Abstract
G proteins are major transducers of signals from G-protein coupled receptors (GPCRs). They are made up of α, β, and γ subunits, with 16 Gα, 5 Gβ and 12 Gγ subunits. Though much is known about the specificity of Gα subunits, the specificity of Gβγs activated by a given GPCR and that activate each effector in vivo is not known. Here, we examined the in vivo Gβγ specificity of presynaptic α2a-adrenergic receptors (α2aARs) in both adrenergic (auto-α2aARs) and non-adrenergic neurons (hetero-α2aARs) for the first time. With a quantitative MRM proteomic analysis of neuronal Gβ and Gγ subunits, and co-immunoprecipitation of tagged α2aARs from mouse models including transgenic FLAG-α2aARs and knock-in HA-α2aARs, we investigated the in vivo specificity of Gβ and Gγ subunits to auto-α2aARs and hetero-α2aARs activated with epinephrine to understand the role of Gβγ specificity in diverse physiological functions such as anesthetic sparing, and working memory enhancement. We detected Gβ2, Gγ2, Gγ3, and Gγ4 with activated auto α2aARs, whereas we found Gβ4 and Gγ12 preferentially interacted with activated hetero-α2aARs. Further understanding of in vivo Gβγ specificity to various GPCRs offers new insights into the multiplicity of genes for Gβ and Gγ, and the mechanisms underlying GPCR signaling through Gβγ subunits.
Introduction
G-protein coupled receptors (GPCRs) are the largest and most diverse superfamily of transmembrane receptors that convey signal transduction across cell membranes, and mediate a vast array of cellular responses necessary for human physiology1–3. Upon their activation, GTP-Gα and Gβγ subunits are released from the GPCR and interact with various effectors to initiate downstream signaling cascades. Theoretically, 60 different combinations of Gβγ dimers are possible (5 Gβ × 12 Gγ subunits)4–8. However, not all theoretical Gβγ dimers exist, are equally expressed, or interact with Gα subunits, receptors, effectors, and downstream signaling factors5,9–17. For example, Gβ1 and Gβ4 dimerize with all Gγ subunits, while Gβ2 and Gβ3 are unable to dimerize with Gγ1 and Gγ118. In addition, Gβ5 has low-affinity interaction with Gγ subunits18,19 and preferentially forms a stable dimer with the RGS R7 subfamily20–24. Similarly, Gβ2γ1 shows a stronger association than Gβ2γ417,25,26. The expression levels, localizations, and affinities of each Gβ and Gγ subunit influences intracellular signaling cascades through the formation of specific Gβγ dimers and the specificity of each dimer for GPCRs5,25,27,28.
Given the diversity seen for the expression and affinity of Gβ and Gγ subunits, as well as the affinity of Gβγ-effector interactions, it is likely that specific dimers could permit specialized roles in signal transduction pathways through association with particular GPCRs. Despite many attempts to understand G protein βγ specificity for particular GPCRs, much remains unclear due to a lack of specific antibodies or other methods of confidently assaying such preferences. Indeed, as yet only in vitro data exists which describes Gβγ specificity, and for only a few GPCRs29–31. For example, activated α2a-adrenergic receptors (α2aARs) are found to interact with Gαi1, Gβ1, Gβ2, Gγ2, Gγ3, Gγ4, and Gγ7 as shown by a fluorescence resonance energy transfer (FRET) assay32,33 while M4 muscarinic receptors interact with Gαo, Gβ3, and Gγ434. Lack of tissue -specific determinants of specificity in heterologous expression systems created a gap between understanding in vitro and in vivo specificity of G protein βγ. As the interaction Gβγ dimers with particular GPCRs in the CNS may determine their role in regulating synaptic transmission, or their impact in neurological disease and GPCR targeted drug mechanism, further elucidation of G protein specificities in vivo is necessary.
α2aARs are Gi/o-coupled GPCRs35,36 that are widely distributed in the peripheral and central nervous systems37,38, are expressed in both adrenergic and non-adrenergic neurons, and are located in both pre- and post-synaptic39 terminals. Presynaptic α2aARs in adrenergic neurons are called autoreceptors (auto-α2aARs) and act to inhibit exocytosis and prevent norepinephrine release. α2aARs in non-adrenergic neurons are called heteroreceptors (hetero-α2aARs)37, and these also inhibit neurotransmitter release. Hetero-α2aARs activity is known to play a role in working memory, hypotension, bradycardia, sedation, analgesia, and hypnosis37. Using mRNA in situ hybridization and immunohistochemical analysis, auto- and hetero-α2aARs have been found in the locus coeruleus, cerebral cortex, hypothalamus, hippocampus, and amygdala37,40–43. Multiple polymorphisms within the ADRA2A gene have been identified, which variously increase α2aARs expression and alcohol dependence, reduce glucose-stimulated insulin release and antidepressant responsiveness, and alter memory and behavior44–46. In addition, the dysregulation of α2aARs, by increasing the amount of norepinephrine released, enhances fear memory and impairs spatial working memory47,48. Though the main mechanism of inhibition of exocytosis is via Gβγ subunits49–51, it is unclear which G protein βγs are involved in these downstream signals of α2aARs.
With the development of transgenic mice including Hemagglutinin tagged (HA)-α2aARs knock-in (HA-α2aARs) and FLAG-α2aARs transgenic mice, the physiological implications of α2aARs can be further studied. HA-α2aARs mice were generated utilizing a homologous recombination gene targeting strategy to express HA-α2aARs in the endogenous mouse ADRA2A gene locus52. Expression and distribution of HA-α2aARs in these mice is identical to those of wildtype mice52, as they are expressed in both adrenergic and non-adrenergic neurons which represent both auto- and hetero-α2aARs. Conversely, FLAG-α2aARs transgenic mice express FLAG-α2aARs only in adrenergic neurons, as the transgene is under the control of the dopamine-β-hydroxylase (Dbh) promoter37. These mice were then crossed with α2aAR knockout (α2aARs KO) mice, such that only FLAG-α2aARs autoreceptors are present. The expression and function of this mice is identical to that of α2aARs autoreceptor49. By comparing with the wildtype, FLAG-α2aARs, and α2aARs knock-out mice, the different physiological functions of auto- and hetero-α2aARs were characterized. Auto-α2aARs play a role in bradycardia and hypotension while hetero-α2aARs are involved in anesthetic sparing, hypothermia, analgesia, bradycardia, and hypotension37. Given the physiological importance of α2aARs, and the different roles of auto-and hetero-α2aARs, the signaling mechanisms of α2aARs in both adrenergic and non-adrenergic neurons need to be further elucidated.
Together with our previous study quantifying the change in abundance and localization of each neuronal Gβ and Gγ subunit28, the differences in physiological functions of auto- and hetero-α2aARs37 suggests that the different α2aARs may utilize unique Gβγ dimers to regulate auto- vs. hetero-α2aARs specific downstream signaling pathways. Although Gβ1γ2 is the most abundant neuronal Gβγ dimer, other Gβγ combinations may be mediating auto- or hetero-α2aAR signaling. For example, Gβ2γ and Gβ4γ dimers may specifically interact with adrenergic and opioid GPCRs30. In this paper, we test this hypothesis by using FLAG-α2aARs, HA-α2aARs, α2aAR KO, and wildtype mice, together with various biochemical approaches such as a co-immunoprecipitation (co-IP) and a quantitative multiple reaction monitoring (MRM) method to identify and quantify Gβ and Gγ subunits. We measured and compared the interaction of overall (HA-α2aARs) or auto-α2aARs with neuronal Gβ and Gγ subunits for the first time, and depict the in vivo Gβγ specificity to auto- and hetero-α2aARs.
Results
The interaction of α2a adrenergic receptors and Gβγ
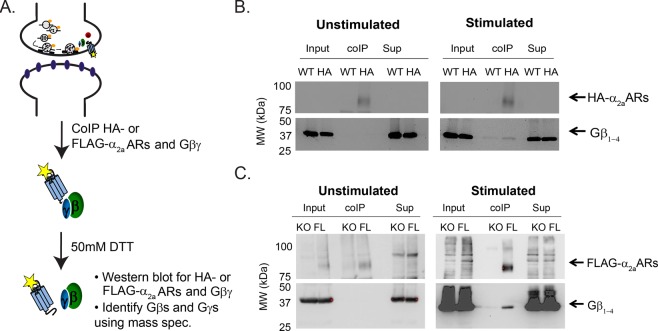
To study the specificity of neuronal Gβγ subunits to synaptic α2aARs, we used brain synaptosomes from wildtype, α2aAR KO, HA- and FLAG-α2aAR mice. Because no GPCR antibodies are specific enough to co-IP α2aARs and Gβγ, we used HA- and FLAG-α2aARs expressing mice to overcome this limitation. Wildtype and α2a-ARs KO mice were used as controls for HA- and FLAG-α2aARs mice. Synaptosomes from these mice were resuspended in a buffer with (stimulated) or without (unstimulated) epinephrine. DSP, a lipid-soluble thiol cleavable crosslinker, was added to ensure the receptor and Gβγ remained intact during co-IP experiments. The synaptosomes were then lysed and co-IPed for HA- or FLAG-α2aARs and Gβγ (Fig. 1A), which was validated by Western blot. Input represents total proteins present in lysate after the preclear while supernatant (Sup) represents what proteins are left in lysate after the co-IP with HA or FLAG specific antibodies (see Materials and Methods for more details). In wildtype and α2aARs KO mice, no α2aAR and Gβγ interactions were detected following receptor stimulation (Fig. 1B,C). Here, we detected HA- and FLAG-α2aARs interacting with Gβγ only following α2aAR stimulation (Fig. 1B,C).
Figure 1.
Co-immunoprecipitation of adrenergic α2a receptors and Gβγ. Workflow of co-immunoprecipitation (coIP) experimental protocol (A), and representative Western blot of coIP of the HA-α2aARs (B) or FLAG-α2aARs (C) and Gβs following the resuspension of synaptosomes with unstimulated or stimulated buffers (stimulated, 100 μM epinephrine). Gels are cut out at 50 kDa to separate receptor (HA- or FLAG-α2aARs) and Gβ blots. The exposure times of receptor (HA- or FLAG-α2aARs) blots are 300 secs and 120 secs, respectively. The exposure times of Gβ blots are 300 secs for HA-α2aARs and 100 secs for FLAG-α2aARs coIP. The co-IP lane represents proteins immunoprecipitated with HA or FLAG specific antibodies. HA-α2aARs and FLAG-α2aARs are ~75 kDa while Gβs are ~33 kDa. HA-α2aARs and FLAG-α2aARs interact with Gβγ upon the activation of the receptors (stimulated). Sup: depleted supernatant.
Limit of Gβ1 detection and quantification
To determine the number of co-IPs needed to detect Gβ and Gγ subunits in our MRM method, we used a serial dilution of purified Gβ1γ1 and monitored four non-heavy labeled proteolytic peptides of Gβ1 to determine the limits of detection and quantitation (LOD/LOQ) (Supplementary Table 1)53. Because Gβ1γ1 is easily purified from the bovine retina, we chose it as our standard. It is used as a control to make sure that our method is running correctly and accurately. Previously, we have validated how each Gβ and Gγ are detected in our quantitative method28. Because Gγ1 is not present in the brain but only in photoreceptors, we only monitored Gβ1 with mass spec. Below 10 pg of Gβ1γ1, we couldn’t confidently identify the presence of Gβ1 in samples. Between 10 pg to 250 pg, we were able to detect Gβ1 but total area under the curve (AUC) didn’t increase as the amount of purified Gβ1γ1 was increased (Supplementary Fig. 1). This suggests that we need more than 250 pg of Gβ1 to detect and quantify proteins using our MRM method. We subsequently found using quantitative Western blots, that ~400–700 ng of Gβγ was pulled down with FLAG-α2aARs per half mouse brain used (10 co-IPs/half mouse brain) (data not shown). However, the previous limit of quantification experiment suggests that we need more than 4 ng of Gβγ for quantification28. Thus, using a half brain per condition, we can detect and quantify neuronal Gβ and Gγ despite our previously described technical challenges28.
Gβ2, Gβ4, Gγ2, Gγ3, Gγ4, and Gγ12 specifically interact with neuronal α2a adrenergic receptors
We examined the Gβ and Gγ subunits interacting with α2aARs to distinguish which Gβ and Gγ subunits interact with auto- vs. hetero-α2aARs. In Figs 2 and 3, we applied the quantitative MRM method28 to co-IP samples of wildtype (WT) and HA-α2aARs mouse synaptosomes. Using SDS-PAGE gel, we excised Gβ and Gγ bands and added the heavy labeled proteolytic peptides to quantify each neuronal Gβ and Gγ subunit28 (see Materials and Methods). Because Gβγ can be sticky, we built in a number of negative controls. To identify nonspecific interactions of Gβ and Gγ subunits, we used both unstimulated WT (WT no epi) and HA-α2aAR (HA-α2aAR no epi) samples as controls. In addition, we used stimulated WT (WT +epi) samples to detect nonspecific interactions with other receptors (non-HA-α2aAR-mediated interactions). Thus the first three conditions in each graph in Figs 2 and 3 were to detect non-specific interactions of Gβγ, while the last detected interaction of Gβγ isoforms with epi-stimulated HA-α2aAR.
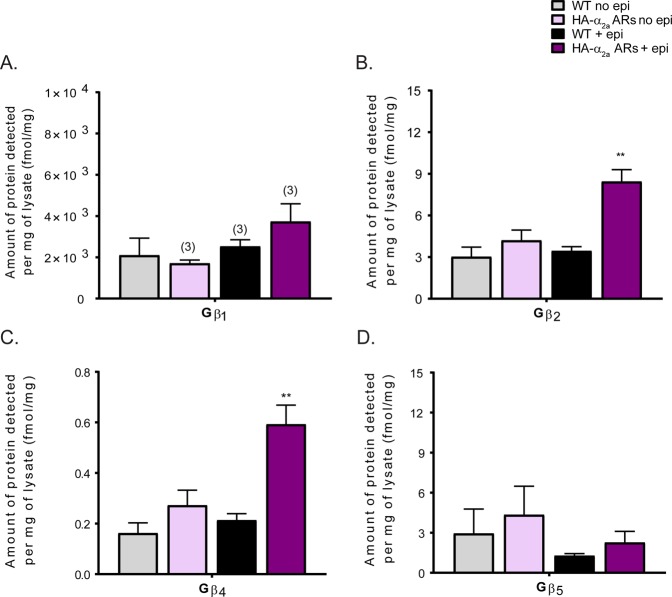
Figure 2.
Gβ subunit specificity to α2a adrenergic receptors. Quantification of Gβ subunits interacting with α2aARs in both adrenergic and non-adrenergic neurons (N = 4 unless otherwise noted on the graph with parentheses). Gβ subunits detected (fmol) from quantitative measurements were normalized by the amount of protein (mg), calculated using the volume and the protein concentration of precleared lysate used in co-IPs. We included several controls: unstimulated WT (WT no epi), HA-α2aAR (HA-α2aAR no epi), and stimulated WT (WT + epi) samples are all controls for the key sample, the Gβ and γ isoforms interacting with HA-α2aAR. Gβ2 and Gβ4 specifically interact with activated α2aARs present in all synaptic terminals. Data were presented as mean ± SEM and compared by a one-way ANOVA, **P < 0.01. Post hoc analysis was performed with Tukey’s multiple comparison test.
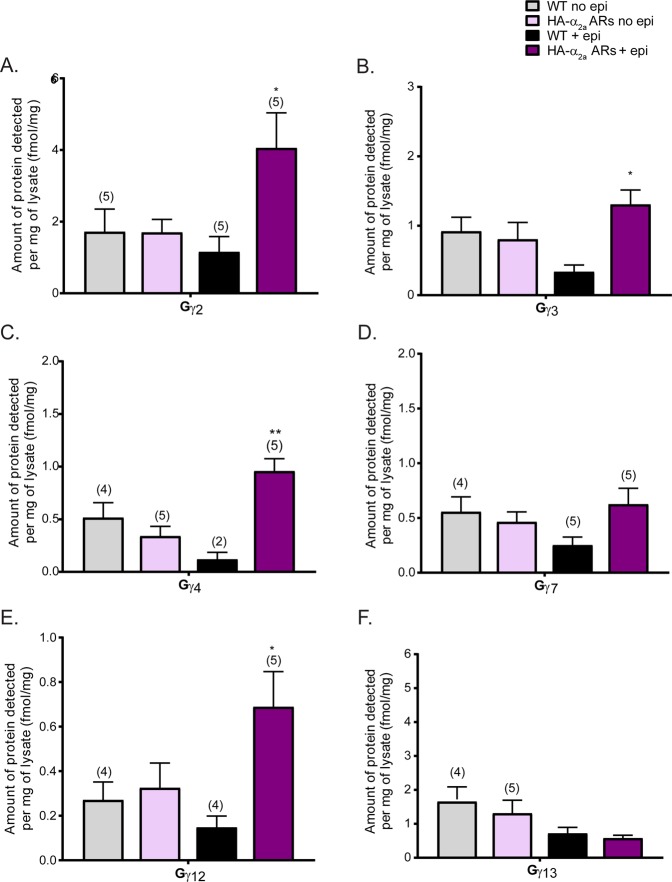
Figure 3.
Gγ subunit specificity to α2a adrenergic receptors. Quantification of Gγ subunit interactions with α2aARs in both adrenergic and non-adrenergic neurons (N = 4 unless otherwise noted on the graph). Gγ subunits detected (fmol) from quantitative measurements were normalized by the amount of protein (mg), calculated using the volume of precleared lysate used and the protein concentration of precleared lysate from BCA assay, used in co-IPs. Several controls were run: unstimulated WT (WT no epi), HA-α2aAR (HA-α2aAR no epi), and stimulated WT (WT + epi) samples. These are all controls for the key sample, the Gβ and γ isoforms interacting with HA-α2aAR. Gγ2, Gγ3, Gγ4, and Gγ12 specifically interact with HA-α2aARs present in all synaptic terminals. Data were presented as mean ± SEM and compared by one-way ANOVA, *P < 0.05 and **P < 0.01. Post hoc analysis was performed with Tukey’s multiple comparison test.
Gβ2 and Gβ4 were significantly enriched with HA-α2aARs stimulated with epi (Fig. 2B,C). More Gβ4 was detected than Gβ2 In contrast, Gβ5 did not interact with HA-α2aARs. Next, we examined the specificity of Gγ subunits to α2aARs to determine possible Gβγ dimer interactions with α2aARs. From the 6 detectable and quantifiable neuronal Gγ subunits28, Gγ2, Gγ3, Gγ4, and Gγ12 were significantly enriched with HA-α2aARs upon epinephrine stimulation (Fig. 3A–C and E). We detected Gγ2 > Gγ3 ≈ Gγ4 > Gγ12. Gγ7 and Gγ13 in stimulated HA-α2aARs + epi samples were equal to, or less, than corresponding control samples, suggesting these Gγs are present nonspecifically (Fig. 3D,F). From the subunits we have detected, we postulate that there may be as many as 8 different combinations of Gβγ dimers in vivo (Gβ2γ2, Gβ2γ3, Gβ2γ4, Gβ2γ12, Gβ4γ2, Gβ4γ3, Gβ4γ4, and Gβ4Gγ12) which may interact with α2aARs in adrenergic and non-adrenergic neurons. Based on their detection levels, Gβ2γ2, Gβ2γ3, and Gβ2γ4 may be more likely to interact with α2aARs than other Gβγ dimers. Gβ2γ12, Gβ4γ2, Gβ4γ3, Gβ4γ4, and Gβ4Gγ12 are less abundant Gβγ dimers interacting with α2aARs. Further biochemical analysis will be needed to validate the presence of these Gβγ dimers and their specificities with α2aARs in both adrenergic and non-adrenergic neurons.
Gβ2, Gγ2, Gγ3, and Gγ4 specifically interact with auto-adrenergic α2a receptors
After identifying the specificities of Gβ and Gγ for α2aARs in both adrenergic and non-adrenergic neurons, we decided to examine the specificity to auto-α2aARs which are only present in adrenergic neurons. In previous studies, auto-α2aARs and hetero-α2aARs were shown to have very different physiological functions37. We wondered if these different physiological functions may be mediated by unique Gβ and Gγ specificities for the different receptor types or through specific effector interactions. We again applied a quantitative MRM method to TCA-precipitated and trypsin-digested co-IP samples of α2aARs KO and FLAG-α2aARs mouse synaptosomes.
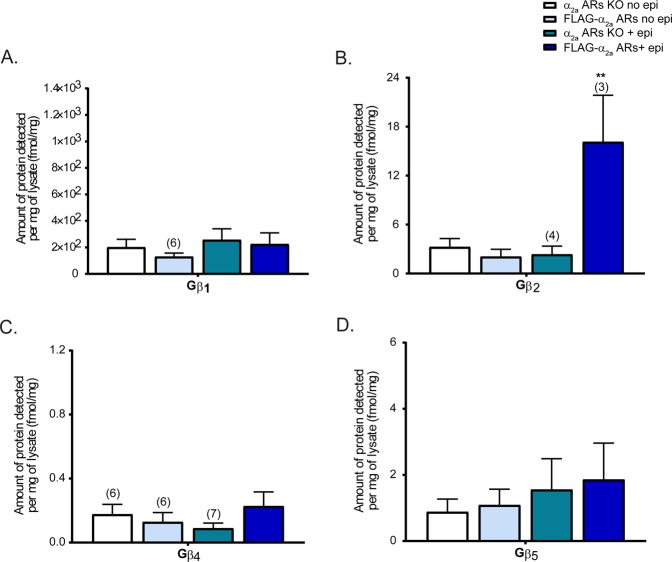
FLAG-α2aARs only express auto-α2aARs at the sympathetic presynaptic terminal, allowing us to study Gβ and Gγ subunit specificities to autoreceptors uniquely in sympathetic neurons. Similar to the previous experiment, α2aARs KO no epi and FLAG-α2aARs no epi samples were used as controls to identify nonspecific interactions, and α2aARs KO + epi samples were used to detect non-α2aARs associations. Here, Gβ2 but not Gβ4, showed a significant enrichment with auto-α2aARs (FLAG-α2aARs) (Fig. 4B). Again, Gβ1 and Gβ5 did not specifically interact with auto-α2aARs upon stimulation (Fig. 4A,D).
Figure 4.
Gβ subunit specificity to auto-α2a adrenergic receptors. Quantification of Gβ subunits interacting with auto-α2aARs (FLAG-α2a-ARs) in adrenergic neurons (N = 5 unless otherwise noted on the graph). The data were analyzed identical to the study of α2aARs in both adrenergic and non-adrenergic neurons. Unstimulated α2aARs KO (KO no epi), FLAG-α2aAR (FLAG-α2aAR no epi), and stimulated KO (KO + epi) samples are controls. The difference between these epi-stimulated α2aARs KO and FLAG-α2aAR represents the interaction of Gβ isoforms upon auto-α2aARs activation. Gβ2 specifically interacts with auto-α2aARs. Data were presented as mean ± SEM and compared by one-way ANOVA, **P < 0.01. Post hoc analysis was performed with Tukey’s multiple comparison test.
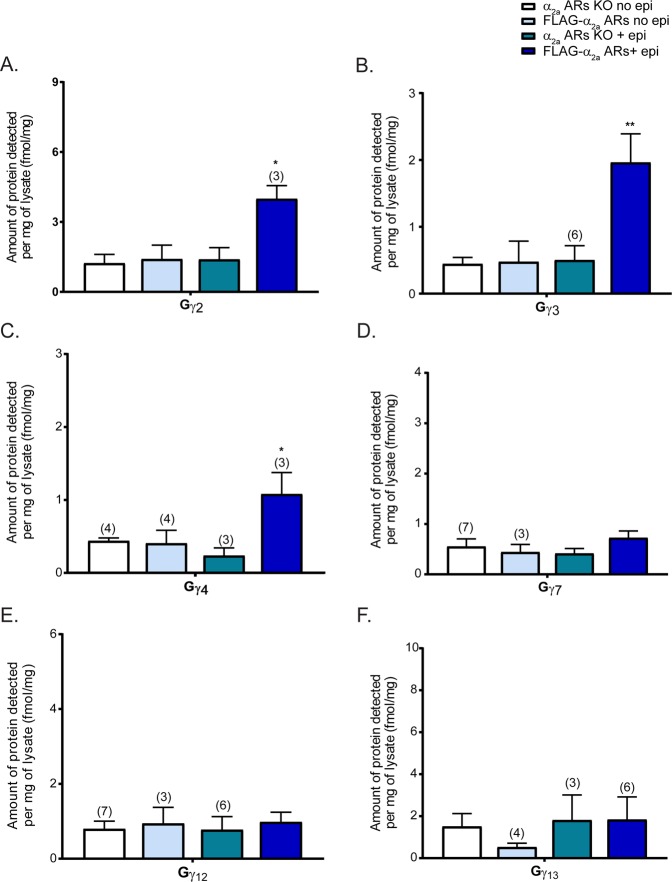
In contrast to the 4 Gγ subunits enriched with HA-α2aARs, we were able to detect Gγ2, Gγ3, and Gγ4 enriched with FLAG-α2aARs (Fig. 5A–C). Interestingly, we no longer saw enrichment of Gγ12 with FLAG-α2aARs (Fig. 5E) suggesting that Gγ12 may be a hetero-α2aAR-specific Gγ subunit. As expected from the HA-α2aAR study, Gγ7 and Gγ13 did not interact with FLAG-α2aARs (Fig. 5D,F). Although further validation is necessary, we speculate that Gβ2γ2, Gβ2γ3, and Gβ2γ4 may be the possible Gβγ dimers interacting with auto-α2aARs in sympathetic adrenergic neurons.
Figure 5.
Gγ subunit specificity to auto-α2a adrenergic receptors. Quantification of Gγ subunits interacting with auto-α2aARs on adrenergic neurons (N = 5 unless otherwise noted on the graph). The data were analyzed identical to the study of α2aARs in both adrenergic and non-adrenergic neurons. Unstimulated α2aARs KO (KO no epi), FLAG-α2aAR (FLAG-α2aAR no epi), and stimulated KO (KO + epi) samples are controls. The difference between these epi-stimulated α2aARs KO and FLAG-α2aAR represents the interaction of Gγ isoforms upon auto-α2aARs activation. Gγ2, Gγ3, and Gγ4 specifically interact with auto-α2aARs. Data were presented as mean ± SEM and compared by one-way ANOVA, *P < 0.05 and **P < 0.01. Post hoc analysis was performed with Tukey’s multiple comparison test.
Gβ4 and Gγ12 may specifically interact with heteroreceptors
Only a subset of Gβ and Gγ subunits from the HA-α2aARs study exhibited specificity to auto-α2aARs, suggesting that hetero-α2aARs may utilize those Gβ and Gγ subunits not associated with auto-α2aARs to regulate unique downstream signaling pathways. Without a transgenic tagged hetero-α2aARs mouse; however, we cannot directly measure the Gβ and Gγ subunits specific to hetero-α2aARs. However, in this study, we can infer the Gβ and Gγ specific to hetero-α2aARs by comparing and subtracting the results of our HA- and FLAG-α2aARs studies. By comparing the Gβ and Gγ subunits detected each set of experiments (which represent overall synaptic α2aARs and presynaptic α2aARs at the sympathetic terminal, respectively), we determined that Gβ4 (Figs 2 and 4C) and Gγ12 (Figs 3 and 5E) may be heteroreceptor specific. As a result, it is possible that Gβ2γ12, Gβ4γ2, Gβ4γ3, Gβ4γ4, and Gβ4γ12 dimers may be left to interact with hetero-α2aARs.
Discussion
It is well defined that Gβγ dimers are released upon the activation of Gi/o-coupled GPCRs, such as the α2aAR, and act as important signaling units to various downstream signaling cascades to ultimately mediate various physiological functions54–61. It is not known whether all 32 possible neuronal Gβγs (combined from the known expression of 4 neuronal Gβs and 8 neuronal Gγs28), are functional in vivo, however, how such sorting may take place to determine the formation of particular Gβγ dimers is not known, and very little is known of how the specificity of particular Gβγs plays a role in defining the specificity of signaling pathways5,25,27–34.
In vivo specificity of α2aARs for Gβγ
In this study, we have addressed the in vivo specificity of Gβ and γ interaction with the α2aAR using MRM proteomics. We demonstrate that α2aARs preferentially interact with a subset of Gβ and Gγ subunits at synaptic terminals in vivo. Neuronal α2aARs (both auto- and hetero-α2aARs) interacted with Gβ2, Gβ4, Gγ2, Gγ3, Gγ4, and Gγ12 while auto-α2aARs interacted with Gβ2, Gγ2, Gγ3, and Gγ4 only. These findings suggest that Gβγs may shape signaling pathway specificity and that receptor and Gβγ interactions may be important in determining specific effector interactions.
In our previous study, we found Gβ1 as the most abundant Gβ subunit in whole synaptosomes as well as at both pre- and post-synaptic fractions28. Interestingly, however, in this study we did not find a statistically significant interaction between Gβ1 and HA-α2aARs upon receptor activation (Fig. 2A). Interestingly, we found Gβ2 and Gβ4 with activated α2aAR instead, though there was more than 1,000-fold more Gβ1 present at synapses. Despite the low abundance of Gβ4 at the membrane28, Gβ4 binding to α2aARs, as well as the exclusion of the highly abundant Gβ1, suggests a high specificity of this interaction. The numbers of receptors and effectors that specifically bind to unique Gβ and Gγ subunits may influence the abundance of certain Gβ and Gγ subunits at the membrane. For example, Gβ1 may be specific to other receptors that are more abundant than α2aARs at synaptic terminals. Further studies are needed to determine these specificities, but these findings suggest that each receptor may utilize a unique set of Gβγ dimers to finely regulate receptor-specific downstream signaling.
Moreover, we detected a minor interaction between Gγ12 and HA-α2aARs but not with auto-α2aARs (Figs 3 and 5E). Although Gγ12 was one of most abundant Gγ subunits at the membrane fraction in our previous study28, it was not specifically associated with auto-α2aARs, providing evidence for high specificity of the Gγ12 subunit at the hetero-α2aARs. This suggests a Gβ4γ12 dimer at hetero-α2aARs. In addition, Gβ5 showed no specific interaction with α2aARs (Figs 2 and 3D), which supports previous studies that demonstrate it preferentially forms a stable dimer with the RGS R7 subfamily in vivo to modulate postsynaptic Gαi–mediated signal transduction pathways20–24.
As previously addressed28, we experienced some technical challenges in detecting and quantifying Gγ subunits with this method. The amount of detected Gγ subunits was not similar to the amount of detected Gβ subunits. This difference may be due to the differences in peptide yield, which could stem from post–translational modifications, sample preparation artifacts, and differences in peptide re-solubilization efficiencies, all of which can lead to systematic errors in quantification62. Because of these, we are unable to calculate absolute protein quantities, but we can accurately determine the expression pattern of neuronal Gβ and Gγ subunits and compare within Gβ and Gγ subunits.
No evidence for pre-coupling of α2aAR GPCRs in vivo
The association of receptor and G protein prior to receptor activation (“pre-coupling”) has been suggested in some studies, but still remains unclear1,63–68. For example, in in vitro FRET assay, activated α2aARs were found to interact with Gβ132,33. However, in our study using synaptosomes from brain tissue, we do not see significant basal association between α2aARs and Gβ and Gγ. And we see only non-specific interaction between Gβ1 and α2aAR, even though it is highly abundant pre-synaptically. By contrast, we saw significant interactions of Gβ2 and Gβ4 with α2aARs, but only after epinephrine activation of α2aARs.
α2aAR autoreceptors vs. heteroreceptors
Our findings suggest that unique Gβγ combination may play specific roles in mediating interactions with receptors. We found different Gβ and Gγ subunits in FLAG-tagged autoreceptors as compared to total HA-tagged α2aARs. This suggests that Gβγ specificities to receptors may change based on the cell type and localization of receptors. We estimate Gβ and Gγ subunit interactions with hetero-α2aARs by subtraction of presynaptic autoreceptor-associated Gβs and Gγs from total HA-α2aAR-associated Gβs and Gγs, yielding the finding that Gβ2 may be auto-α2aAR specific, while Gβ4 may be hetero-α2aARs specific. For Gγ subunits, Gγ2, Gγ3 and Gγ4 were determined to be auto-α2aARs specific, while Gγ12 was hetero-α2aARs specific. (Table 1). Overall, hetero-α2aARs may associate with G protein heterotrimers paired with Gβ4γ12 to mediate hetero-α2aAR-specific phenotypes such as sedation and anesthetic sparing37. One difference between these two mice is that heteroreceptors may be found either pre- or post-synaptically, whereas autoreceptors are only pre-synaptic.
Table 1.
Gβ and Gγ specificities to hetero-α2aARs.
| G proteins | α2a ARs | Auto-α2a ARs | Hetero-α2a ARs (estimated) |
|---|---|---|---|
| Gβ2 | ++ | ++ | − |
| Gβ4 | + | − | + |
| Gγ2 | +++ | +++ | − |
| Gγ3 | ++ | ++ | − |
| Gγ4 | + | + | − |
| Gγ12 | + | − | + |
The number of + denotes abundance. +: interaction with receptor detected; −: no interaction was detected.
We were not able to separate these two populations of heteroreceptors to determine whether this localization makes a difference. We were able to compare the results of these two studies side-by-side as similar levels of proteins were detected for most Gβ and Gγ subunits, however, one limitation of our studies is that we were unable to determine the differences in co-IP efficiency of HA- and FLAG- antibodies and the number of receptors in digested samples to calculate the relative Gβ and Gγ enrichment with hetero-α2aARs. Again, future studies with refined methodologies are needed to determine the functional consequences of identified specificities.
Because HA-α2aARs represent both auto- and heteroreceptors and are found throughout the brain, we did not specify the neuronal type nor the location of receptors in the synaptosomes. Gβ2 and Gβ4 were previously identified to interact with α2aARs30, and in this study these Gβ subunits are identified to interact with Gγ2, Gγ3, Gγ4, Gγ12 subunits. The rank order of Gγ specificity to overall neuronal α2aARs is similar to the Gγs found in whole and fractionated synaptosomes in the previous study28. It still remains unclear which Gγ subunits associate with each Gβ subunit. Though the rules for specificity determination are unknown, we assume that multiple factors affect the specificity: the preference of these Gβ subunits for Gγ subunits, the localization of receptors, and effector availability. The protein abundance and location of Gγ subunits will affect the Gβγ dimerization and their specificity to α2aARs.
Gβ and Gγ subunit specificity to α2aARs studied in vitro
Numerous in vitro studies have attempted to determine the specificity of Gβγ dimerization and their selectivity in interacting with various GPCRs and effectors11,69,70. Similar to our observations, Gβ2, Gβ4, Gγ2, Gγ3, and Gγ4 were previously shown to be strongly associated with α2aARs32,71. Using FRET, Gibson and Gilman demonstrated that endogenous α2aARs preferentially stimulated Gαi1 heterotrimers paired with Gβ1 or Gβ4, and Gαi3 heterotrimers paired with Gβ232. They also found that Gβ2 association permitted 2-fold higher receptor activation, which was lost when Gβ2 was replaced with Gβ1. This result and our studies suggest that α2aARs with Gαi3β2γ heterotrimers may be most likely to be present at the in vivo synaptic terminals. Moreover, Gβ2γ and Gβ4γ dimers were determined to interact with adrenergic and opioid GPCRs, while Gβ1γ and Gβ3γ dimers, particularly Gβ1γ3 and Gβ3γ4, may preferentially couple with somatostatin and muscarinic M4 GPCRs29–31. However, no specificity was identified based on the localization of receptors. In addition to the identify of Gα and Gγ subunits, the localization of receptor may play a role in α2aAR selectivity of Gβ2 and Gβ4 over Gβ1. Depending on the localization of receptor, α2aARs may also preferentially interact with specific effectors. Based on our results and previous biochemical studies, Gβ2γ2, Gβ2γ3, and Gβ2γ4 may be auto-α2aARs specific, while Gβ4γ12 may be hetero-α2aARs specific.
Other in vitro G protein specificity studies71–74 depict a different Gβ and Gγ specificity than seen in our study. The gap between in vitro and in vivo detection of G protein specificity may be explained by tissue-specific determinants of specificity that are not present in heterologous expression systems, or difference in expression and availability of Gβ and Gγ subunits for in vitro studies. It is clear that Gβγ subunits are sticky, and this is why we provided multiple controls for non-specific effects. Future studies will be needed to address these differences.
Role of Gα subunits in determining Gβγ specificity to α2aAR receptors
In addition to Gβγ, Gα may also define the selectivity of Gi/o–coupled GPCRs such as α2aARs. Unlike Gαs, much less is known about how GPCRs selectively activate inhibitor Gαi1–3 and Gαo subunits. Recent cryo-electron microscopy (cryoEM) studies reporting the structures of Gi/o bound GPCRs, such as μ-opioid75, adenosine A176, 5HT1B77, and light receptor rhodopsin78, determine the interaction of these receptors with Gi or Go and suggest the conformational re-arrangements on the GPCR cytoplasmic site may affect the binding of specific G proteins. Interestingly, they found different interactions of Gi/o bound GPCRs and Gβ subunits79. However, the role of Gβγ in GPCRs-G protein specificity is unclear in these studies due to the modification of the proteins and the resolution of cryoEM structures. Moreover, the studies of GABAB heteromeric receptors with GABAB1 and GABAB2 have suggested hetero-dimerization of GPCRs may also affect the binding interactions of Gβγ with the receptor80,81. Further studies are needed to determine how Gα subunits affect the specificity of Gβγ.
As a Gi/o–coupled GPCR, α2aARs couple to Gαi1–3 and Gαo1–2. In a previous study by Richardson and Robishaw, Gαi-containing heterotrimers were highly coupled to α2aARs71. Further, Gαi subunits were demonstrated to mediate sedative anesthetic-sparing effects, but not inhibition of evoked release82, and Gαi1 were found to preferentially associate with Gβ1γ3 over Gβ1γ1 or Gβ1γ1071. This suggests that Gα−mediated selectivity additionally contributes to the specificity of α2aAR signaling through G proteins and their physiological functions. Further studies will be needed to understand the specific associations of Gα subunits with the Gβ and Gγ subunits observed here and their roles in known α2aAR-mediated physiological effects.
Conclusions
With the quantitative MRM method28, we now can further elucidate the in vivo Gβ and Gγ specificities to other GPCRs as well as Gβγ effectors, and validate previous in vitro studies of the Gβγ dimerization and their selectivity in interacting with various GPCRs and effectors11,69,70. In the CNS, numerous Gβ and Gγ subunits exhibit interesting subcellular localizations28,83. We do not yet fully understand the importance of these localizations and their physiological role, however. This study begins to piece together the puzzle why multiple different isoforms of Gβ and Gγ subunits exist. Further efforts and development of tools, such as knockout or tissue-specific knockout animals, will be needed to determine the specificity and roles of each unique Gβγ dimer in regulating various GPCR signaling cascades, and their impacts on neurological diseases and GPCR targeted drug mechanisms. Eventually this will allow us to determine how cells precisely regulate multiple downstream mechanisms to modulate signal intensity and specificity.
GPCR specificity to G proteins is defined by the Gα subunit preferred by a given GPCR. Whether GPCRs also have preference for Gβ and Gγ subunits is not well investigated. Here, we measured the in vivo specificity of presynaptic α2aARs to a subset of neuronal Gβ and Gγ subunits using a previously published proteomic approach. We found that Gβγ dimers, other than the most abundant Gβ1γ2, are also involved in α2aARs-mediated signaling cascades in vivo. In addition, auto- and hetero-α2aARs exhibit specificity to different Gβ and Gγ subunits. The variety of potential Gβγ dimers identified implies that the specificity of Gβγs to signaling pathways could be in part mediated through the receptors and their locations on particular types of neurons.
Materials and Methods
See supplementary for more details.
Animals
Adult, male HA- and FLAG-alpha2a adrenergic receptors (α2aARs), α2aARs knockout (KO), and wildtype mice37,52 were used. All animal handling and procedures were conducted in accordance with the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Vanderbilt Institutional Animal Care and Use Committee.
Drugs
Epinephrine (catalog E4642), prazosin (catalog P7791), and propranolol (catalog P0884) were purchased from Sigma-Aldrich.
Antibodies
Mouse anti-HA-agarose (Sigma, A2095), mouse anti-FLAG (Sigma, F3165) mouse anti-HA (Covance, 901514, 1:750), rabbit anti-FLAG (Sigma, F7425, 1:100), and rabbit anti-Gβ (Santa Cruz, sc-378, 1:10,000 and 1:5000) were used.
Synaptosome
Crude synaptosomes were isolated from mouse brain tissue, as described previously53,84,85 and stimulated with 100 µM epinephrine (epi). This mimics the local synaptic concentration of epinephrine and it is a commonly used concentration in alpha2a adrenergic receptor studies86–88. They were frozen in liquid nitrogen and stored at −80 °C.
Co-immunoprecipitation (Co-IP)
Crude synaptosomes were gently resuspended in 4 mL of RIPA buffer using a 25-gauge needle to lyse membranes and diluted to 1 mg/ml. Homogenates were centrifuged to separate the triton-soluble and insoluble fractions. Triton-soluble fractions were used for co-IP by incubating with either an anti-HA or FLAG antibody and Protein G agarose beads overnight. For elution, 100 µL of 1X sample buffer with DTT and 5% βME were used for HA-α2aARs and wildtype samples while 15.09 µg FLAG peptide was used for FLAG-α2aARs and α2aARs KO samples. Elutants were TCA precipitated and resuspended in 100 µL of 1x sample buffer with DTT and 5% βME. All samples were stored at −80 °C freezer for Western blot or MRM analysis.
Immunoblot analysis
To examine the results of IP, Western blot analysis was performed on equal volumes of input, co-IP, and supernatant samples using 10% SDS-PAGE gels. Using Western Lightning™ Chemiluminescence Reagent Plus (Perkin-Elmer) and Bio-rad Western blot imager, Western blots were developed.
Heavy labeled peptide cocktail
A heavy labeled peptide cocktail was made as described previously28.
Quantitative MRM of Gβ and Gγ subunits
Co-IP samples containing Gβ and Gγ subunits were separated, digested, and analyzed by a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific)28. To allow comparisons between G proteins co-IPed from multiple mice, quantitative Gβ and Gγ subunits detected (fmol) were normalized by the amount of protein (mg) used in co-IPs. The amount of protein used in co-IPs was calculated using the volume of precleared lysate used and the protein concentration of precleared lysate from BCA assay.
Statistical analysis
One-way analysis of variance (ANOVA) with a Tukey post hoc test was used to account for differences in protein expression of Gβ and Gγ subunits (*p < 0.05, **p < 0.01, ***p < 0.001). All statistical tests were performed using GraphPad Prism v.7.0 for Windows, (GraphPad Software, La Jolla, California, USA, www.graphpad.com).
Supplementary information
The in vivo specificity of synaptic Gβ and Gγ subunits to the α2a adrenergic receptor at CNS synapses
Acknowledgements
We thank the proteomics core of the Mass Spectrometry research Center for advice and technical assistance. This work was supported by the National Institutes of Health (EY10291, MH101679, MH081917, and T32 GM07628).
Author Contributions
Y.Y., K.B., W.H.M. and H.E.H. participated in research design. Y.Y., K.B., and W.H.M. conducted experiments. K.H., R.G., L.H., Y.C. and Q.W. contributed in mouse breeding and sampling. Y.Y. performed data analysis. Y.Y., W.H.M., K.B. and H.H. wrote or contributed to the writing of manuscript. All authors reviewed the results and approved the final version of the manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37222-1.
References
- 1.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nature reviews. Molecular cell biology. 2008;9:60. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 2.Eglen RM, Reisine T. New insights into GPCR function: implications for HTS. Methods in molecular biology. 2009;552:1–13. doi: 10.1007/978-1-60327-317-6_1. [DOI] [PubMed] [Google Scholar]
- 3.Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010;24:261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downes GB, Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt JD. Role of subunit diversity in signaling by heterotrimeric G proteins. Biochemical Pharmacology. 1997;54:325–339. doi: 10.1016/S0006-2952(97)00269-4. [DOI] [PubMed] [Google Scholar]
- 6.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 7.Dingus J, et al. G Protein betagamma dimer formation: Gbeta and Ggamma differentially determine efficiency of in vitro dimer formation. Biochemistry. 2005;44:11882–11890. doi: 10.1021/bi0504254. [DOI] [PubMed] [Google Scholar]
- 8.Dingus J, Hildebrandt JD. Synthesis and assembly of G protein betagamma dimers: comparison of in vitro and in vivo studies. Sub-cellular biochemistry. 2012;63:155–180. doi: 10.1007/978-94-007-4765-4_9. [DOI] [PubMed] [Google Scholar]
- 9.Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cellular and molecular life sciences: CMLS. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan K, Kalyanaraman V, Gautam N. Differential ability to form the G protein βγ complex among members of the β and γ subunit families. The Journal of biological chemistry. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]
- 11.Robishaw JD, Berlot CH. Translating G protein subunit diversity into functional specificity. Curr Opin Cell Biol. 2004;16:206–209. doi: 10.1016/j.ceb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Schwindinger WF, et al. Loss of G protein γ7 alters behavior and reduces striatal alpha(olf) level and cAMP production. The Journal of biological chemistry. 2003;278:6575–6579. doi: 10.1074/jbc.M211132200. [DOI] [PubMed] [Google Scholar]
- 13.Schwindinger WF, et al. Mice with Deficiency of G Protein γ3 Are Lean and Have Seizures. Mol. Cell. Biol. 2004;24:7758–7768. doi: 10.1128/MCB.24.17.7758-7768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwindinger WF, et al. Adenosine A2A Receptor Signaling and Golf Assembly Show a Specific Requirement for the γ7 Subtype in the Striatum. Journal of Biological Chemistry. 2010;285:29787–29796. doi: 10.1074/jbc.M110.142620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwindinger WF, et al. Synergistic roles for G-protein γ3 and γ7 subtypes in seizure susceptibility as revealed in double knockout mice. Journal of Biological Chemistry. 2011;287:7121–7133. doi: 10.1074/jbc.M111.308395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan SM, et al. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacological reviews. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 17.Pronin AN, Gautam N. Interaction between G-protein beta and gamma subunit types is selective. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6220–6224. doi: 10.1073/pnas.89.13.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang JJ, Cockett M, Khawaja XZ. Immunohistochemical localization of G protein beta1, beta2, beta3, beta4, beta5, and gamma3 subunits in the adult rat brain. Journal of neurochemistry. 1998;71:345–355. doi: 10.1046/j.1471-4159.1998.71010345.x. [DOI] [PubMed] [Google Scholar]
- 19.Hillenbrand M, Schori C, Schoppe J, Pluckthun A. Comprehensive analysis of heterotrimeric G-protein complex diversity and their interactions with GPCRs in solution. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E1181–1190. doi: 10.1073/pnas.1417573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachariou V, et al. Essential role for RGS9 in opiate action. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Fando A, Rodriguez-Munoz M, Sanchez-Blazquez P, Garzon J. Expression of neural RGS-R7 and Gbeta5 Proteins in Response to Acute and Chronic Morphine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005;30:99–110. doi: 10.1038/sj.npp.1300515. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GR, et al. R7BP complexes with RGS9-2 and RGS7 in the striatum differentially control motor learning and locomotor responses to cocaine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:1040–1050. doi: 10.1038/npp.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Psifogeorgou K, et al. A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:5617–5624. doi: 10.1523/JNEUROSCI.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuho I, Xie K, Martemyanov KA. Macromolecular composition dictates receptor and G protein selectivity of regulator of G protein signaling (RGS) 7 and 9-2 protein complexes in living cells. The Journal of biological chemistry. 2013;288:25129–25142. doi: 10.1074/jbc.M113.462283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cellular and molecular life sciences: CMLS. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, et al. Identification of protein-protein interactions and topologies in living cells with chemical cross-linking and mass spectrometry. Molecular & cellular proteomics: MCP. 2009;8:409–420. doi: 10.1074/mcp.M800232-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens GJ. G-protein-coupled-receptor-mediated presynaptic inhibition in the cerebellum. Trends Pharmacol Sci. 2009;30:421–430. doi: 10.1016/j.tips.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Yim YY, et al. Quantitative Multiple-Reaction Monitoring Proteomic Analysis of Gbeta and Ggamma Subunits in C57Bl6/J Brain Synaptosomes. Biochemistry. 2017;56:5405–5416. doi: 10.1021/acs.biochem.7b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosohata K, et al. The role of the G protein gamma(2) subunit in opioid antinociception in mice. European journal of pharmacology. 2000;392:R9–R11. doi: 10.1016/S0014-2999(00)00132-1. [DOI] [PubMed] [Google Scholar]
- 30.Asano T, Morishita R, Ueda H, Kato K. Selective association of G protein beta(4) with gamma(5) and gamma(12) subunits in bovine tissues. The Journal of biological chemistry. 1999;274:21425–21429. doi: 10.1074/jbc.274.30.21425. [DOI] [PubMed] [Google Scholar]
- 31.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 32.Gibson SK, Gilman AG. Gi alpha and G beta subunits both define selectivity of G protein activation by alpha 2-adrenergic receptors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:212–217. doi: 10.1073/pnas.0509763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson M, Robishaw JD. The alpha(2A)-adrenergic receptor discriminates between G(i) heterotrimers of different beta gamma subunit composition in Sf9 insect cell membranes. Journal of Biological Chemistry. 1999;274:13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- 34.Krumins AM, Gilman AG. Targeted knockdown of G protein subunits selectively prevents receptor-mediated modulation of effectors and reveals complex changes in non-targeted signaling proteins. The Journal of biological chemistry. 2006;281:10250–10262. doi: 10.1074/jbc.M511551200. [DOI] [PubMed] [Google Scholar]
- 35.Bylund DB, et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacological reviews. 1994;46:121–136. [PubMed] [Google Scholar]
- 36.Bylund, David B. et al. The alpha-2 Adrenergic Receptors. (Humana Press 1988, 1988).
- 37.Gilsbach R, Hein L. Are the pharmacology and physiology of alpha(2) adrenoceptors determined by alpha(2)-heteroreceptors and autoreceptors respectively? British journal of pharmacology. 2012;165:90–102. doi: 10.1111/j.1476-5381.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daunt DA, et al. Subtype-specific intracellular trafficking of alpha2-adrenergic receptors. Molecular pharmacology. 1997;51:711–720. doi: 10.1124/mol.51.5.711. [DOI] [PubMed] [Google Scholar]
- 39.Gannon, M. & Wang, Q. In Encyclopedia of Signaling Molecules (ed. Choi, Sangdun) 1–4 (Springer New York, 2016).
- 40.Gyires K, Zadori ZS, Torok T, Matyus P. alpha(2)-Adrenoceptor subtypes-mediated physiological, pharmacological actions. Neurochemistry international. 2009;55:447–453. doi: 10.1016/j.neuint.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Szabadi E. Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol. 2013;27:659–693. doi: 10.1177/0269881113490326. [DOI] [PubMed] [Google Scholar]
- 42.Gobert A, Billiras R, Cistarelli L, Millan MJ. Quantification and pharmacological characterization of dialysate levels of noradrenaline in the striatum of freely-moving rats: release from adrenergic terminals and modulation by alpha(2)-autoreceptors. J Neurosci Meth. 2004;140:141–152. doi: 10.1016/j.jneumeth.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 43.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 44.Gribble FM. α2A-adrenergic receptors and type 2 diabetes. N Engl J Med. 2010;362:361–362. doi: 10.1056/NEJMcibr0911499. [DOI] [PubMed] [Google Scholar]
- 45.Comings DE, et al. Association between the adrenergic alpha(2A) receptor gene (ADRA2A) and measures of irritability, hostility, impulsivity and memory in normal subjects. Psychiatr Genet. 2000;10:39–42. doi: 10.1097/00041444-200010010-00007. [DOI] [PubMed] [Google Scholar]
- 46.Wakeno M, et al. The alpha 2A-adrenergic receptor gene polymorphism modifies antidepressant responses to milnacipran. J Clin Psychopharm. 2008;28:518–524. doi: 10.1097/JCP.0b013e31818455fc. [DOI] [PubMed] [Google Scholar]
- 47.Davies MF, et al. Augmentation of the noradrenergic system in alpha-2 adrenergic receptor deficient mice: anatomical changes associated with enhanced fear memory. Brain Res. 2003;986:157–165. doi: 10.1016/S0006-8993(03)03248-7. [DOI] [PubMed] [Google Scholar]
- 48.Marrs W, Kuperman J, Avedian T, Roth RH, Jentsch JD. Alpha-2 adrenoceptor activation inhibits phencyclidine-induced deficits of spatial working memory in rats. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005;30:1500–1510. doi: 10.1038/sj.npp.1300700. [DOI] [PubMed] [Google Scholar]
- 49.Gilsbach R, et al. Genetic dissection of alpha2-adrenoceptor functions in adrenergic versus nonadrenergic cells. Molecular pharmacology. 2009;75:1160–1170. doi: 10.1124/mol.109.054544. [DOI] [PubMed] [Google Scholar]
- 50.Philipp M, Brede M, Hein L. Physiological significance of α(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- 51.Philipp M, Hein L. Adrenergic receptor knockout mice: distinct functions of 9 receptor subtypes. Pharmacology & therapeutics. 2004;101:65–74. doi: 10.1016/j.pharmthera.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Lu RJ, et al. Epitope-tagged Receptor Knock-in Mice Reveal That Differential Desensitization of alpha(2)-Adrenergic Responses Is because of Ligand-selective Internalization. Journal of Biological Chemistry. 2009;284:13233–13243. doi: 10.1074/jbc.M807535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betke KM, et al. Differential localization of G protein betagamma subunits. Biochemistry. 2014;53:2329–2343. doi: 10.1021/bi500091p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blackmer T, et al. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nature neuroscience. 2005;8:421–425. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- 55.Blackmer T, et al. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 56.Yoon EJ, Gerachshenko T, Spiegelberg BD, Alford S, Hamm HE. Gbg interferes with Ca2+-dependent binding of synaptotagmin to the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. Mol. Pharmacol. 2007;72:1210–1219. doi: 10.1124/mol.107.039446. [DOI] [PubMed] [Google Scholar]
- 57.Wells CA, et al. Gbetagamma inhibits exocytosis via interaction with critical residues on soluble N-ethylmaleimide-sensitive factor attachment protein-25. Molecular pharmacology. 2012;82:1136–1149. doi: 10.1124/mol.112.080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown DA, Sihra TS. Presynaptic signaling by heterotrimeric G-proteins. Handb Exp Pharmacol. 2008;184:207–260. doi: 10.1007/978-3-540-74805-2_8. [DOI] [PubMed] [Google Scholar]
- 59.Herlitze S, et al. Modulation of Ca2+ channels by G-protein bg subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 60.Michaeli A, Yaka R. Dopamine inhibits GABAA currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience. 2010;165:1159–1169. doi: 10.1016/j.neuroscience.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 61.Fasshauer D. Structural insights into the SNARE mechanism. Biochimica et biophysica acta. 2003;1641:87–97. doi: 10.1016/S0167-4889(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 62.Hoofnagle AN, et al. Recommendations for the Generation, Quantification, Storage, and Handling of Peptides Used for Mass Spectrometry-Based Assays. Clinical chemistry. 2016;62:48–69. doi: 10.1373/clinchem.2015.250563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neubig RR, Gantzos RD, Thomsen WJ. Mechanism of agonist and antagonist binding to alpha 2 adrenergic receptors: evidence for a precoupled receptor-guanine nucleotide protein complex. Biochemistry. 1988;27:2374–2384. doi: 10.1021/bi00407a019. [DOI] [PubMed] [Google Scholar]
- 64.Lohse MJ, et al. Kinetics of G-protein-coupled receptor signals in intact cells. British journal of pharmacology. 2008;153(Suppl 1):S125–132. doi: 10.1038/sj.bjp.0707656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin K, Dong C, Wu G, Lambert NA. Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers. Nat Chem Biol. 2011;7:740–747. doi: 10.1038/nchembio.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ayoub MA, et al. Real-time analysis of agonist-induced activation of protease-activated receptor 1/Galphai1 protein complex measured by bioluminescence resonance energy transfer in living cells. Molecular pharmacology. 2007;71:1329–1340. doi: 10.1124/mol.106.030304. [DOI] [PubMed] [Google Scholar]
- 67.Hein P, Bunemann M. Coupling mode of receptors and G proteins. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:435–443. doi: 10.1007/s00210-008-0383-7. [DOI] [PubMed] [Google Scholar]
- 68.Vilardaga JP, et al. GPCR and G proteins: drug efficacy and activation in live cells. Mol Endocrinol. 2009;23:590–599. doi: 10.1210/me.2008-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleuss C, et al. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 70.Albert PR, Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/S0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 71.Richardson M, Robishaw JD. The alpha2A-adrenergic receptor discriminates between Gi heterotrimers of different betagamma subunit composition in Sf9 insect cell membranes. The Journal of biological chemistry. 1999;274:13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- 72.Hou Y, Azpiazu I, Smrcka A, Gautam N. Selective role of G protein gamma subunits in receptor interaction. The Journal of biological chemistry. 2000;275:38961–38964. doi: 10.1074/jbc.C000604200. [DOI] [PubMed] [Google Scholar]
- 73.Hou Y, Chang V, Capper AB, Taussig R, Gautam N. G Protein beta subunit types differentially interact with a muscarinic receptor but not adenylyl cyclase type II or phospholipase C-beta 2/3. The Journal of biological chemistry. 2001;276:19982–19988. doi: 10.1074/jbc.M010424200. [DOI] [PubMed] [Google Scholar]
- 74.McIntire WE, MacCleery G, Garrison JC. The G protein beta subunit is a determinant in the coupling of Gs to the beta 1-adrenergic and A2a adenosine receptors. The Journal of biological chemistry. 2001;276:15801–15809. doi: 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- 75.Koehl, A. et al. Structure of the micro-opioid receptor-Gi protein complex. Nature (2018). [DOI] [PMC free article] [PubMed]
- 76.Draper-Joyce, C. J. et al. Structure of the adenosine-bound human adenosine A1 receptor-Gi complex. Nature (2018). [DOI] [PubMed]
- 77.Garcia-Nafria J, Nehme R, Edwards PC, Tate CG. Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature. 2018;558:620–623. doi: 10.1038/s41586-018-0241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang, Y. et al. Cryo-EM structure of human rhodopsin bound to an inhibitory G protein. Nature (2018). [DOI] [PMC free article] [PubMed]
- 79.Capper MJ, Wacker D. How the ubiquitous GPCR receptor family selectively activates signalling pathways. Nature. 2018;558:529–530. doi: 10.1038/d41586-018-05503-4. [DOI] [PubMed] [Google Scholar]
- 80.Margeta-Mitrovic M, Jan YN, Jan LY. Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14649–14654. doi: 10.1073/pnas.251554498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pin JP, et al. Activation mechanism of the heterodimeric GABA(B) receptor. Biochem Pharmacol. 2004;68:1565–1572. doi: 10.1016/j.bcp.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 82.Albarran-Juarez J, et al. Modulation of alpha2-adrenoceptor functions by heterotrimeric Galphai protein isoforms. J Pharmacol Exp Ther. 2009;331:35–44. doi: 10.1124/jpet.109.157230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Betke, K. M. Investigating The Role of Gprotein βγ Specificity In Modulation of Synaptic Transmission Doctor of Philosophy thesis, Vanderbilt University (2014).
- 84.Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. Journal of anatomy. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 85.Whittaker VP, Michaelson IA, Kirkland RJ. The separation of synaptic vesicles from nerve-ending particles (‘synaptosomes’) The Biochemical journal. 1964;90:293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brady AE, et al. Alpha 2-adrenergic agonist enrichment of spinophilin at the cell surface involves beta gamma subunits of Gi proteins and is preferentially induced by the alpha 2A-subtype. Molecular pharmacology. 2005;67:1690–1696. doi: 10.1124/mol.104.005215. [DOI] [PubMed] [Google Scholar]
- 87.Wang Q, et al. Spinophilin Blocks Arrestin Actions in Vitro and in Vivo at G Protein-Coupled Receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- 88.Wang Q, Limbird LE. Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. The Journal of biological chemistry. 2002;277:50589–50596. doi: 10.1074/jbc.M208503200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The in vivo specificity of synaptic Gβ and Gγ subunits to the α2a adrenergic receptor at CNS synapses
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).