Abstract
Fire-derived organic matter, often referred to as pyrogenic organic matter (PyOM), is present in the Earth’s soil, sediment, atmosphere, and water. We investigated interactions of PyOM with ammonia (NH3) gas, which makes up much of the Earth’s reactive nitrogen (N) pool. Here we show that PyOM’s NH3 retention capacity under ambient conditions can exceed 180 mg N g−1 PyOM–carbon, resulting in a material with a higher N content than any unprocessed plant material and most animal manures. As PyOM is weathered, NH3 retention increases sixfold, with more than half of the N retained through chemisorption rather than physisorption. Near-edge X-ray absorption fine structure and nuclear magnetic resonance spectroscopy reveal that a variety of covalent bonds form between NH3-N and PyOM, more than 10% of which contained heterocyclic structures. We estimate that through these mechanisms soil PyOM stocks could retain more than 600-fold annual NH3 emissions from agriculture, exerting an important control on global N cycling.
Fire-derived organic matter (OM) is present throughout the environment, and its impact on nutrient cycling remains poorly understood. Here, the authors show that this pyrogenic OM can retain large quantities of ammonia through covalent bond formation, thereby exerting an important control on nitrogen cycling.
Introduction
The Earth’s soil, atmosphere, marine sediment, and ocean water contain large quantities of pyrogenic C (54–109, 0.26 10−3, 480–1440, and 26–145 Pg of C, respectively1,2). In soil, most of this pyrogenic C originates from burnt biomass generated during vegetation fires, which contributes up to 129 Tg yr−1 of PyOM–carbon (PyOM–C) to soil C stocks1. Many aspects of pyrogenic C biogeochemistry remain poorly understood, including interactions between pyrogenic C’s heterogeneous surface—containing both aromatic and aliphatic C, condensates, and other elements such as N, H, and O—and environmental N sources. Interactions between PyOM and environmental N may influence gaseous N emissions, N leaching, N availability to living organisms, and global N transport3. Here, we focus on PyOM’s interactions with NH3—the atmosphere’s most abundant alkaline gas. Global NH3 emissions are projected to double by 2050 and constitute a large part of the Earth’s reactive N pool4–6. Common sources of NH3 in soils include decomposing organic matter, rainwater, and N fertilizer. Laboratory studies show that various forms of natural and industrially modified organic matter can retain NH37–18, but the NH3 retention capacity of natural PyOM stocks and the mechanisms responsible for NH3 retention under ambient conditions have not been established. Therefore, the extent to which these studies can inform our understanding of PyOM’s role in global biogeochemical cycles is unknown.
Proposed mechanisms for NH3 retention by natural PyOM include physisorption, electrostatic interactions, and precipitation of ammonium (NH4+) salts7–9. Although these retention mechanisms would allow PyOM to act as a temporary N sink, N retained in these ways would be readily available for plant and microbial uptake, or loss through gas or solute transport7. Conversely, the formation of stronger covalent bonds between PyOM and NH3 would result in more persistent N retention, allowing PyOM to serve as a dynamic, long-term N source and sink—both capturing NH3 from its surroundings and slowly releasing it over time. This could also result in greater coupling of global C and N cycling, as covalently bound NH3–N would be carried with the PyOM–C as it traveled over great distances1. However, until now, covalent bond formation between natural PyOM and NH3 under ambient terrestrial conditions has not been observed.
Some evidence exists that under certain laboratory conditions, covalent bonds can form between NH3 and industrially produced relatives of PyOM or secondary organic aerosols found in the atmosphere. Graphene oxides and activated carbons can form a variety of cyclic and non-cyclic N structures when exposed to NH3 at temperatures exceeding 200 °C10–14,17,18. However, these materials are often modified (e.g., through exposure to chemical oxidants and heat or impregnated with metals), differ considerably from natural PyOM in surface area and functional group composition, and are exposed to NH3 under conditions that are not representative of the natural environment19–21. Thus, it is unknown whether these studies of graphene oxides and activated carbons can be used to predict interactions between natural PyOM and NH3, and whether the same variety of covalent N structures would develop under natural environmental conditions. Following exposure to NH3 at ambient temperatures, industrial relatives of PyOM can form non-cyclic amine and amide bonds22. Secondary organic aerosols found in the atmosphere—which contain functional groups present in terrestrial PyOM—can form both non-cyclic N structures as well as N heterocycles following exposure to NH3, NH4+, and amino acids under atmospheric conditions23,24. However, the formation of aromatic and non-aromatic heterocyclic N structures between terrestrial PyOM and NH3 has never been observed under ambient environmental conditions. This is of great interest because enrichment with these heterocyclic N structures influences the electrochemical properties, absorptive capacity, and environmental persistence of both natural and industrial pyrogenic C materials16,22–27. If heterocyclic N structures develop between PyOM and NH3 under natural conditions, this interaction would have important consequences for global C and N cycling.
In order to assess the impact of PyOM stocks on global nutrient cycles, it is also necessary to consider PyOM’s dynamic nature. Similar to the variety found in other sources of organic matter, different types of PyOM have different physical and chemical characteristics, including elemental makeup, functional group composition, surface area, pH, and other properties28. Additionally, PyOM properties change over time, as the material is exposed to water, sunlight, microbial activity, and other oxidizing forces29–31. Such variation in physiochemical properties can drastically alter PyOM’s role in the environment. Thus, to understand the influence of PyOM–NH3 interactions on global N cycling, it is important to consider how PyOM’s NH3 retention capacity might change over time. In this study, we investigate PyOM’s NH3 retention capacity under ambient conditions, N retention mechanisms, and whether retention capacity develops as PyOM stocks are weathered. We find that PyOM retains a surprising quantity of NH3–N and that this retention capacity increases significantly as PyOM is exposed to conditions mimicking natural weathering processes. More than half of the NH3–N is retained through chemisorption, including the formation of a variety of covalent bonds. We estimate that through these mechanisms soil PyOM stocks could play an important role in the global N cycle.
Results
Weathering increases PyOM N retention capacity
PyOM produced from woody biomass was oxidized to generate a gradient of weathered PyOM30,31 and subsequently exposed to NH3 vapor at ambient temperature and pressure (35 °C and 80–800 Torr). Total NH3 capture increased more than sixfold after oxidation, from 2.3 mmol g−1 PyOM–C in unoxidized PyOM to 13.5 mmol g−1 PyOM–C in highly oxidized PyOM (Fig. 1a), showing that PyOM can retain substantial quantities of N from this form of NH3. Although specific surface area (SSA) and low pH may contribute to the NH3 retention capacity of some pyrogenic C materials7,8,32, these characteristics did not explain the trends observed here. PyOM SSA decreased with oxidation and therefore could not have contributed to the increase in NH3 retention observed in highly oxidized PyOM samples (Fig. 1b). PyOM pH also decreased with oxidation (Fig. 1c). However, when unoxidized PyOM was incubated with hydrochloric acid, which lowered its pH without altering key oxygen-containing functional groups (Supplementary Fig. 1), NH3 retention remained unchanged (Supplementary Fig. 2). This shows that although oxidation and low pH are correlated, pH itself did not drive NH3 retention and cannot be used to predict PyOM oxidation or NH3 retention capacity. Instead, our analyses indicate that functional group composition may be a more reliable determinant of PyOM’s NH3 retention capacity11,21. Peak height ratios measured by Fourier transform infrared spectroscopy (FTIR) and integrated peak areas measured by solid-state 13C nuclear magnetic resonance (NMR) spectroscopy suggest that with progressive oxidation, PyOM’s oxygen-containing functional groups increase relative to aromatic C structures (Fig. 1d and Supplementary Figs. 3 and 4). Quantitative stoichiometric measurements show that an increase in PyOM O:C ratio corresponds with the same trends observed through these spectral analyses. Taken together, these results highlight PyOM’s substantial and dynamic N retention capacity, and the relevance of weathering and exposure to oxidizing agents (e.g., microbial activity or ozone) when considering PyOM’s potential role in N cycling.
Fig. 1.
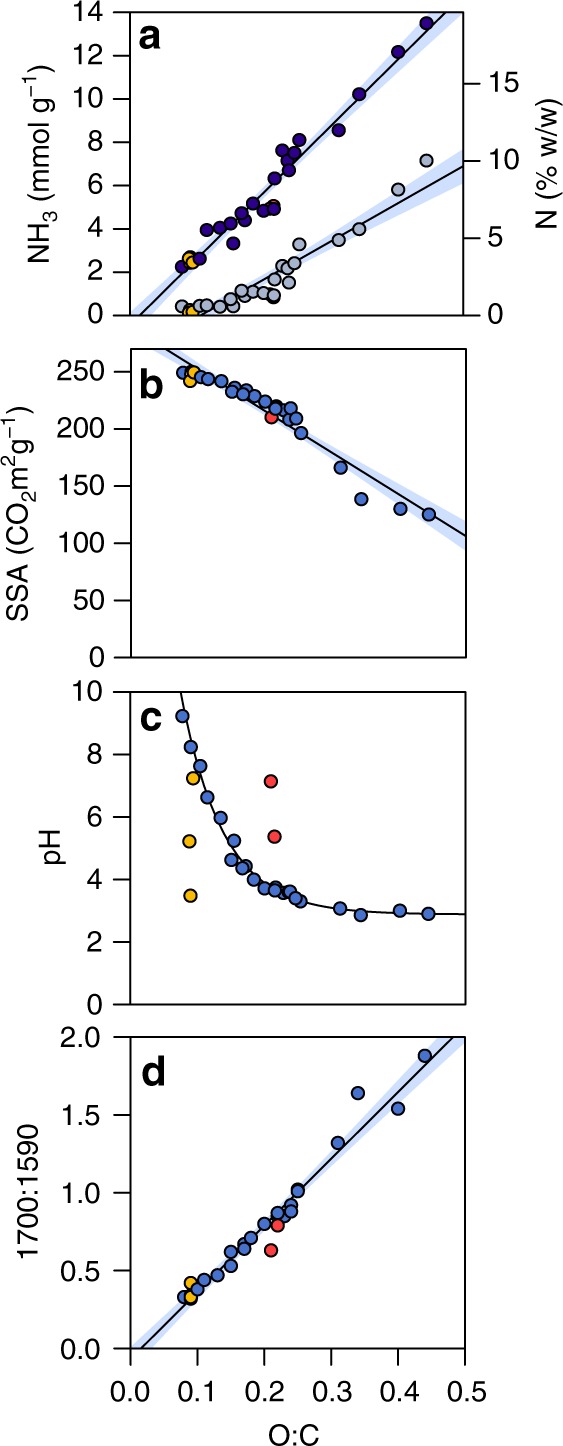
Changes in pyrogenic organic matter ammonia retention and physiochemical characteristics as a function of molar O:C ratio. a ammonia (NH3) retention capacity—expressed in mmol of NH3 g−1 of pyrogenic organic matter-carbon (PyOM–C, left y axis) and percent nitrogen (N) of PyOM–C (right y axis)—increases as a function of molar O:C ratio. Each point represents the average oxygen:carbon (O:C) ratio for two replicates. NH3 chemisorption = 17.49x − 1.84, R2 = 0.89, p < 0.001, F1,25 = 204.7, S25 = 0.59 (light blue); NH3 combined chemical and physical adsorption = 30.51x − 0.44, R2 = 0.96, p < 0.001, F1,25 = 567.2, S25 = 0.62 (dark blue). b Specific surface area (SSA) decreases as PyOM O:C ratio increases. SSA = −365x + 288.8, R2 = 0.931, p < 0.001, F1,25 = 338.5, S25 = 9.591. c PyOM pH decreases as oxidation increases. Blue symbols represent unoxidized PyOM and PyOM incubated with deionized water (DIH2O) and hydrogen peroxide (H2O2) and are fitted with a significant curve (y = 20.8*e−14.8(O:C) + 2.84, S19 = 0.199). d The intensity of Fourier transform infrared (FTIR) peak heights associated with C=O stretching (1691–1715 cm−1) increases in proportion to the intensity of peak heights associated with C=C vibrations and stretching (1582–1609 cm−1) as PyOM O:C ratio increases (y = 4.29x − 0.0670, R2 = 0.963, p < 0.001, F1,25 = 650, S25 = 0.081). For all figures, yellow symbols represent PyOM that was incubated with 1 M hydrochloric acid (HCl); pink symbols represent PyOM that was incubated with H2O2 and then with 1 M sodium hydroxide (NaOH); shaded bands represent the 95% confidence intervals
PyOM retains NH3–N through chemisorption
In addition to revealing PyOM’s considerable NH3 retention capacity, adsorption isotherms showed that up to 53% of the NH3 was retained through chemisorption rather than physisorption, and that this proportion was greatest in oxidized PyOM (Fig. 1a). A commonly proposed mechanism for NH3 chemisorption by PyOM is protonation of NH3 to form NH4+ and subsequent electrostatic interaction between the NH4+ and PyOM’s negatively-charged functional groups7, but our data indicate that this mechanism cannot solely be responsible for PyOM NH3–N retention. Direct exposure of oxidized PyOM to NH4+ resulted in much lower N retention than exposure to NH3 gas (Fig. 2a), suggesting that electrostatic interactions alone cannot explain PyOM’s NH3 retention capacity. Furthermore, if physisorption or electrostatic interactions are predominantly responsible for PyOM–N retention from NH3 and NH4+, then stoichiometry dictates that on a molar basis, increases in PyOM–N following exposure should be accompanied by a threefold to fourfold increase in PyOM–H. However, when oxidized PyOM (molar O:C ratio 0.402) was exposed to NH3, the increase in PyOM molar H:N ratio was smaller than 0.5, suggesting that a substantial portion of NH3–N is retained without retention of NH3–H (Fig. 2b). When the same PyOM sample was exposed to NH4+, the molar H:N ratio increased by 5.03, suggesting that most of the NH4+–N was retained along with NH4+’s H atoms. This stoichiometric comparison of H:N ratios in PyOM samples before and after exposure to NH3 and NH4+ indicates that the respective mechanisms for N retention differ substantially, and that alternatives to physisorption and electrostatic interaction are likely responsible for PyOM’s NH3 retention capacity.
Fig. 2.
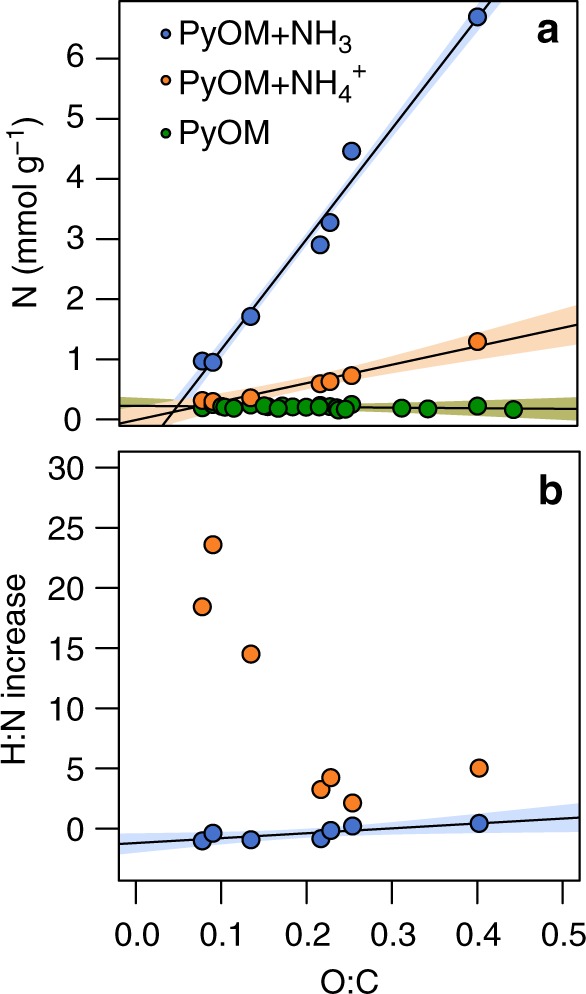
Pyrogenic organic matter N content and H:N ratio following exposure to ammonium or ammonia. a Pyrogenic organic matter (PyOM) nitrogen (N) retention in mmol g−1 PyOM–carbon (C) measured by dry combustion is significantly associated with molar O:C ratios following exposure to NH3 and NH4+ (y = 18.25x – 0.0665, p < 0.001 and y = 3.045x − 0.008, p < 0.001, respectively). b H:N molar increases are significantly associated with O:C ratios following exposure to NH3 (y = 6.23x − 1.70, R2 = 0.59, p < 0.005, F1,10 = 14.45, RSE10 = 0.53), but not NH4+. PyOM samples retain <0.5 moles of NH3–H for every mole of NH3–N retained, compared to 2.13–23.60 moles of NH4+–H for every mole of NH4+–N retained. Green symbols represent original PyOM samples without N addition, orange symbols represent PyOM following exposure to NH4+, and blue symbols represent PyOM following exposure to NH3. Shaded bands represent the 95% confidence intervals
PyOM and NH3–N form covalent bonds
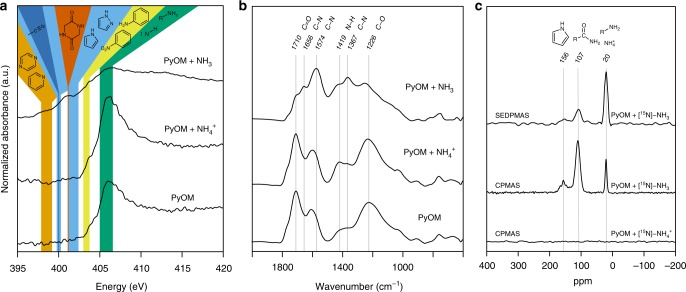
To examine these alternative mechanisms for NH3 retention, we compared the N near-edge X-ray absorption fine structure (K-edge NEXAFS) spectra of oxidized PyOM (O:C ratio 0.402) to those of oxidized PyOM following exposure to either NH3 or NH4+ (Fig. 3a and Supplementary Fig. 5). This method cannot be used to quantify the absolute amount of N retained by PyOM, but does provide information about the types of covalent N bonds present. Exposure to NH3 resulted in the formation of a variety of covalent N bonds that differ from those originating from PyOM feedstock N, N structures formed during thermal decomposition, N structures formed between PyOM and NH4+, and N–H bonds in pure NH3 or NH4+33–36. Interactions between oxidized PyOM and NH3 led to the strong development of absorption peaks between 397.88 and 402.40 eV, many of which are consistent with aromatic and non-aromatic heterocyclic N structures14,36 (see Supplementary Tables 1–3). Compared to spectra collected from PyOM that was not exposed to additional N, spectra collected from PyOM following exposure to NH3 showed a threefold increase in the 1s → π* area consistent with aromatic six-membered heterocycles containing either one or two N atoms (model peaks located at 397.88, 398.76, and 399.2 eV), a threefold increase in the area consistent with nitrile bonds and aromatic five-membered heterocycles containing either one or two N atoms (400.05, 401.43, and 402.40 eV), and a 1.3-fold increase in the area consistent with aliphatic N bonded to aromatic rings (403.00 and 403.65 eV) (Supplementary Table 2). Additionally, while no feature consistent with non-aromatic six-membered N heterocycles (401.15 eV) was identified in spectra collected from oxidized PyOM not exposed to NH3, this feature accounted for two percent of the area underneath the spectrum collected from oxidized PyOM following exposure to NH3. In contrast, development of peaks in the 1s → π* region following exposure to NH4+ was very small, suggesting that there was little change in N functional group composition with NH4+ addition. Both the spectra of PyOM exposed to NH4+ as well as those of unexposed PyOM are strongly dominated by 1s → σ* features with peak centers between 405.00 and 406.58 eV, which dwarf the 1s → π* region area in these spectra. Although these 1s → σ* features are often associated with N–H bonds, they cannot be assigned definitively due to severe overlap of spectral features in this region36.
Fig. 3.
Nitrogen K-edge NEXAFS, FTIR, and NMR spectra of oxidized PyOM samples. Nitrogen (N) K-edge near-edge X-ray absorption fine structure (NEXAFS) (a), Fourier transform infrared (FTIR) (b), and nuclear magnetic resonance (NMR) (c) spectra collected from oxidized PyOM, oxidized PyOM following exposure to ammonium (NH4+), and oxidized pyrogenic organic matter (PyOM) following exposure to ammonia (NH3). a Shaded bands represent the range of peak centers consistent with selected spectral features: 397.88–399.20 eV for C=N bonds in 1N and 2N aromatic six-membered rings (orange), 400.00 for nitrile bonds (dark blue), 399.76–400.27 for C=N bonds in 2N five-membered rings (light blue), 401.15 for C–N bonds in non-aromatic six-membered rings (red), 401.20–402.40 for C–N bonds in 1N and 2N aromatic five-membered rings (light blue), 403.00–403.75 for aliphatic N bonded to aromatic rings (yellow), and 405.00–406.58 for aliphatic amines and N–H bonds (green). Model chemical structures are shown at the top of the figure. b FTIR spectra of oxidized PyOM, oxidized PyOM following exposure to NH4+, and oxidized PyOM following exposure to NH3. c 15N-NMR spin echo direct polarization magic angle spinning (SEDPMAS) spectrum of oxidized PyOM following exposure to [15N]-NH3, and 15N-NMR cross-polarization magic angle spinning (CPMAS) spectra of oxidized PyOM following exposure to [15N]-NH3 and [15N]-NH4+. The spectra suggest that NH3–N retention mechanisms could include NH4+ adsorption (represented by the peak at 20 ppm), and the formation of covalent C–N bonds such as amines (20 ppm), amides (~107 ppm), and aromatic five-membered heterocycles (chemical shifts between ~130–165 ppm)16,33,39,40
To further compare mechanisms for PyOM retention of NH3 in comparison to NH4+, we also collected FTIR spectra from oxidized PyOM, and oxidized PyOM following exposure to NH3 and NH4+ (Fig. 3b). Similar to the NEXAFS spectra (Fig. 3a), the FTIR spectra show clear differences between functional groups present in PyOM, PyOM following exposure to NH3, and PyOM following exposure to NH4+. Exposure to NH3 resulted in the emergence of new peaks at 1656 and 1367 cm−1 and an increase in peak height at 1574 cm−1, all of which are consistent with C–N stretching, including C–N resonance stretching in aromatic rings at 1656 cm−137,38. Exposure to NH3 also resulted in a marked decrease in the peaks associated with C=O and C–O carbonyl/carboxyl and ketonic stretching at 1710 and 1226 cm−1, respectively, suggesting that these functional groups decrease relative to other functional groups in this sample. In contrast, exposure to NH4+ resulted in only two new spectral features, medium-sized peaks at 1419 and 1372 cm−1, which are consistent with N–H and C–N stretching, respectively.
Definitive functional group assignment using FTIR spectra collected from heterogeneous materials such as PyOM is challenging because the regions associated with different bonds often overlap with one another. However, the major treatment difference between our PyOM samples is whether or not they were exposed to gaseous NH3 or aqueous NH4+. Therefore, although FTIR spectral features between 1200 and 1700 cm−1 are sometimes associated with bonds between other elements (including C and O), it is probable that the emergence of distinct features in the FTIR spectra collected from our oxidized PyOM samples following exposure to either NH3 or NH4+ is a result of bonds that formed between PyOM and N. Since the FTIR spectrum of pure NH3 contains predominant peaks around 950 cm−1, it is also unlikely that physical adsorption of NH3 alone could account for the differences observed between the FTIR spectra collected from our PyOM samples before and after NH3 exposure38. In contrast, the FTIR spectra of NH4+ standards contain predominant peaks around 1440 cm−1, indicating that NH4+ adsorption was responsible for the relative increase in this region of the spectra collected from our PyOM samples following exposure to NH4+. This is consistent with our NEXAFS deconvolution analysis, which shows that exposure to NH4+ does not result in the substantial formation of a variety of N functional groups, despite the increased N content relative to unexposed PyOM samples. It also is consistent with elemental analyses, which suggest that oxidized PyOM retains NH4+–N in stoichiometric balance with NH4+–H, but retains NH3–N without NH3–H.
To further investigate mechanisms for NH3–N and NH4+–N retention, we collected solid-state 15N-NMR spectra after separate exposure of oxidized PyOM to enriched [15N]-NH3 gas or [15N]-NH4+ solution (Fig. 3c). Use of 15N-enriched reagents was necessary because there was insufficient signal from 15N at natural abundance in PyOM samples exposed to unlabeled NH3 or NH4+. Substantial differences were observed in the 15N-NMR CPMAS spectra collected from PyOM exposed to enriched [15N]-NH3 gas and these differences confirmed the formation of a variety of new N functional groups, including NH4+ and amines (~20 ppm), and C–N groups such as amides (~107 ppm) and N heterocycles (~156 ppm)16,33,39,40. Similar to the results of our NEXAFS spectral deconvolution analyses, integration of the 15N-NMR SEDPMAS spectrum collected from oxidized PyOM following exposure to [15N]-NH3 gas shows that over 40% of the newly-incorporated NMR-detectable N is consistent with covalent C–N bonds, including more than 11% in heterocyclic structures (Supplementary Table 4). On the other hand, the 15N-NMR spectrum collected from PyOM exposed to [15N]-NH4+ did not show any evidence of NH4+–N incorporation into PyOM, also corresponding with the results of our NEXAFS spectral deconvolution analysis, which show very little difference between the N functional group composition of PyOM and PyOM following exposure to NH4+. It is possible that some NH4+–N was incorporated into PyOM, but that the quantity retained was below the detection limit for NMR, implying that it is essential for an acid-base reaction (e.g., –CO2H + NH3 → –CO2–NH4+) to occur for NH4+ retention.
Direct comparison of NEXAFS and NMR results is difficult because of fundamental differences between the two methods. In particular, NMR detects functional groups, while NEXAFS detects individual bonds, some of which may be present together in one functional group. Additionally, due to severe overlap of features in the 1s → σ* region of NEXAFS N spectra, the portion of N–H and C–NH2 bonds present in a sample cannot be determined with this method. However, the overall results from the two analyses are consistent. In combination with stoichiometric analyses, the NEXAFS, NMR, and FTIR spectra show that PyOM interactions with NH3 under ambient conditions can result in substantial N retention and are fundamentally different from interactions with NH4+. The mechanism may involve nucleophilic NH3 reacting with predisposed functional groups of PyOM, such as acid anhydrides, or diketo-fragments to form a range of covalent C–N bonds, including amides and N heterocycles (Fig. 3). Similar reactions have been described between NH3/NH4+ and small organic molecules such as carbonyls, glyoxals, and secondary organic aerosols found in the atmosphere23,24,41. Both the NH3 retention capacity and mechanisms of natural PyOM are similar to those of some industrially produced graphene oxides and activated carbons8,19,42 even when exposure occurs at ambient temperature and pressure. These results demonstrate for the first time that the enrichment of PyOM with N functional groups such as N heterocycles, aromatic N heterocycles, and amides may occur under natural environmental conditions and that PyOM’s interaction with NH3 versus NH4+ has very different implications for the global N cycle.
Discussion
Our data show that natural PyOM—a ubiquitous component of soil, atmosphere, and water—can react with NH3 gas to form covalent bonds under conditions approximating the natural environment. This is decisive because such covalent bond formation would result in more persistent N retention than physisorption, electrostatic interactions, and precipitation of NH4+ salts, which are currently thought to be the dominant mechanisms for PyOM NH3 retention7–9. Since covalently bound N might be less accessible to living organisms and less susceptible to volatilization, diffusion, and leaching than weakly sorbed NH3 and NH4+, it would also have very different implications for local N availability and global N cycling. By incorporating NH3–N into covalent C–N bonds, PyOM could provide a more dynamic mechanism for N storage, transport, and release. The discovery of aromatic and non-aromatic heterocyclic N bond formation between natural PyOM and NH3 at ambient temperatures is also noteworthy, as this has not been observed for terrestrial pyrogenic C material, including coal, activated carbon, and graphene oxides. This is particularly relevant for industrial applications, where N-doping is used to improve the performance of C-based supercapacitors, catalysts, and other materials15,16,25.
The formation of covalent bonds between PyOM and NH3–N under ambient conditions is a surprising outcome that has not been considered by most scientists investigating PyOM interactions with N. While the work presented here did not determine the chemical reactions responsible for such bond formation, similar reactions are well documented. For example, the reaction between carboxylic acids and amines (including NH3 as the simplest case) to form amides is the basis of protein synthesis from amino acids. It is also well established that subsequent condensation, cyclization, and aromatization to form N-aromatics are also possible. For example, the Paal–Knorr pyrrole synthesis reaction—which produces pyrroles through the condensation of a dicarbonyl compound with an amine or NH3—is thought to be responsible for N heterocycle formation between secondary organic aerosols and NH3 or amines in the atmosphere23,24,41. The discovery of covalent bond formation between PyOM and NH3 under ambient conditions may direct us to rethink PyOM material science and N biogeochemistry on local and global scales. Since many forms of organic matter present in the Earth’s soil, atmosphere, and water contain the same functional groups found in PyOM, it is possible that similar reactions occur between these materials and NH3. Future research should investigate the extent to which organic matter retains NH3–N through covalent bonds, the mechanisms responsible, and the implications for global N biogeochemistry.
Given the existing uncertainties in global PyOM and NH3 budgets, it is difficult to calculate exactly how much N might potentially be retained or transported by the Earth’s PyOM stocks. Based on estimates of 54–109 Pg PyOM–C in soil1 and an NH3 adsorption capacity of 13.5 mmol g−1 for oxidized PyOM (Fig. 1a), we calculate that soil PyOM stocks have the potential to store or transport up to 7.3–14.7 × 1014 mol NH3 through PyOM–NH3 interactions, equaling up to 645-fold more than estimated annual NH3 emissions from global agriculture, or up to 251-fold more than the estimated quantity of annually applied synthetic N fertilizer43. If NH3 interactions with soil PyOM are representative of those with other PyOM stocks, the atmospheric, ocean sediment, and marine PyOM pools could store or transport an additional 214 × 1014 mol NH3–N through similar mechanisms. Combined, all of these PyOM stocks could retain ~320 Pg N, or more than 1500-fold the contribution of global anthropogenic N inputs per year44.
These calculations predict a large potential influence of PyOM on global N cycling, and should motivate further work to constrain estimates so that they reflect the amount of NH3–N retained and transported by PyOM. It is important to consider factors influencing NH3 volatilization (e.g., pH, moisture, and temperature), PyOM–NH3 retention capacity (e.g., functional group composition, surface area, and fouling of PyOM surfaces), and other variables that affect interactions between PyOM and NH3 (e.g., the temperature of exposure, distance from the NH3 source, and biological competition for NH3). However, even at relatively low NH3 concentrations or PyOM–N retention levels, PyOM could influence NH3 loss, N availability to plants and microbes, and global N transport. Additional experiments are necessary to investigate the frequency of PyOM–NH3 interactions and to examine them in more complex and heterogeneous environments, especially in marine waters and sediments, which hold the vast majority of the Earth’s PyOM stocks. The coupling of global C and N cycles through such interactions could also be significant and warrants further research, particularly as global fire patterns change.
Methods
PyOM preparation
Maple (Acer rubrum) wood chips were pyrolyzed at 500 °C for 30 min in a modified muffle furnace28. In order to produce a homogenous product, the furnace employs a custom-made inline mixing unit, regulates temperature, and maintains an internal atmosphere of inert gas throughout pyrolysis. These highly standardized process conditions ensure that the pyrolysis products are as homogenous as possible. The resulting PyOM was ground and sieved to 149–850 µm, divided into subsamples, and incubated with hydrogen peroxide (H2O2) or deionized water (DIH2O) at 30 °C for up to three months (PyOM:H2O2 ratio of 1:10 g mL−1). After oxidation, PyOM was rinsed thoroughly with DIH2O and dried. Some PyOM samples were rewetted with DIH2O (PyOM: DIH2O ratio of 1:20 g mL−1) and treated with 1 M HCl or NaOH until the desired pH was achieved. These PyOM samples were also rinsed with DIH2O and dried.
PyOM characterization
PyOM pH was measured in DIH2O at a ratio of 1:20 g mL−1. SSA was quantified using the B.E.T. method with CO2 at 273.15 K (ASAP 2020, Micromeritics, Atlanta, Georgia). Total C, N, H, and O were measured using a Delta V Isotope Ratio Mass Spectrometer (Thermo Scientific, Germany) coupled to a Carlo Erba NC2500 Elemental Analyzer (Italy).
NH3 and NH4+ adsorption
NH3 adsorption isotherms were measured with an Autosorb iQ gas sorption analyzer (Quantachrome Instruments, Boynton Beach, Florida). Briefly, samples were degassed at 300 °C for 3 h prior to NH3 adsorption isotherm determination, which was conducted from 80 to 800 Torr at 35 °C. Chemisorption values indicate NH3 that was retained by PyOM under vacuum. See Supplementary Fig. 6 for comparison of N measured by chemisorption isotherms to N measured by dry combustion. For NH4+- adsorption measurements, PyOM samples were mixed with 100 mM ammonium chloride solution for 16 h, filtered, rinsed with ethanol, and dried. Retained N was measured using an elemental analyzer, as described above.
FTIR
FTIR spectroscopy was used to characterize oxidized PyOM samples and investigate changes in PyOM functional group composition after exposure to NH3 and NH4+. Two replicates of each PyOM sample were scanned 200 times from 575 to 3500 cm−1 at a resolution of 8 cm−1 using a Bruker Hyperion FT-IR Spectrometer (Bruker, Billerica, Massachusetts) equipped with a ZnSe crystal source (PIKE Technologies, Inc., Madison, Wisconsin). Atmospheric background spectra were subtracted from each sample spectrum. Replicate sample spectra were averaged, baseline corrected, and normalized. Wavenumbers were assigned and peak ratios were calculated for the following functional groups: 752–761, 813–823, and 875–915 cm−1 to aromatic C–H out of plane deformation, 1690–1715 cm−1 to carbonyl/carboxyl and ketonic C=O stretching, and 1581–1609 cm−1 to aromatic C=C vibrations and stretching (OPUS, Bruker, Billerica, Massachusetts).
NEXAFS
Nitrogen K-edge NEXAFS was used to discern how NH3 and NH4+ were retained by PyOM following exposure. Briefly, samples were mounted onto gold-coated silicon wafers and scanned in 49 different locations for 20 seconds each, without any spatial overlap to prevent radiation damage to the sample. N Kα partial fluorescence yield was collected using silicon drift detectors in the slew scanning mode of the spherical grating monochromator beamline at the Canadian Light Source (Saskatoon, Canada). For each sample, all 49 scans were averaged across four detectors and normalized by the beamline incident flux obtained by measuring the drain current in a gold mesh (IGOR Pro 6.36, WaveMetrics, Lake Oswego, Oregon). Following a modification of the method used by Gillespie et al.45, spectra were shifted based on the N2 absorption spectrum measured from ammonium sulfate, background corrected, smoothed, and normalized to an edge-step of 1 (Athena 0.8.056, Bruce Ravel; Ifeffit 1.2.11, Matt Newville, University of Chicago, Chicago, Illinois). Deconvolution was performed using Gaussian curves and peak characteristics of N-containing standards (Fityk 0.9.8, Marcin Wojdyr; see Supplementary Fig. 7 for N standard spectra, Supplementary Table 1 for peak assignments used in deconvolution, and Supplementary Table 3 for features in spectra collected from standard compounds). The fraction of π* area associated with specific N bonds (compared to total area of all deconvolution products) was calculated for each sample (Supplementary Table 2). If they were present as physically adsorbed molecules, neither NH3 nor NH4+ could have been responsible for the development of the numerous pre-edge features in the spectra collected from PyOM that was exposed to NH3 and NH4+ (see Supplementary Figs. 7 and 8, Supplementary Table 2, and refs. 34–38).
To confirm that radiation damage was not responsible for spectral features, samples were also scanned 15 additional times in the same location. These 15 spectra were then averaged, shifted, background corrected, smoothed, and normalized as described above. If the samples were susceptible to beam damage, we would expect to see new spectral features that would become more pronounced as each additional scan exposed the sample to increasing radiation. However, as shown in Supplementary Fig. 9, this did not occur—even after a 15-fold increase in radiation, the spectral features remain the same as those presented in Fig. 3a. As other authors have noted, this indicates that radiation damage was not responsible for the features present in NEXAFS sample spectra46.
Solid-state NMR spectroscopy
Solid-state NMR spectroscopy was used to investigate how NH3 and NH4+ were retained by oxidized PyOM following exposure. In order to obtain a sufficiently strong signal during 15N-NMR experiments, oxidized PyOM samples were exposed to gaseous [15N]-NH3 with 98 atom% 15N (Air Liquide America Specialty Gases, Plumsteadville, PA) and [15N]-NH4+ with 10 atom% 15N (Cambridge Isotope Labs, Tewksbury, MA) at 35 °C, under atmospheric pressure.
1D 1H, 13C and 15N solid-state NMR spectra were obtained at a magnetic field of 7 Tesla (1H, 13C and 15N Larmor frequency of 300 MHz, 75 and 30 MHz, respectively) using a Bruker Avance III NMR spectrometer fitted with a 4 mm magic angle spinning (MAS) double resonance probe. For both the 13C and 15N experiments, ~50 mg of PyOM was packed into a 4 mm zirconia rotor sealed with a Kel-F cap. For 13C experiments, CP (cross-polarization) was achieved with 6.5 kHz MAS; contact time, 1 ms, ramped from 70 to 100%; recycle delay, 3–20 s (Supplementary Figs. 10 and 11); 83 kHz 1H decoupling via spinal-64 sequence; ca 2k scans; TOSS (TOtal Suppression of Spinning side-bands) removed side-bands from the aromatic peaks that obscured the aliphatic region, and also gave better quality response from the sample without pulse breakthrough (Supplementary Figs. 4 and 12); SEDP (spin echo direct polarization with 1H decoupling) was achieved with 12 kHz MAS; recycle delay of 2–150 s; 13C excitation pulse of 4.5 µs (90°); echo time of ~75 µs; 71 kHz 1H decoupling via spinal-64 sequence (Supplementary Fig. 13). Complementary spectra were also acquired with dipolar-dephasing delays of 40 µs with both CP and SEDP to assess non-protonated carbon content. For 15N experiments, CP was achieved at 5 kHz MAS; contact time, 2 ms, ramped from 70 to 100%; recycle delay, 3 s; 83 kHz 1H decoupling via spinal-64 sequence47. TOSS was also used to suppress pulse breakthrough. For 15N SEDPMAS experiments, 10 kHz MAS was used with 100 µs echo time and a 50 kHz 1H decoupling field during acquisition with the spinal-64 sequence. The relaxation behavior was tested using a series of experiments with a fixed number of scans (400) and with increasing recycle delays from 5 to 400 s (Supplementary Figs. 14 and 15).
All spectra were processed using the Bruker software, TOPSPIN 3.5pl7. Spectra were produced from the free induction decays by first zero filling, applying Gaussian multiplication (e.g., LB = −10, GB = 0.03), Fourier transformation, and phase correction. Chemical shift values were referenced to the C=O of glycine, δC 176 ppm for 13C and to (NH4)2SO4, δN 24 ppm on the NH3 scale for 15N. All literature quoted in the text were converted to the NH3 scale for 15N by adding 380 ppm.
Data analysis
All statistical analyses were performed using the lsmeans and nlstools packages48,49 in the statistical computing language and environment R50.
Supplementary information
Acknowledgements
This work was supported by the National Science Foundation's BREAD Program (IOS-0965336), Cornell University’s David R. Atkinson Center for a Sustainable Future, the Towards Sustainability Foundation, the Atkinson Center for a Sustainable Future Impact through Innovation Fund, USDA Hatch (NYC-125443), and the McKnight Foundation. Some of the research described in this paper was performed at the Canadian Light Source Inc., which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan. This work also made use of the Cornell Center for Materials Research Shared Facilities which are supported through the NSF MRSEC program (DMR-1120296, DMR-1719875). The Mark Wainwright Analytical Centre at the University of New South Wales is acknowledged for access to solid-state NMR spectrometers funded through Australian Research Council LIEF LE0989541. R.H. acknowledges support from the NSF IGERT Program (DGE-0903371 and DGE-1069193) and the NSF GRFP (DGE-1144153). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the donors. Special thanks to Akio Enders, Kelly Hanley, and Cornell University Stable Isotope Laboratory staff for their help with sample analysis.
Author contributions
R.H. and J.L. conceived the experiments; R.H. performed the experiments and analyzed the data; D.T.-R., J.D., A.G. and T.R. assisted with NEXAFS measurements and interpretation; J.H. conducted the NMR experiments; J.H. and R.S. led NMR data interpretation; R.H. wrote the paper; all authors contributed to the final draft.
Data availability
The data that support the findings of this study are available in Cornell University’s digital repository eCommons with the identifier 10.7298/X0B7-PX55.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Pellegrino Conte and the other anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-019-08401-z.
References
- 1.Bird MI, Wynn JG, Saiz G, Wurster CM, McBeath A. The pyrogenic carbon cycle. Annu. Rev. Earth Planet. Sci. 2015;43:273–298. doi: 10.1146/annurev-earth-060614-105038. [DOI] [Google Scholar]
- 2.Lauer A, Hendricks J. Simulating aerosol microphysics with the ECHAM4/MADE GCM—Part II: Results from a first multiannual simulation of the submicrometer aerosol. Atmos. Chem. Phys. 2006;6:5495–5513. doi: 10.5194/acp-6-5495-2006. [DOI] [Google Scholar]
- 3.Clough TJ, Condron LM, Kammann C, Müller C. A review of biochar and soil nitrogen dynamics. Agron. J. 2013;3:275–293. doi: 10.3390/agronomy3020275. [DOI] [Google Scholar]
- 4.Galloway JN, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70:153–226. doi: 10.1007/s10533-004-0370-0. [DOI] [Google Scholar]
- 5.Clarisse L, Clerbaux C, Dentener F, Hurtmans D, Coheur PF. Global ammonia distribution derived from infrared satellite observations. Nat. Geosci. 2009;2:479–483. doi: 10.1038/ngeo551. [DOI] [Google Scholar]
- 6.Krupa SV. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environ. Pollut. 2003;124:179–221. doi: 10.1016/S0269-7491(02)00434-7. [DOI] [PubMed] [Google Scholar]
- 7.Taghizadeh-Toosi A, Clough TJ, Sherlock RR, Condron LM. Biochar adsorbed ammonia is bioavailable. Plant Soil. 2012;350:57–69. doi: 10.1007/s11104-011-0870-3. [DOI] [Google Scholar]
- 8.Kastner JR, Miller J, Das KC. Pyrolysis conditions and ozone oxidation effects on ammonia adsorption in biomass generated chars. J. Hazard. Mater. 2009;164:1420–1427. doi: 10.1016/j.jhazmat.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 9.Day D, Evans RJ, Lee JW, Reicosky D. Economical CO2, SOx, and NOx capture from fossil-fuel utilization with combined renewable hydrogen production and large-scale carbon sequestration. Energy. 2005;30:2558–2579. doi: 10.1016/j.energy.2004.07.016. [DOI] [Google Scholar]
- 10.Zawadzki J, Wisniewski A. In situ characterization of interaction of ammonia with carbon surface in oxygen atmosphere. Carbon N. Y. 2003;41:2257–2267. doi: 10.1016/S0008-6223(03)00251-3. [DOI] [Google Scholar]
- 11.Stohr B, Boehm HP, Schlogl R. Enhancement of the catalytic activity of activated carbons in oxidation reactions by thermal treatment with ammonia or hydrogen cyanide and observation of a superoxide species as a possible intermediate. Carbon N. Y. 1991;29:707–720. doi: 10.1016/0008-6223(91)90006-5. [DOI] [Google Scholar]
- 12.Jansen RJJ, Van Bekkum H. Amination and ammoxidation of activated carbons. Carbon N. Y. 1994;32:1507–1516. doi: 10.1016/0008-6223(94)90146-5. [DOI] [Google Scholar]
- 13.Schultz BJ, et al. X-ray absorption spectroscopy studies of electronic structure recovery and nitrogen local structure upon thermal reduction of graphene oxide in an ammonia environment. Rsc Adv. 2014;4:634–644. doi: 10.1039/C3RA45591B. [DOI] [Google Scholar]
- 14.Geng D, et al. Nitrogen doping effects on the structure of graphene. Appl. Surf. Sci. 2011;257:9193–9198. doi: 10.1016/j.apsusc.2011.05.131. [DOI] [Google Scholar]
- 15.Li X, et al. Simultaneous nitrogen doping and reduction of graphene oxide. J. Am. Chem. Soc. 2009;131:15939–15944. doi: 10.1021/ja907098f. [DOI] [PubMed] [Google Scholar]
- 16.Latham KG, Rawal A, Hook JM, Donne SW. Molecular structures driving pseudo-capacitance in hydrothermal nanostructured carbons. Rsc Adv. 2016;6:12964–12976. doi: 10.1039/C5RA26136H. [DOI] [Google Scholar]
- 17.Mortland MM. Reactions of ammonia in soils. Adv. Agron. 1958;10:325–348. doi: 10.1016/S0065-2113(08)60069-3. [DOI] [Google Scholar]
- 18.Richardson LB. The adsorption of carbon dioxide and ammonia by charcoal. J. Am. Chem. Soc. 1917;39:1828–1848. doi: 10.1021/ja02254a005. [DOI] [Google Scholar]
- 19.Zhang TY, et al. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004;105:53–59. doi: 10.1016/j.cej.2004.06.011. [DOI] [Google Scholar]
- 20.Azargohar R, Dalai AK. Biochar as a precursor of activated carbon. Appl. Biochem. Biotechnol. 2006;131:762–773. doi: 10.1385/ABAB:131:1:762. [DOI] [PubMed] [Google Scholar]
- 21.Seredych M, Bandosz TJ. Mechanism of ammonia retention on graphite oxides: Role of surface chemistry and structure. J. Phys. Chem. C. 2007;111:15596–15604. doi: 10.1021/jp0735785. [DOI] [Google Scholar]
- 22.Petit C, Seredych M, Bandosz TJ. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J. Mater. Chem. 2009;19:9176–9185. doi: 10.1039/b916672f. [DOI] [Google Scholar]
- 23.Updyke KM, Nguyen TB, Nizkorodov SA. Formation of brown carbon via reactions of ammonia with secondary organic aerosols from biogenic and anthropogenic precursors. Atmos. Environ. 2012;63:22–31. doi: 10.1016/j.atmosenv.2012.09.012. [DOI] [Google Scholar]
- 24.De Haan DO, et al. Secondary organic aerosol-forming reactions of glyoxal with amino acids. Environ. Sci. Technol. 2009;43:2818–2824. doi: 10.1021/es803534f. [DOI] [PubMed] [Google Scholar]
- 25.Li XF, et al. Unraveling the formation mechanism of graphitic nitrogen-doping in thermally treated graphene with ammonia. Sci. Rep. 2016;6:23495. doi: 10.1038/srep23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Rosa JM, Knicker H. Bioavailability of N released from N-rich pyrogenic organic matter: an incubation study. Soil Biol. Biochem. 2011;43:2368–2373. doi: 10.1016/j.soilbio.2011.08.008. [DOI] [Google Scholar]
- 27.Smernik RJ, Baldock JA. Does solid-state 15N NMR spectroscopy detect all soil organic nitrogen? Biogeochemistry. 2005;75:507–528. doi: 10.1007/s10533-005-2857-8. [DOI] [Google Scholar]
- 28.Enders A, Hanley K, Whitman T, Joseph S, Lehmann J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012;114:644–653. doi: 10.1016/j.biortech.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Velasco-Molina M, Berns AE, Macias F, Knicker H. Biochemically altered charcoal residues as an important source of soil organic matter in subsoils of fire-affected subtropical regions. Geoderma. 2016;262:62–70. doi: 10.1016/j.geoderma.2015.08.016. [DOI] [Google Scholar]
- 30.Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006;37:1477–1488. doi: 10.1016/j.orggeochem.2006.06.022. [DOI] [Google Scholar]
- 31.Cheng CH, Lehmann J, Engelhard MH. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta. 2008;72:1598–1610. doi: 10.1016/j.gca.2008.01.010. [DOI] [Google Scholar]
- 32.Park SJ, Jin SY. Effect of ozone treatment on ammonia removal of activated carbons. J. Colloid Interf. Sci. 2005;286:417–419. doi: 10.1016/j.jcis.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 33.Knicker H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry. 2007;85:91–118. doi: 10.1007/s10533-007-9104-4. [DOI] [Google Scholar]
- 34.Jaeger R, Stohr J, Kendelewicz T. X-ray induced electron stimulated desorption versus photon stimulated desorption: NH3 on Ni(110) Surf. Sci. 1983;134:547–565. doi: 10.1016/0039-6028(83)90440-5. [DOI] [Google Scholar]
- 35.Wight GR, Brion CE. K-shell excitation of CH4, NH3, H2O, CH3OH, CH3OCH3 and CH3NH2 by 2.5 eV electron impact. J. Electron Spectrosc. 1974;4:25–42. doi: 10.1016/0368-2048(74)80040-X. [DOI] [Google Scholar]
- 36.Leinweber P, et al. Nitrogen K-edge XANES—an overview of reference compounds used to identify ‘unknown’ organic nitrogen in environmental samples. J. Synchrotron Radiat. 2007;14:500–511. doi: 10.1107/S0909049507042513. [DOI] [PubMed] [Google Scholar]
- 37.Gunasekaran S, Sailatha E, Seshadri S, Kumaresan S. FTIR, FT Raman spectra and molecular structural confirmation of isoniazid. Indian J. Pure Appl. Phys. 2009;47:12–18. [Google Scholar]
- 38.National Institute of Standards and Techology. NIST Chemistry WebBook. 10.18434/T4D303 (2016).
- 39.Knicker H. The feasibility of using DCPMAS 15N 13C NMR spectroscopy for a better characterization of immobilized 15N during incubation of 13C- and 15N-enriched plant material. Org. Geochem. 2002;33:237–246. doi: 10.1016/S0146-6380(01)00155-3. [DOI] [Google Scholar]
- 40.Smernik RJ, Baldock JA. Solid-state 15N NMR analysis of highly 15N-enriched plant materials. Plant Soil. 2005;275:271–283. doi: 10.1007/s11104-005-2153-3. [DOI] [Google Scholar]
- 41.Amarnath V, et al. Intermediates in the Paal–Knorr synthesis of pyrroles. J. Org. Chem. 1991;56:6924–6931. doi: 10.1021/jo00024a040. [DOI] [PubMed] [Google Scholar]
- 42.Guo X, Tak JK, Johnson RL. Ammonia removal from air stream and biogas by a H2SO4 impregnated adsorbent originating from waste wood-shavings and biosolids. J. Hazard. Mater. 2009;166:372–376. doi: 10.1016/j.jhazmat.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 43.Beusen AHW, Bouwman AF, Heuberger PSC, Van Drecht G, Van Der Hoek KW. Bottom-up uncertainty estimates of global ammonia emissions from global agricultural production systems. Atmos. Environ. 2008;42:6067–6077. doi: 10.1016/j.atmosenv.2008.03.044. [DOI] [Google Scholar]
- 44.Fowler D, et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B. 2013;368:20130164. doi: 10.1098/rstb.2013.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillespie AW, et al. Nitrogen input quality changes the biochemical composition of soil organic matter stabilized in the fine fraction: a long-term study. Biogeochemistry. 2014;117:337–350. doi: 10.1007/s10533-013-9871-z. [DOI] [Google Scholar]
- 46.Gillespie AW, et al. Advances in using soft X-ray spectroscopy for measurement of soil biogeochemical processes. Adv. Agron. 2015;133:1–32. doi: 10.1016/bs.agron.2015.05.003. [DOI] [Google Scholar]
- 47.Mao J, Cao X, Olk DC, Chu W, Smidt-Rohr K. Advanced solid-state NMR spectroscopy of natural organic matter. Prog. Nucl. Mag. Reg. Spectrosc. 2017;100:17–51. doi: 10.1016/j.pnmrs.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Lenth R. Least-squares means. The R package lsmeans. J. Stat. Softw. 2016;69:1–33. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- 49.Baty F, et al. A toolbox for nonlinear regression in R: the package nlstools. J. Stat. Softw. 2015;66:1–21. doi: 10.18637/jss.v066.i05. [DOI] [Google Scholar]
- 50.R Development Core Team. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in Cornell University’s digital repository eCommons with the identifier 10.7298/X0B7-PX55.