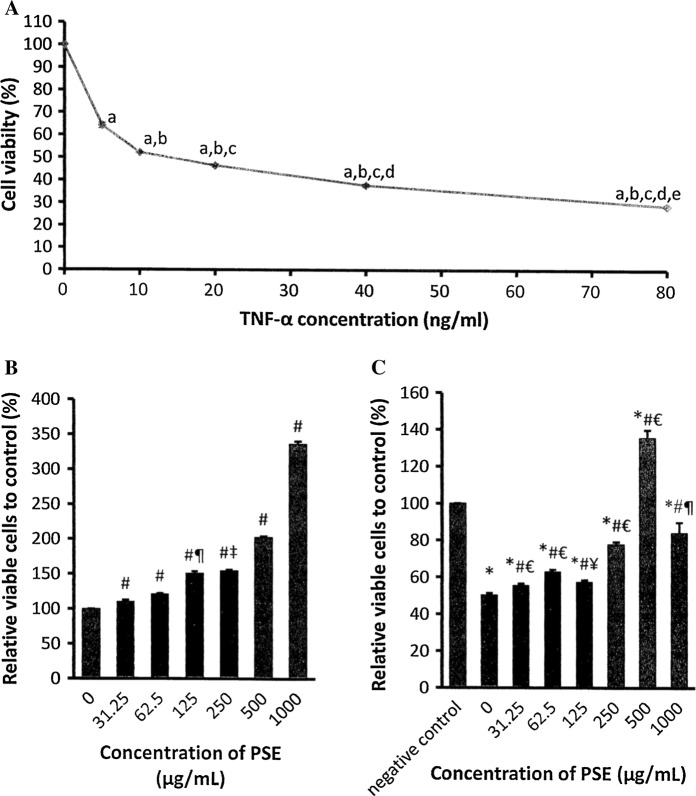
Exposure to TNF-α (5–80 ng/mL) for 24 h significantly reduced H9c2 cell viability in a concentration-dependent manner. The median inhibitory concentration (IC50) value for TNF-α was 12 ng/mL (Fig. 1a). The number of viable H9c2 cells that were treated with PSE at concentrations of 31.25–1000 μg/mL was concentration-dependently increased compared to the negative control group (0 μg/mL) (p < 0.05) (Fig. 1b).
Fig. 1.
Effects of various concentrations of a TNF-α after 24 h incubation, bParkia speciosa extract (PSE) on after 1 h incubation, and c pretreatment of PSE for 1 h, followed by 24 h incubation with TNF-α (12 ng/mL) on H9c2 cell viability. The data are expressed as mean ± SEM (n = 3). ap < 0.05 versus 0 ng/mL, bp < 0.05 versus 5 ng/mL, cp < 0.05 versus 10 ng/mL, dp < 0.05 versus 20 ng/mL, ep < 0.05 versus 40 ng/mL μM, *p < 0.05 versus negative control, #p < 0.05 versus 0 (μg/mL, €p < 0.05 versus other concentrations of PSE, ¥p < 0.05 versus other concentrations of PSE except 31.25 μg/ml, ‡p < 0.05 versus other concentrations of PSE except 125 ng/ml, ¶p < 0.05 versus other concentrations of PSE except 250 μg/ml
Exposure to 12 ng/mL TNF-α for 24 h diminished the number of viable cells to almost half of that of the negative control. Pretreatment of PSE at 31.25–1000 μg/mL for 1 h prior to exposure to TNF-α concentration-dependently raised the viable cell number (p < 0.05). The highest cell viability with PSE was observed at 500 μg/mL. No significant difference in cell viability was observed between the extract concentration of 31.25 μg/mL and 125 μg/mL and between 250 and 1000 μg/mL (Fig. 1c).
NFκB p65 and p38 MAPK protein expression
Exposure to TNF-α increased NFκB p65 and p38 MAPK expression in H9c2 cells compared to the negative control (Fig. 2). Pretreatment with PSE or quercetin reduced expression significantly (p < 0.05). Both expressions in the quercetin-pretreated group were significantly lower than that of the PSE group (p < 0.05). There was no expression of NFκB p65 detected in either the negative control or quercetin + TNF-α groups.
Fig. 2.
Effects of Parkia speciosa extract (PSE, 500 ng/mL) and quercetin (1000 µM) on NFkB (a) and p38 MAPK protein expression (b) in TNF-α-activated (12 ng/mL) H9c2 cardiomyocytes. The data are expressed as mean ± SEM (n = 3). *p < 0.05 versus negative control, #p < 0.05 versus TNF-α, ¥p < 0.05 versus PSE + TNF-α
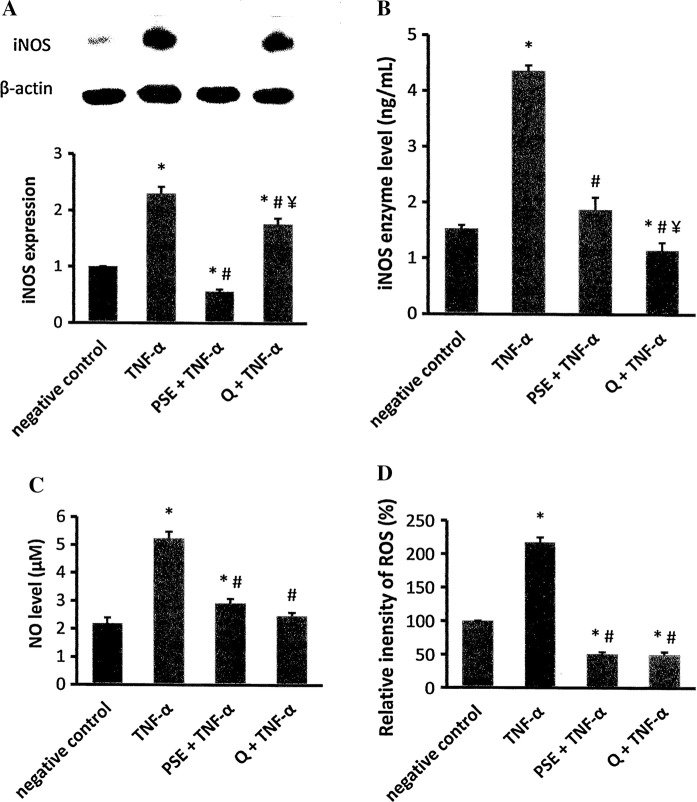
iNOS protein expression and activity, NO and ROS levels
iNOS protein expression and activity were elevated in H9c2 cells exposed to TNF-α (p < 0.05) compared to the negative control (Fig. 3a, b). In the PSE- and quercetin-pretreated cells, expression and activity was significantly lower than that of the TNF-α group. The expression in the PSE-pretreated group was significantly lower than the negative control and quercetin-pretreated groups. However, the PSE-pretreated group (0.68 ± 0.07 ng/mg protein) had comparable iNOS activity to the negative control (0.48 ± 0.02 ng/mg protein) and greater activity than the quercetin-pretreated group (0.38 ± 0.04 ng/mg protein) (p < 0.05).
Fig. 3.
Effects of Parkia speciosa extract (PSE, 500 μg/mL) and quercetin (1000 μM) on inducible nitric oxide synthase (iNOS) protein expression (a), activity (b), nitric oxide (NO) (c) and reactive oxygen species (ROS) (d) levels in TNF-α-activated (12 ng/mL) H9c2 Cardiomyocytes. The data are expressed as mean ± SEM (n = 3). *p < 0.05 versus negative control, #p < 0.05 versus TNF-α, ¥p < 0.05 versus PSE + TNF-α
Incubation with TNF-α significantly augmented NO levels (5.24 ± 0.23 μM) more than twofold in H9c2 cells compared to the negative control group (2.19 ± 0.20 μM) (p < 0.05) (Fig. 3c). Both the PSE (2.92 ± 0.16 μM) and quercetin (2.47 ± 0.13 μM) groups that were exposed to TNF-α had significantly lower NO level than the TNF-α group (p < 0.05). A significantly greater level of NO was observed in the PSE + TNF-α group than the negative control group (p < 0.05). The NO level in the quercetin-pretreated group was comparable to the extract-pretreated and negative control groups.
Intracellular ROS levels were elevated in the TNF-α group compared to the negative control (Fig. 3d). This increment was significantly inhibited in the groups pretreated with PSE or quercetin (p < 0.05). Both pretreated groups had comparable ROS levels, but significantly lower levels than the negative control.
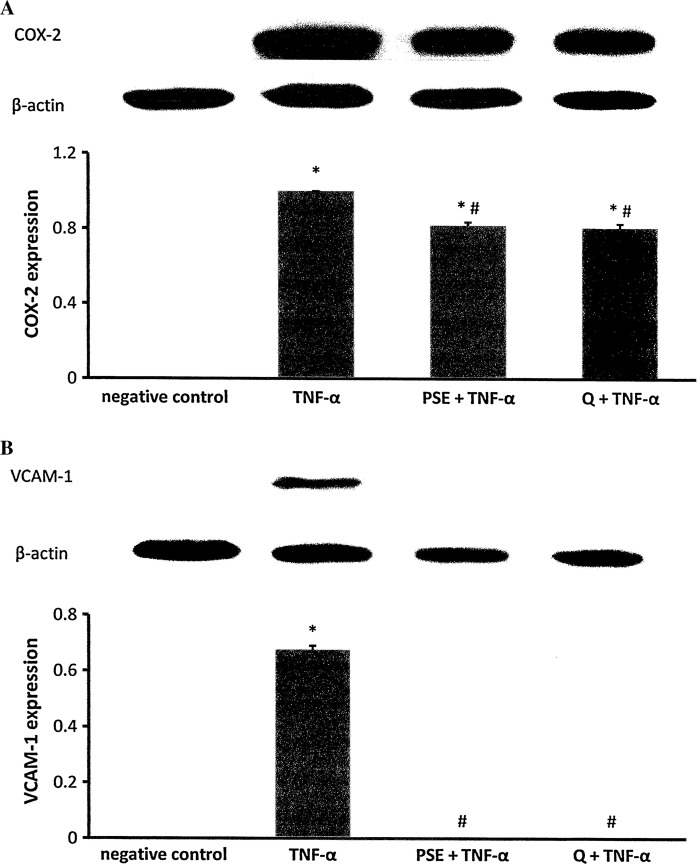
COX-2 and VCAM-1 protein expression
Treatment with TNF-α increased COX-2 expression in H9c2 cells. Pre-incubation with PSE or quercetin attenuated the TNF-α-induced COX-2 expression. Both pre-incubated groups had comparable expression and were significantly higher than that of the negative control group (p < 0.05). No COX-2 expression was observed in the negative control group (Fig. 4a).
Fig. 4.
Effects of Parkia speciosa extract (PSE, 500 μg/mL) and quercetin (1000 μM) on COX-2 (a) and VCAM-1 protein expression (b) in TNF-α-activated (12 ng/mL) H9c2 cardiomyocytes. The data are expressed as mean ± SEM (n = 3). *p < 0.05 versus negative control, #p < 0.05 versus TNF-α
H9c2 cells exposed to TNF-α had significantly increased expression of the VCAM-1 protein (0.68 ± 0.01) versus the negative control group (p < 0.05) (Fig. 4b). Pretreatment with PSE or quercetin significantly inhibited VCAM-1 expression induced by TNF-α. There was no VCAM-1 expression detected in the negative control.
Discussion
Inflammation plays an important role in the pathogenesis of cardiovascular diseases, like left ventricular hypertrophy and congestive heart failure (Vaziri 2008). Pro-inflammatory cytokines, such as TNF-α, could trigger inflammatory responses, which then activate NFκB (Xia et al. 2014). NFκB is a transcription factor and one of the vital regulators in the expression of pro-inflammatory genes through regulating the transcription of pro-inflammatory cytokines, chemokines, iNOS, COX-2 and cell adhesion molecules that function within the inflammatory processes (Baeuerle and Baichwal 1997).
In this study, we confirmed the cytotoxic effect of TNF-α in H9c2 cardiomyocytes and the IC50 of TNF-α was determined to be 12 ng/mL, just as similarly reported by another study (Bergmann et al. 2001). Increasing concentrations of PSE were able to decrease the detrimental effect of TNF-α on cell viability, and this could be because of a proliferative effect of the extract. The highest percentage of viable cells was at 500 μg/mL of PSE, and the concentration was employed in the subsequent experiments. The highest content of quercetin was detected in the ethyl acetate fraction in our previous study (Mustafa et al. 2018) compared to other fractions (hexane and ethanol residue), of which quercetin was used as the positive control. Based on Chen et al. (2013), it was demonstrated there was a protective effect exerted upon H2O2-induced H9c2 cell injury, where quercetin at 1000 μM was used.
Activation of NFκB p65 and p38 MAPK pathways by TNF-α is based on the binding of TNF-α to tumor necrosis factor receptor 1 (TNFR1) (Huang et al. 2012), which then associates with tumor necrosis factor receptor type 1-associated death domain protein (TRADD) and tumor necrosis factor receptor-associated factor (TRAF), including TRAF-2 and -5, to form a complex. This complex further causes phosphorylation of an inhibitory protein (IκB) and activation of NFκB (Gordon et al. 2011; Verma 2004), as well as activation of the p38 MAPK signaling pathway (Sabio and Davis 2014). Activated NFκB and MAPK orchestrate the recruitment and regulation of gene transcription and protein expression of numerous genes and proteins involved in inflammation (Lawrence 2009; Kyriakis and Avruch 2012). PSE diminished these activations, leading to reduced expression and concentrations of downstream molecules (iNOS, VCAM-1, COX-2, NO and ROS), most likely attributable to its detected content of quercetin (5.84 μg/100 g dry weight) (Mustafa et al. 2018). It was also most probable that PSE triggered MAPK phosphatases (MKPs) to deactivate MAPK by dephosphorylating threonine and tyrosine residues at the site of MAPK activation, and decrease the phosphorylation of IκBα (type of IκB), thus deactivating NFκB p65 (Xia et al. 2014).
Increased expression of iNOS would then elevate the production of the iNOS enzyme in cardiomyocytes, translating to greater NO formation. iNOS catalyzes the production of NO through the synthesis of L-citrulline from l-arginine (Dhawan 2014). At low concentrations, NO bestowed beneficial effects upon cells, such as protection against ischemic injuries (Strijdom et al. 2009). On the contrary, excessive NO production causes apoptosis, necrosis and inflammation by producing potent radicals, such as peroxynitrite radicals owing to the interaction of NO and ROS (Strijdom et al. 2009), observed in the current study as elevated ROS levels. This effect was reduced by pretreatment of PSE and quercetin, possibly because of the inhibitory impact on phosphorylation of both NFκB p65 and p38 MAPK, thereby inhibiting iNOS transcription (Xia et al. 2014). Even though no expression of both NFκB p65 and p38 MAPK was observed in the quercetin-treated group, the expression of iNOS and other inflammatory proteins could still be detected, suggestive of involvement of other inflammatory pathways induced by TNF-α. TNF-α has also been reported to induce inflammation through the extracellular signal-regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK) pathways, in addition to the NFκB and p38 MAPK pathways (Sabio and Davis 2014; Chan and Riches 1998).
PSE had a more robust inhibitory effect on iNOS expression, but a weaker effect on iNOS activity than quercetin. This might be because of post-translational modification leading to reduced synthesis of the enzyme by quercetin. The extract contained roughly 2.17 μM quercetin, which was far lower than the positive control. The concentration of quercetin in the cells following exposure to the extract or quercetin was not measured in the current study. However, plasma quercetin concentration was reported to rise to approximately 90 nM in healthy volunteers after consumption of 97 mg/L (320 μM) quercetin contained in a blueberry-apple juice mixture for 2 weeks. Even though the oral absorption of quercetin was less than one-thousandth, at this level, it still managed to increase total antioxidant status in vivo (Boots et al. 2008).
Activated NFκB p65 induced by TNF-α caused its binding to DNA sequences at the COX-2 and VCAM-1 gene promoters (Chen et al. 2000; Modlich et al. 2000), subsequently encoding the COX-2 and VCAM-1 protein, thus increasing expression (Xia et al. 2014; Huang et al. 2012). COX-2 is responsible for prostaglandin synthesis, which is involved in inflammation (Li et al. 2014), while VCAM-1 is one of the adhesion molecules that plays a role in leukocyte migration to inflammation sites (Bhaskar et al. 2016). The latter is an indicator that features prominently in cardiovascular diseases, such as unstable angina and acute myocardial infarction (Macías et al. 2003). The effects of both PSE and quercetin pretreatments on COX-2 and VCAM-1 protein expression induced by TNF-α were previously reported (Mustafa et al. 2018; García-Mediavilla et al. 2007). The reduced expression of VCAM-1 by the extract and quercetin might be because of inhibited NFκB binding to the VCAM-1 gene promoter, hence inhibiting VCAM-1 transcription.
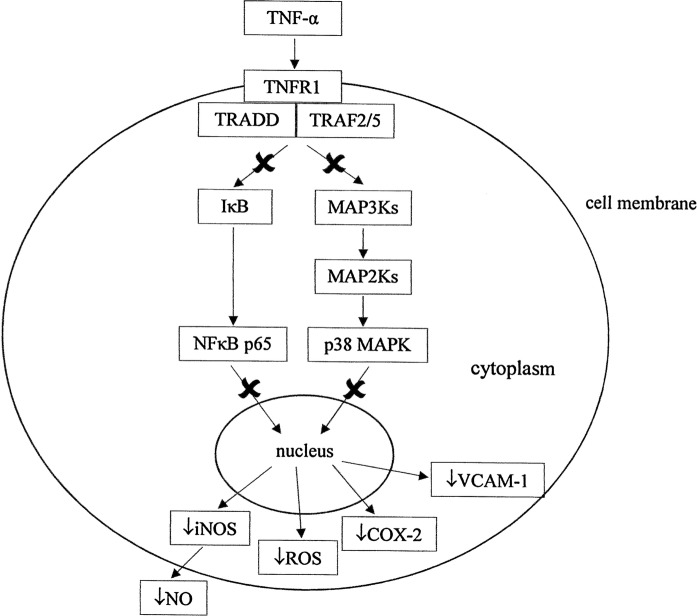
The protective effects of quercetin seemed to be more pronounced with the upstream molecules of the inflammatory pathway (NFκB p65 and p38 MAPK inhibitions) rather than the downstream molecules (iNOS expression and activity, NO and ROS levels, COX-2 and VCAM-1 expressions). Postulated effects of PSE on inflammatory signaling pathways are shown in Fig. 5. Downstream, PSE had a similar protective effect to quercetin, but quercetin was more effective in reducing iNOS activity. These preliminary findings describe the beneficial effects of PSE in preventing cardiovascular diseases associated with inflammation. Further in vivo studies should be conducted to confirm PSE’s in vitro beneficial effects.
Fig. 5.
Postulated mechanistic action (×) of Parkia speciosa extract (PSE) on inflammatory pathway. PSE reduces the TNF-α-induced activation of p38 MAPK and NFkB p65 by reducing the phosphorylation of IκBα, thus reducing iNOS, COX-2 and VCAM-1 expressions, as well as NO and ROS levels
Conclusions
PSE exerted anti-inflammatory protective effects in H9c2 cardiomyocytes against TNF-α-induced inflammation by modulating the NFκB p65 and p38 MAPK signaling pathways, which was reflected by reduced expression of iNOS, COX-2 and VCAM-1, iNOS activity, as well as NO and ROS levels. The protective effect of the plant could be attributable to its polyphenol content, in particular, quercetin as we have previously reported.
Acknowledgements
The authors would like to acknowledge the financial support from Universiti Kebangsaan Malaysia (UKM) Grant (AP-2014-013) and technical help from Puan Nurul Hafizah Abas, Encik Fadhlullah Zuhair Japar Sidik and Puan Juliana Abdul Hamid.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baeuerle PA, Baichwal VR. NF-kappa B as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv Immunol. 1997;65:111–137. doi: 10.1016/S0065-2776(08)60742-7. [DOI] [PubMed] [Google Scholar]
- Bergmann MW, Loser P, Dietz R, von Harsdorf R. Effect of NF-kappa B Inhibition on TNF-alpha-induced apoptosis and downstream pathways in cardiomyocytes. J Mol Cell Cardiol. 2001;33:1223–1232. doi: 10.1006/jmcc.2001.1385. [DOI] [PubMed] [Google Scholar]
- Bhaskar S, Sudhakaran PR, Helen A. Quercetin attenuates atherosclerotic inflammation and adhesion molecule expression by modulating TLR-NF-κB signaling pathway. Cell Immunol. 2016;310:131–140. doi: 10.1016/j.cellimm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Boots AW, Wilms LC, Swennen EL, Kleinjans JC, Bast A, Haenen GR. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 2008;24:703–710. doi: 10.1016/j.nut.2008.03.023. [DOI] [PubMed] [Google Scholar]
- Chan ED, Riches DW. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochem Biophys Res Commun. 1998;253:790–796. doi: 10.1006/bbrc.1998.9857. [DOI] [PubMed] [Google Scholar]
- Chen CC, Sun YT, Chen JJ, Chiu KT. TNF-α-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-γ2, protein kinase C-α, tyrosine kinase, NF-κB-inducing kinase, and I-κB kinase 1/2 pathway. J Immunol. 2000;165:2719–2728. doi: 10.4049/jimmunol.165.5.2719. [DOI] [PubMed] [Google Scholar]
- Chen YW, Chou HC, Lin ST, Chen YH, Chang YJ, Chen L, Chan HL. Cardioprotective effects of quercetin in cardiomyocyte under ischemia/reperfusion injury. Evid Based Complement Alternat Med. 2013;2013:364519. doi: 10.1155/2013/364519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denise Martin E, De Nicola GF, Marber MS. New therapeutic targets in cardiology: p38 alpha mitogen-activated protein kinase for ischemic heart disease. Circulation. 2012;126:357–368. doi: 10.1161/CIRCULATIONAHA.111.071886. [DOI] [PubMed] [Google Scholar]
- Dhawan V. Reactive oxygen and nitrogen species: general considerations. In: Saha GK, Jindal SK, Biswal S, Barnes PJ, Pawankar R, editors. Studies on respiratory disorders. New York: Humana Press; 2014. pp. 27–47. [Google Scholar]
- García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, González-Gallego J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang liver cells. Eur J Pharmacol. 2007;557:221–229. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Huang SS, Deng JS. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS ONE. 2012;7:e35922. doi: 10.1371/journal.pone.0035922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisah Y, Othman F, Qodriyah HM, Jaarin K. Parkia speciosa Hassk.: a potential phytomedicine. Evid Based Complement Alternat Med. 2013;2013:1–9. doi: 10.1155/2013/709028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisah Y, Zuhair JSF, Juliana AH, Jaarin K. Parkia speciosa empty pod prevents hypertension and cardiac damage in rats given N(G)-nitro-l-arginine methyl ester. Biomed Pharmacother. 2017;96:291–298. doi: 10.1016/j.biopha.2017.09.095. [DOI] [PubMed] [Google Scholar]
- Karunaweera N, Raju R, Gyengesi E, Münch G. Plant polyphenols as inhibitors of NF-kB induced cytokine production—a potential anti-inflammatory treatment for Alzheimer’s disease? Front Mol Neurosci. 2015;8:1–5. doi: 10.3389/fnmol.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HJ, Ang LH, Ng LT. Antioxidant activities and polyphenolic constituents of bitter bean Parkia speciosa. Int J Food Prop. 2014;17:1977–1986. doi: 10.1080/10942912.2013.775152. [DOI] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang L, Wu Z, Yao L, Wu Y, Huang L, Liu L, Zhou X, Gou D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci Rep. 2014;4:6234. doi: 10.1038/srep06234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TK. Edible medicinal and non-medicinal plants, volume 2 (fruits) Dordrecht: Springer; 2012. Parkia speciosa; pp. 798–803. [Google Scholar]
- Macías C, Villaescusa R, del Valle L, Boffil V, Cordero G, Hernández A, Hernández P, Ballester JM. Endothelial adhesion molecules ICAM-1, VCAM-1 and E-selectin in patients with acute coronary syndrome. Rev Esp Cardiol. 2003;56:137–144. doi: 10.1016/S0300-8932(03)76837-7. [DOI] [PubMed] [Google Scholar]
- Mahmood T, Yang PC. Western blot: technique, theory, and troubleshooting. N Am J Med Sci. 2012;4:429–434. doi: 10.4103/1947-2714.94940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vázquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm. 2010;7:1–9. doi: 10.1186/1476-9255-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda KM, Espey MG, Wink DA. Rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Modlich U, Pugh CW, Bicknell R. Increasing endothelial cell specific expression by the use of heterologous hypoxic and cytokine-inducible enhancers. Gene Ther. 2000;7:896–902. doi: 10.1038/sj.gt.3301177. [DOI] [PubMed] [Google Scholar]
- Mustafa NH, Ugusman A, Jalil J, Kamisah Y. Anti-inflammatory properties of Parkia speciosa empty pod extract in human umbilical vein endothelial cells. J Appl Pharm Sci. 2018;8:152–158. [Google Scholar]
- Rozaq P, Sofriani N. Organic pesticide from urine and spices modification. Asian J Food Agro Ind. 2009;Special Issue:S105–S111. [Google Scholar]
- Sabio G, Davis RJ. TNF and MAP kinase signaling pathways. Sem Immunol. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Inc.; 2001. [Google Scholar]
- Samuel A, Kalusalingam A, Chellappan D, Gopinath R, Radhamani S, Husain H, Muruganandham V, Promwichit P. Ethnomedical survey of plants used by the Orang Asli in Kampung Bawong, Perak, West Malaysia. J Ethnobiol Ethnomed. 2010;6:5. doi: 10.1186/1746-4269-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NF-κB-dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- Strijdom H, Chamane N, Lochner A. Nitric oxide in the cardiovascular system: a simple molecule with complex actions. Cardiovasc J Afr. 2009;20:303–310. [PMC free article] [PubMed] [Google Scholar]
- Vaziri ND. Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis. 2008;2:1–10. [PubMed] [Google Scholar]
- Verma IM. Nuclear factor (NF)-κB proteins: therapeutic targets. Ann Rheum Dis. 2004;63:57–61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K, Sun H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J Atheroscler Thromb. 2014;21:768–783. doi: 10.5551/jat.23697. [DOI] [PubMed] [Google Scholar]
- Yang W, Yu M, Fu J, Bao W, Wang D, Hao L, Yao P, Nüssler AK, Yan H, Liu L. Deoxynivalenol induced oxidative stress and genotoxicity in human peripheral blood lymphocytes. Food Chem Toxicol. 2014;64:383–396. doi: 10.1016/j.fct.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Yullia T (2008) Prakata. In: Variasi Masakan Petai & Jengkol, Tim Dapur DeMedia, DeMedia Pustaka, 1st edn. Jakarta Selatan, Indonesia, p 2
- Zhou-Stache J, Buettner R, Artmann G, Mittermayer C, Bosserhoff AK. Inhibition of TNF-alpha induced cell death in human umbilical vein endothelial cells and Jurkat cells by protocatechuic acid. Med Biol Eng Comput. 2002;40:698–703. doi: 10.1007/BF02345308. [DOI] [PubMed] [Google Scholar]