Abstract
The seasonal timing of recurring biological processes is essential for organisms living in temperate regions. While ample knowledge of these processes exists for terrestrial environments, seasonal timing in the marine environment is relatively understudied. Here, we characterized the annual rhythm of habitat use in six fish species belonging to the Sparidae family, highlighting the main environmental variables that correlate to such rhythms. The study was conducted at a coastal artificial reef through a cabled observatory system, which allowed gathering underwater time-lapse images every 30 minutes consecutively over 3 years. Rhythms of fish counts had a significant annual periodicity in four out of the six studied species. Species-specific temporal patterns were found, demonstrating a clear annual temporal niche partitioning within the studied family. Temperature was the most important environmental variable correlated with fish counts in the proximity of the artificial reef, while daily photoperiod and salinity were not important. In a scenario of human-induced rapid environmental change, tracking phenological shifts may provide key indications about the effects of climate change at both species and ecosystem level. Our study reinforces the efficacy of underwater cabled video-observatories as a reliable tool for long-term monitoring of phenological events.
Introduction
In temperate regions, characterized by strong seasonality, the annual temporal organization of biological processes (i.e. phenology) provides evident ecological advantages1,2. One of the mechanisms governing annual rhythms implies the existence of an internal time-keeping mechanism (or circannual clock) that is able to synchronize with external cues in order to cope with, and anticipate, predictable changes in the environment3. Photoperiod is the most important proximate variable controlling phenology in animals but a certain degree of plasticity allows non-photoperiodic variables to modulate annual timing programs, to cope with unpredictable changes of environment. For example, the duration of the photophase is the principal environmental variable controlling full gonadal maturation in birds, but temperature, rainfall, and food contribute to the fine tuning of the process4. The regulation of phenology is not well known in marine organisms, and much less studied, compared to terrestrial ones5,6. For example, photoperiod in combination with temperature and food availability seem to have a synergistic effect on the synchronization of annual biological processes in fish7,8. Moreover, investigations in situ are rarely performed, due to the technical and pragmatic difficulties of surveying the marine environment at high frequency and large temporal scales.
Several authors have already stressed that the ultimate significance of biological rhythms cannot be completely understood by presenting a single variable to individuals maintained in the laboratory, because the observed response may not be normally expressed, or relevant to fitness, as in the wild3,9,10. Studying species in their natural ecosystems allows exploring the temporal modulation of biological processes in the presence of photoperiodic and non-photoperiodic signals, as well as under intra- and interspecific competition11,12. Accordingly, expanding the study of biological rhythms to natural contexts and to multiple species allows a comprehensive understanding of evolutionary mechanisms of phenology13. Such an approach is complementary to more mechanistic ones in the laboratory and it may add new perspectives to track the effects of key environmental drivers such as temperature, photoperiod, and salinity on phenology of marine species. This knowledge is of particular relevance today, to better understand how marine organisms are responding to climate change14,15, with cascade effects influencing both community structures and ecosystems’ functioning (for a review see11).
In the last two decades, there have been substantial developments in telemetry systems, allowing continuous long-term tracking of aquatic animals16–18. At the same time, multiparametric video-platforms cabled to shore for continuous data transmission and powering (i.e. cabled observatories) have become an effective tool to investigate underwater ecosystems19–22. Although the spatial area that cabled observatories can monitor is limited, they have the advantage to enable monitoring specific habitats for very long periods of time23,24 and in automatic way22. In this context, the coastal Seafloor Observatory OBSEA25, has already demonstrated its value for the video-monitoring of daily activity rhythms of fishes at an artificial reef located in north western Mediterranean Sea, 4 Km off the coast and at a depth of 21 m22,26,27.
Seasonality in the Mediterranean Sea is well marked and the reproductive timing of coastal rocky fishes is correlated with seasonal changes of photoperiod, temperature, and salinity28. One of the most abundant taxa in the Mediterranean coastal fish assemblages are those belonging to sea breams, family Sparidae29,30. They are also commonly observed at artificial reefs31 and have a great commercial value in fishery32. Despite the fact that some of the species belonging to the Sparidae family have been observed to use artificial reefs differently throughout the year31, knowledge about the environmental variables that affect annual changes in species presence is limited31. Here, we aimed to identify how water temperature, daily photoperiod, and salinity correlate with the annual habitat use of an artificial reef by six species belonging to the Sparidae family by using 30-minute time-lapse images shot at the OBSEA cabled observatory over a three year period. The artificial reef is located in north western Mediterranean Sea, 4 Km off the coast and at a depth of 21 m. The six selected species were: the common dentex, Dentex dentex (Linnaeus, 1758), the white seabream, Diplodus sargus (Linnaeus, 1758), the two-banded seabream, Diplodus vulgaris (Geoffroy Saint-Hilaire, 1817), the annular seabream, Diplodus annularis (Linnaeus, 1758), the sharpsnout seabream, Diplodus puntazzo (Walbaum, 1792), and the zebra seabream, Diplodus cervinus (Lowe, 1838).
Materials and Methods
Data collection
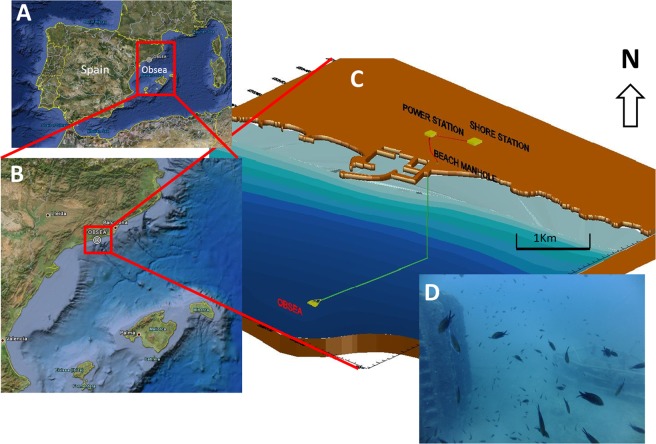
The Western Mediterranean Expandable SEAfloor Observatory (OBSEA; www.obsea.es) is a cabled video-platform located at a depth of 21 m, 4 km off Vilanova i la Geltrú, Spain (41°10′54″ N; 01°45′08″ E, geodetic datum WGS84; see Fig. 1A). The observatory is equipped with an underwater camera (OceanOptic Cam) that can store online all acquired time-lapse images. The OBSEA is also equipped with a custom developed LED lighting system to allow shooting at night. There are two light sources located beside the camera at 1 m distance from each other. Each source has one LED emitting 2900 lumen with an angle of 120°. The light has a color temperature of 2700 kelvin (for more details see25). The OBSEA is placed in front of an artificial reef at a distance of 3.5 m.
Figure 1.
Location of the OBSEA platform in the North Western Mediterranean Sea (A,B,C) Indicates the location of the platform off the harbor of Vilanova i la Geltrú (Spain). (D) Depicts the view of the platform (right) and part of the artificial reef (left). Satellite images (A,B) have been obtained on google maps (©2018 Google, Inst. Geogr., last accessed on 16 August 2018).
We acquired images every 30 min during 3 years (2012–2014), preserving the same field of view centered on the artificial reef (Fig. 1D). Shooting at night was carried out by switching on the lighting system just before the shooting of the camera, and switching off immediately after (total time of light-on was at about 30 s; for more details see27). Temperature and salinity were measured by the CTD probe installed aside the camera. Global irradiance (W m−2) has been retrieved from a nearby meteorological station at Sant Pere de Ribes, 6 km away from the OBSEA (http://www.obsea.es).
Statistical analysis
The acquired images were manually analyzed by a trained operator to count all the individuals belonging to the 6 target species. The final matrix of daily abundances and averaged environmental data was used for the statistical analysis and it was composed by: one dependent variable (daily sum of fish counts); three fixed effects (mean daily water temperature, mean daily salinity, and daily hours of light received at the meteorological station). We investigated which environmental variable was related the most with the changes in fish counts as a proxy of annual rhythms in their habitat use, through Generalized Additive Models for Location, Scale, and Shape (GAMLSS). GAMLSS offers the possibility to select the distribution for the response variable from a very general family of distributions, including highly skewed or kurtotic continuous and discrete distributions. This fits well with the distribution of our dependent variable that is a count variable33. We looked at the frequency distribution of the count data of each of the 6 species (Fig. S1), then we used a null model to test three preselected family distributions (Negative Binomial, NBI; Zero-Inflated Poisson, ZIP; Poisson distribution, PO). Afterwards, we used the results of the model to assess the best fitting distribution comparing the Akaike’s Information Criterion (AIC)34 and examining normality of residuals by plotting theoretical quantiles versus standardized residuals (Q–Q plots) (Table S1). According to the results, we decided to use the NBI family distribution for all the species. The potential temporal autocorrelation was incorporated using Generalized Auto-Regressive Moving Average models (GARMA) with “garmaFit” command in “gamlss.utils” library33. We used a null model to test different combination of GARMA structure (See Table S1). Then, we used the results of the model to assess the best autocorrelation structure using AIC and examining the presence of autoregressive conditional heteroscedasticity by examining Auto-Correlation Function plots (ACF) of squared residuals from the models (Fig S2). According to the results, we decided the best autocorrelative structure for each species-specific model (Table S2).
Since the number of photos counted for each day was different due to technical difficulties (e.g. transient turbidity or camera malfunctioning, see Table S3), we used the logarithm of number of photo counted for each day as an offset variable in the model equation. Prior to analysis, multicollinearity among predictors in the GAMLSS models was tested, using Variance Inflation Factor (VIF). The estimated VIF showed low levels of collinearity among predictors (VIF < 5 for all the cases). Thus, all the predictors were included in the GAMLSS models. We first tested whether the 3-years’ fish count time series indicated an annual periodicity, by implementing a null model with presence/absence of the independent variable periodicity (a vector from 1 to 12 representing the months of the year). In a second step, we implemented all the possible models (n = 8) combining presence/absence of the fixed effects except periodicity (Table S4). In both cases, the AIC was used to assess models’ performance. In addition, we computed Akaike’s weight (wi) for each candidate model (Franklin et al., 2001) through its computed AIC and the Δ values. The weights’ range between 0 and 1 has been interpreted as the weights of evidence in favor of model i as the best model among the set of all candidate models examined35. Finally, the models with the smallest AIC and the higher wi values were chosen as the models that best represented the data. In cases where the top models had close convergence (models that did not exceed 0.5 of AIC weight) we implemented a model averaging process to calculate the Relative Importance (RI) of the explicative variables. For this, we used the models that constitute a cumulative AIC weight of 0.9536. We considered an RI around 0.9 as a strong explanatory variable, around 0.9–0.6 as moderate and less than 0.6 as weak36. All analyses were conducted in R 3.3.137.
Results
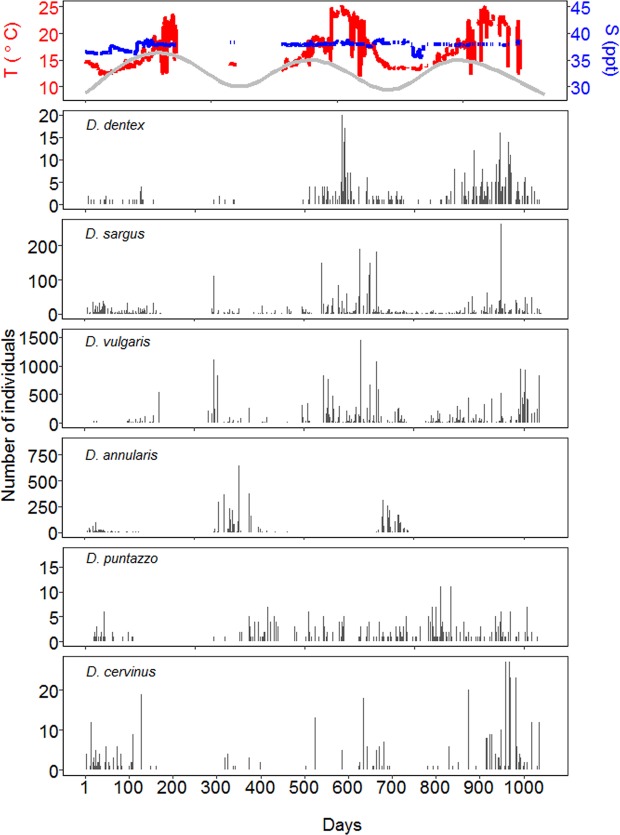
The number of recorded images during the 3-year sampling was 52,609, while those used for species counting were 40,989 (78%). The other photos were of poor quality and were discarded (see Table S3). The total counts for all the targeted species was 125,239: D. vulgaris (89,767); D. annularis (20,577); D. sargus (11,420); D. dentex (1,359); D. cervinus (1,119); D. puntazzo (997). These 6 species together represented 58% of the total fish counts recorded (data for other species not shown).
Modelling results on rhythmicity revealed significant annual oscillations in four out of the six species studied (Table 1) and peaks of fish counts were registered between August and December (Figs 2 and S3). Specifically, D. dentex counts reached their maximum in August; D. vulgaris and D. sargus counts peaked in October and D. annularis in December (Figs 2 and S3). Differently, the abundance of D. cervinus and D. puntazzo did not show clear annual rhythmicity (Figs 2 and S3).
Table 1.
The results of the models used to test periodicity of fish counts in each of the six species.
| Species | Df | Period | AICi | ∆i | w i |
|---|---|---|---|---|---|
| D. dentex | 9 | + | 1719.1 | 0.0 | 0.982 |
| 8 | — | 1727.1 | 8.0 | 0.018 | |
| D. vulgaris | 5 | + | 5022.1 | 0.0 | 1 |
| 4 | — | 5104.0 | 82.0 | <0.001 | |
| D. sargus | 5 | + | 3469.4 | 0.0 | 1 |
| 4 | — | 3500.2 | 30.8 | <0.001 | |
| D. annularis | 7 | + | 1408.8 | 0.0 | 0.8 |
| 6 | — | 1411.6 | 2.8 | 0.2 | |
| D. puntazzo | 7 | — | 1803.1 | 0.0 | 1 |
| 8 | + | 1850.3 | 47.2 | <0.001 | |
| D. cervinus | 5 | — | 1307.2 | 0.0 | 0.8 |
| 6 | + | 1310.0 | 2.8 | 0.2 |
Df indicates degrees of freedom. +/− indicates the presence/absence of the smoothing effect of annual periodicity. The Akaike’s Information Criterion (AICi), the Akaike’s weight (wi) and the Δi values are reported to show the selection information criteria. The models used are highlighted in bold.
Figure 2.
Time series of the daily environmental conditions and the number of individuals counted at the artificial reef according to the days of the three-years study presented here. The plot at the top provides the information on temperature of the water (red), salinity (blue) and daily photoperiod, which is not scaled but ranges from 9 to 16.5 hours (grey line). The bars in the other plots represent the daily counts (number of individuals counted) of each of the six species at the artificial reef.
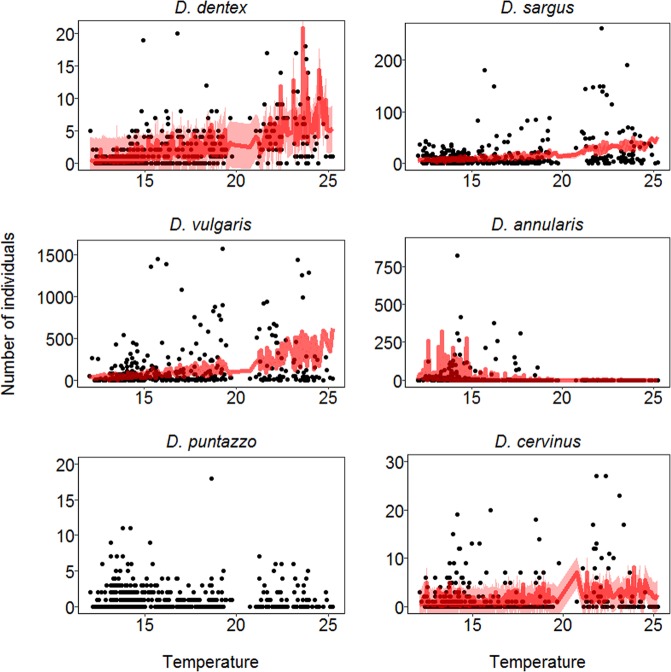
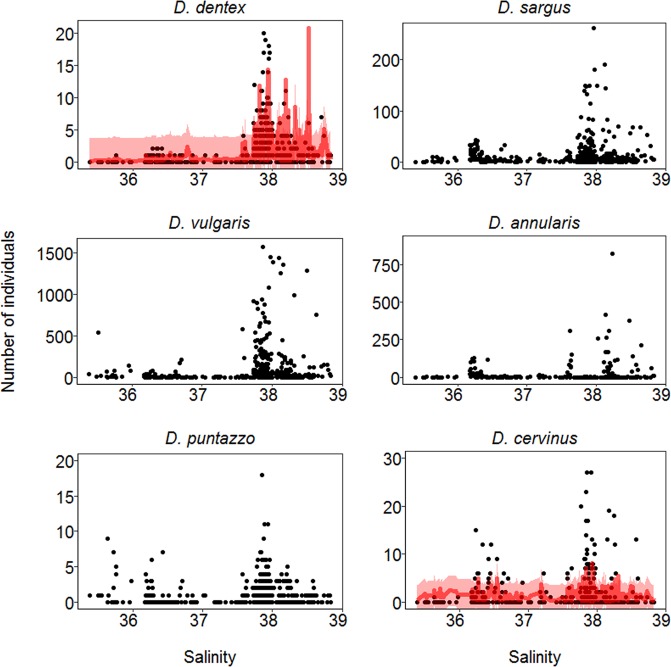
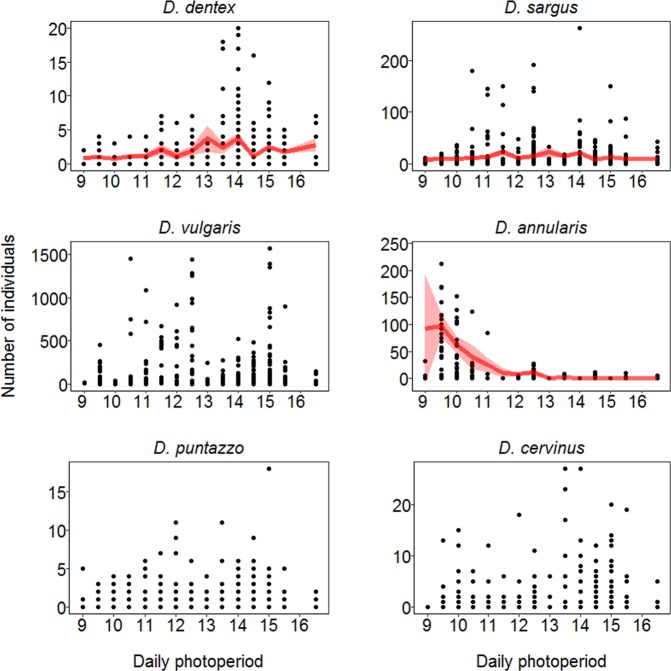
Modelling results indicated that temporal variations in fish counts were related with different variables, except for D. puntazzo counts where none of the fixed effects tested were important (See Table 2). According to the wi and AICi values, the model that best described counts of D. dentex was the one incorporating all the smoothing factors: temperature (Fig. 3 and Table 2), salinity (Fig. 4 and Table 2), and daily photoperiod (Fig. 5 and Table 2). Counts of D. dentex started to increase when the water temperature was above 20 °C, with a clear peak when the salinity was at about 38 ppt and daily photoperiod was at about 14 hours (August; see Fig. S3). In the case of D. sargus, temperature was the most important smoothing factor (Fig. 3 and Table 2), but the daily photoperiod also indicated a weak (RI = 0.67) smoothing effect (Fig. 5 and Table 2). Counts of D. sargus steadily increased from 19 to 25 °C. The model that best described counts of D. vulgaris incorporated only temperature as smoothing factor (Fig. 3 and Table 2) and counts of this species indicated a similar pattern to the one of D. dentex. Then, counts of D. annularis indicated a significant smoothing effect of temperature (Fig. 3 and Table 2) and daily photoperiod (Fig. 5 and Table 2). The presence of the species at the artificial reef was higher at temperatures below 16 °C and with daily photoperiod around 10 hours (December). Finally, the counts of D. cervinus indicated a significant smoothing effect of temperature (Fig. 3 and Table 2) with a similar pattern to the one showed by D. sargus and a weak effect of salinity (Fig. 4 and Table 2).
Table 2.
The results of the models used to test the effect of temperature, salinity and daily photoperiod on the fish counts.
| Species | Model | Df | Temperature | Salinity | Photoperiod | AICi | ∆i | w i | Rsq |
|---|---|---|---|---|---|---|---|---|---|
| D. dentex | m1 | 11 | + | + | + | 1627.1 | 0.0 | 0.99 | 0.33 |
| m3 | 10 | + | + | — | 1637.7 | 10.6 | <0.01 | 0.29 | |
| m2 | 10 | + | — | + | 1640.7 | 13.6 | <0.01 | 0.32 | |
| m6 | 10 | — | + | + | 1641.4 | 14.1 | <0.01 | 0.20 | |
| D. sargus | m1 | 7 | + | + | + | 3429.5 | 0.0 | 0.36 | 0.19 |
| m4 | 6 | + | — | + | 3430.5 | 0.3 | 0.31 | 0.19 | |
| m3 | 6 | + | + | — | 3431.9 | 1.0 | 0.22 | 0.18 | |
| m7 | 5 | + | — | — | 3430.5 | 2.4 | 0.11 | 0.18 | |
| RI | 0.90 | <0.6 | 0.67 | ||||||
| D. vulgaris | m5 | 5 | + | — | — | 5037.9 | 0.0 | 0.40 | 0.21 |
| m3 | 6 | + | + | — | 5038.4 | 0.4 | 0.32 | 0.20 | |
| m4 | 6 | + | — | + | 5039.8 | 1.9 | 0.16 | 0.20 | |
| m1 | 7 | + | + | + | 5040.3 | 2.3 | 0.12 | 0.20 | |
| RI | 0.99 | <0.6 | <0.6 | ||||||
| D. annularis | m4 | 9 | + | — | + | 1644.2 | 0.0 | 0.64 | 0.33 |
| m1 | 10 | + | + | + | 1645.4 | 1.2 | 0.36 | 0.32 | |
| m5 | 8 | + | — | — | 1679.0 | 34.8 | <0.01 | 0.14 | |
| m3 | 9 | + | + | — | 1680.7 | 36.6 | <0.01 | 0.27 | |
| D. puntazzo | m8 | 6 | — | — | — | 1411.2 | 0.0 | 0.64 | — |
| m6 | 7 | — | — | + | 1412.4 | 1.3 | 0.34 | 0.05 | |
| m7 | 7 | — | + | — | 1420.4 | 9.2 | <0.01 | 0.03 | |
| m4 | 8 | + | — | + | 1420.9 | 9.7 | <0.01 | 0.03 | |
| D. cervinus | m3 | 7 | + | + | — | 1303.7 | 0.0 | 0.63 | 0.04 |
| m1 | 8 | + | + | + | 1305.2 | 1.7 | 0.27 | 0.07 | |
| m8 | 5 | — | — | — | 1308.9 | 5.4 | 0.04 | — | |
| m7 | 6 | + | — | — | 1309.6 | 6.1 | 0.03 | 0.03 |
The best four out of eight (see Table S4) models are reported. Df represents the degrees of freedom. +/— indicates the presence/absence of the smoothing effect of the variables. The Akaike’s Information Criterion (AICi), the Akaike’s weight (wi) and the Δi values are reported to show the selection information criteria. Rsq represents the pseudo R-squared, while RI represents the Relative Importance of the explicative variables. In cases where the top models had close convergence (wi < 0.5) we implemented a model averaging process to calculate the RI of the explicative variables. Strong or moderate influences of explanatory variables are highlighted in bold.
Figure 3.
The output of the modeling results according to the best fitting model in Table 2. The points represent the daily counts (number of individuals counted) of the six species at the artificial reef. Each species is represented in relation to daily average of water temperature. The red line represents median prediction (which equals the mean for Negative Binomial I family distribution) together with the 95% confidence interval (red shadowed area).
Figure 4.
The output of the modeling results according to the best fitting model in Table 2. The points represent the daily counts (number of individuals counted) of the six species at the artificial reef. Each species is represented in relation to daily average of water salinity. The red line represents median prediction (which equals the mean for Negative Binomial I family distribution) together with the 95% confidence interval (red shadowed area).
Figure 5.
The output of the modeling results according to the best fitting model in Table 2. The points represent the daily counts (number of individuals counted) of the six species at the artificial reef. Each species is represented in relation to the daily photoperiod. The red line represents median prediction (which equals the mean for Negative Binomial I family distribution) together with the 95% confidence interval (red shadowed area).
Discussion
Here, we characterized the annual rhythms of habitat use in six fish species belonging to the Sparidae family at an artificial reef in the Western Mediterranean Sea by using fish counts at an underwater cabled observatory throughout three years. Fish counts were used as a proxy of habitat used indicating that only four out of the six studied species had significant annual rhythms at the artificial reef. Moreover temporal variations were correlated with different environmental variables, with temperature showing stronger relation with fish counts than salinity and daily photoperiod. Results for each species were discussed comparing our method with telemetry studies and other methodological approaches previously used. Moreover, we interpreted the annual patterns in the context of trophic ecology and reproductive timing for each species. Finally, we highlighted the importance of our results for long-term monitoring of phenology in the context of climate change.
Counts of the common dentex (D. dentex) were significantly correlated to water temperature, salinity, and daily photoperiod with a clear peak in August. This observed pattern is also supported by recent telemetry studies in the NW Mediterranean Sea (at about 200 km north respect to the location of the OBSEA), which highlighted how this species has a clear preference for the suprathermoclinal warm water over the colder layer below the thermocline38. The common dentex is a predator occupying a high trophic level in coastal trophic niches, and mainly feeds on other coastal fishes and cephalopods39–41. The observed peak around August could be related to food availability around the artificial reef or to water temperature that increases metabolic rate and swimming performance of D. dentex42, and so the probability of being detected by the video monitoring. Finally, our results suggest that the artificial reef is not an important habitat for the spawning of the species that usually occur between March-June41.
Counts of the white sea bream (D. sargus) were mainly correlated to water temperature and peaked in October. Interestingly, during a telemetry study Aspillaga, et al.43 observed D. sargus performing movements to deeper areas (>20 m) between November-December and March-April. Such behavior was related to the avoidance of stormy conditions in shallow water (November-December), or to reproductive spawning aggregation (between March-May44). Our results suggest that the species’ counts decrease at the artificial reef (probably due to displacements of individuals to deeper spawning grounds) between March and May. Interestingly, the average ordinary home-range for this species is less than one square km43,45,46 and it could be even smaller in the presence of an artificial reef47. Those structures have been demonstrated to be important feeding sites for the omnivorous D. sargus48, which mainly feed on bivalves, echinoderms, and algae29. Thus, the artificial reef is likely used as a foraging ground, supporting individuals’ dietary requirements, just before the onset of their spawning period.
The counts of the two-banded seabream (D. vulgaris) showed a similar annual pattern to the one of D. sargus (i.e. a peak around October), and they were also significantly correlated to water temperature. Preliminary telemetry study on the D. vulgaris did not show extraordinary movements related to the spawning season49 with average ordinary home range within one square Km46. However, spawning aggregation is reported by other authors50. This species is also omnivorous, preferentially feeding on bivalves, crustaceans, polychaetes, echinoderms, and algae29. Interestingly, crustaceans are mostly consumed during autumn, while echinoderms are less consumed during summer51. Such annual dietary patterns could be the reason of the marked annual rhythm at the artificial reef. Spawning of D. vulgaris occurs between December and January, thus the high consumption on crustaceans and the increase of counts at the artificial reef in autumn could be related to the dietary requirements for reproduction. Crustaceans have indeed a high caloric content52,53 and a marked preference for crustaceans prey just before the reproductive period has been observed in other sparids, such as Pagellus erythrinus (Linnaeus, 1758) and P. acarne (Risso, 1827)54.
The annular seabream (D. annularis) is the only species among the six studied here that showed a peak of annual presence at the artificial reef in December. Such peak was related to water temperature and daily photoperiod. The habitat of D. annularis is mostly related to Posidonia oceanica meadows55 that are abundant around the OBSEA. Individuals probably spend the greater part of the year there, except in winter when they are more present at the artificial reef. Telemetry and tag-recapture experiments showed that the annular seabream has a high site fidelity56, and its diet is based on polychaetes, crustaceans, and algae. Interestingly, artificial reefs have been demonstrated to affect D. annularis diet by providing greater amounts of copepods57,58. It is therefore plausible that the peak of presence at the artificial reef around December is related again to dietary needs, in order to secure energy for the spawning that occurs from February to July59.
The sharpsnout seabream’s (D. puntazzo) presence is constant at the artificial reef with no significant annual variations in counts. This species is known to preferentially feed at deeper waters than other sparids such as D. sargus and D. vulgaris29. Moreover, the diet of D. puntazzo is mainly composed of algae and sponges, while teleosts, mollusks, crustaceans, and annelids represent accessory items of its trophic niche29,60. Such differences ecologically segregate D. puntazzo from the other most common sparid species such as D. sargus and D. vulgaris29 and indicate a different use of the artificial reef. Telemetry could help in elucidating these aspects, but there are no existing tracking studies on this species to the best of our knowledge. Also, D. cervinus did not show significant fluctuations in counts at the artificial reef, although we detected weak correlations of temperature and salinity. Information is scarce on the ecology and biology of this species, however visual census studies reported that D. cervinus is more abundant around natural reefs than artificial ones61.
It is important to notice that we are unable to control the potential effect of artificial light at night on the counts of the six fish species at the artificial reef. The OBSEA mounts a lighting system that allows filming at night, which was used here intermittently every 30 min for about 30 s (see materials and methods). It is unlikely that short intermittent light emissions at night changed the behavior of fish so much as to affect their annual rhythms of habitat use at the artificial reef. However, future long-term monitoring studies need to take into consideration the potential direct and indirect effects of artificial lights on fish behavior in coastal areas62.
Moreover, some of the species belonging to the Sparidae family (D. sargus, D. vulgaris and D. puntazzo) are known to perform ontogenetic shifts in habitat use63 that could have biased the annual patterns described here. For example D.sargus showed peaks of recruitment (6–7 cm) in October-November coinciding with the peaks of counts at the OBSEA. We were not able to discriminate among recruits, sub-adults and adults size classes in the counted individuals at the OBSEA. So, we cannot exclude that the annual patterns observed here can be partially biased by annual recruitment events.
Our findings show the potential of underwater cabled video-observatories to produce complementary data to commonly employed approaches, such as visual census, stomach content analysis29, and telemetry38. Our monitoring by a cabled observatory allowed tracking fish assemblages at high frequencies and in the long term, opening new grounds for investigating biological rhythms at the population level but also to monitor and manage coastal ecosystems22,64. Despite the fact that cabled observatories are limited in space, the main advantage they offer with respect to a visual census approach is a long-term and low-invasive technology coupled with recent potentiality for automatic counting of individuals22 and absolute density estimation65. Low invasive sampling approach is particularly important in areas where fishing is allowed; in fact fish behavior can be strongly modulated by fishing pressure66. In particular, fish escape response could be negatively modulated by the presence of both SCUBA divers67 and free-divers68. So, the use of cabled observatory may reveal patterns of presence that could be biased during visual census. However, further research is needed on this topic for a quantitative assessment of other potential bias.
In a world subjected to rapid environmental changes, the huge amount of data generated by video-based tracking technologies provides new opportunities to quantify changes in phenology, which are particularly sensitive indicators of climate change69. Temperate coastal marine environments may be particularly vulnerable to changes in water temperature. Here, we showed that annual rhythms of habitat use in the Sparidae family had similar patterns to those observed for reproductive timing and other annual migrations previously described with other methods. Moreover fish counts were mainly (but not only) correlated to water temperature changes. Most importantly, the level of response might significantly differ between closely related species supporting the idea that the impact of climate change within an ecological community is expected to be species-specific, leading to potential phenological mismatches between species occupying different trophic levels and eventually to ecosystem-level impacts70.
Supplementary information
Acknowledgements
VS was supported by a Leibniz-DAAD postdoctoral research fellowship (n. 91632699). JA and JdR are members of the CSIC-UPC Associated Unit “Tecnoterra”, managing the OBSEA platform, as EMSO testing-site. JA is also “Science Theme Leader” for the section “Life in the Northeast Pacific” the ONC. This work was partially funded within the framework of the RESBIO project (REdes de Sensores submarinos autónomos y cableados aplicados a la monitorización remota de indicadores BIOlógicos; Comisión Interministerial de Ciencia y Tecnología, MINISTERIO DE ECONOMÍA, INDUSTRIA Y COMPETITIVIDAD, RETOS 2017) under TEC2017-87861-R contract. The Research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2013-2017) under the grant agreement n◦ 312463, FixO3, through the TNA project FISHAUT (grant agreement n◦ FC-01) for accessing the OBSEA data.
Author Contributions
J.d.R., J.A., M.N. conceived the study; V.S., S.C., M.N. collected the data; J.D.N., V.S., D.D., M.P., S.M. contributed to the statistical analysis; V.S., J.A., D.D., M.P., E.F., E.A. interpreted the results; V.S. wrote the manuscript with support of all the other authors.
Data Availability
Original data are available as supplementary file.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-37954-0.
References
- 1.Foster, R. G. & Kreitzman, L. Seasons of life: the biological rhythms that enable living things to thrive and survive. (New Haven, CT, Yale University Press, 2009).
- 2.Helm, B. et al. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. R. Soc. Lond. B280 20130016-20130016, 10.1098/rspb.2013.0016 (2013). [DOI] [PMC free article] [PubMed]
- 3.Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: the internal calendars of vertebrates. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson A. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2008;363:1621–1633. doi: 10.1098/rstb.2007.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulla M, Oudman T, Bijleveld AI, Piersma T, Kyriacou CP. Marine biorhythms: bridging chronobiology and ecology. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2017;372:20160253. doi: 10.1098/rstb.2016.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mat, A. M. & Handlingeditor: Howard, B. Chronobiology and the design of marine biology experiments. ICES J. Mar. Sci. fsy131–fsy131, 10.1093/icesjms/fsy131 (2018).
- 7.Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010;165:469–482. doi: 10.1016/j.ygcen.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Cowan M, Azpeleta C, López-Olmeda JF. Rhythms in the endocrine system of fish: a review. J. Comp. Physiol., B. 2017;187:1057–1089. doi: 10.1007/s00360-017-1094-5. [DOI] [PubMed] [Google Scholar]
- 9.Menaker M. Circadian organization in the real world. Proc. Natl. Acad. Sci. USA. 2006;103:3015–3016. doi: 10.1073/pnas.0600360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calisi RM, Bentley GE. Lab and field experiments: are they the same animal? Horm. Behav. 2009;56:1–10. doi: 10.1016/j.yhbeh.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos Trans R Soc Lond B Biol Sci. 2010;365:3113–3127. doi: 10.1098/rstb.2010.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronfeld-Schor, N., Bloch, G. & Schwartz, W. J. Animal clocks: when science meets nature. Proc. R. Soc. Lond. B 280, 20131354, 10.1098/rspb.2013.1354 (2013). [DOI] [PMC free article] [PubMed]
- 13.Bradshaw WE, Holzapfel CM. Evolution of animal photoperiodism. Annu. Rev. Ecol., Evol. Syst. 2007;38:1–25. doi: 10.1146/annurev.ecolsys.37.091305.110115. [DOI] [Google Scholar]
- 14.Schwartz, M. D. Phenology: an integrative environmental science. (Kluwer Academic Publishers 2003).
- 15.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol., Evol. Syst. 2006;37:637–669. doi: 10.1146/annurev.ecolsys.37.091305.110100. [DOI] [Google Scholar]
- 16.Hussey NE, et al. Aquatic animal telemetry: A panoramic window into the underwater world. Science. 2015;348:1255642–1255642. doi: 10.1126/science.1255642. [DOI] [PubMed] [Google Scholar]
- 17.Dominoni, D. M., Åkesson, S., Klaassen, R., Spoelstra, K. & Bulla, M. Methods in field chronobiology. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 37220160247, 10.1098/rstb.2016.0247 (2017). [DOI] [PMC free article] [PubMed]
- 18.Lennox RJ, et al. Envisioning the future of aquatic animal tracking: technology, science, and application. Bioscience. 2017;67:884–896. doi: 10.1093/biosci/bix098. [DOI] [Google Scholar]
- 19.Aguzzi, J. et al. Challenges to the assessment of benthic populations and biodiversity as a result of rhythmic behaviour. Oceanogr. Mar. Biol. 235–286, 10.1201/b12157-6 (2012).
- 20.Aguzzi J, et al. Coastal observatories for monitoring of fish behaviour and their responses to environmental changes. Rev. Fish Biol. Fish. 2015;25:463–483. doi: 10.1007/s11160-015-9387-9. [DOI] [Google Scholar]
- 21.Danovaro R, et al. An ecosystem-based deep-ocean strategy. Science. 2017;355:452–454. doi: 10.1126/science.aah7178. [DOI] [PubMed] [Google Scholar]
- 22.Marini S, et al. Tracking fish abundance by underwater image recognition. Sci. Rep. 2018;8:13748. doi: 10.1038/s41598-018-32089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier D, et al. Remote high-definition rotating video enables fast spatial survey of marine underwater macrofauna and habitats. Plos One. 2012;7:e30536. doi: 10.1371/journal.pone.0030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matabos M, et al. High-frequency study of epibenthic megafaunal community dynamics in Barkley Canyon: A multi-disciplinary approach using the NEPTUNE Canada network. J. Mar. Syst. 2014;130:56–68. doi: 10.1016/j.jmarsys.2013.05.002. [DOI] [Google Scholar]
- 25.Aguzzi J, et al. The new seafloor observatory (OBSEA) for remote and long-term coastal ecosystem monitoring. Sensors (Basel, Switzerland) 2011;11:5850–5872. doi: 10.3390/s110605850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.del Rio J, et al. A new colorimetrically-calibrated automated video-imaging protocol for day-night fish counting at the OBSEA coastal cabled observatory. Sensors (Basel) 2013;13:14740–14753. doi: 10.3390/s131114740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguzzi J, et al. Daily activity rhythms in temperate coastal fishes: insights from cabled observatory video monitoring. Mar. Ecol. Prog. Ser. 2013;486:223–236. doi: 10.3354/meps10399. [DOI] [Google Scholar]
- 28.Wootton, R. J. & Smith, C. Reproductive biology of teleost fishes. (John Wiley & Sons, 2014).
- 29.Sala E, Ballesteros E. Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar. Ecol. Prog. Ser. 1997;152:273–283. doi: 10.3354/meps152273. [DOI] [Google Scholar]
- 30.Nelson, J. S. Fishes of the world. Fourth Edition edn, (John Wiley & Sons, Inc. 2006).
- 31.Relini M, Torchia G, Relini G. Seasonal variation of fish assemblages in the loano artificial reef (Ligurian Sea Northwestern-Mediterranean) Bull. Mar. Sci. 1994;55:401–417. [Google Scholar]
- 32.Pavlidis, M. A. & Mylonas, C. C. Sparidae: Biology and aquaculture of gilthead sea bream and other species. (John Wiley & Sons 2011).
- 33.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale and shape. J. Roy. Stat. Soc. Ser. C. (Appl. Stat.) 2005;54:507–554. doi: 10.1111/j.1467-9876.2005.00510.x. [DOI] [Google Scholar]
- 34.Akaike, H. In Selected Papers of Hirotugu Akaike Springer Series in Statistics (eds Emanuel Parzen, Kunio Tanabe, & Genshiro Kitagawa) Ch. 15, 199–213 (Springer New York 1998).
- 35.Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociological Methods & Research. 2004;33:261–304. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 36.Anderson, D. R. & Burnham, K. P. Avoiding pitfalls when using information-theoretic methods. J. Wildlife manage. 912–918, 10.2307/3803155 (2002).
- 37.R Core Team. R: A language and environment for statistical computing Version 3, 5 (2018).
- 38.Aspillaga E, et al. Thermal stratification drives movement of a coastal apex predator. Sci. Rep. 2017;7:526. doi: 10.1038/s41598-017-00576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morales-Nin B, Moranta J. Life history and fishery of the common dentex (Dentex dentex) in Mallorca (Balearic Islands, western Mediterranean) Fish. Res. 1997;30:67–76. doi: 10.1016/S0165-7836(96)00560-7. [DOI] [Google Scholar]
- 40.Stergiou KI, Karpouzi VS. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fish. 2002;11:217–254. doi: 10.1023/a:1020556722822. [DOI] [Google Scholar]
- 41.Marengo M, Durieux EDH, Marchand B, Francour P. A review of biology, fisheries and population structure of Dentex dentex (Sparidae) Rev. Fish Biol. Fish. 2014;24:1065–1088. doi: 10.1007/s11160-014-9363-9. [DOI] [Google Scholar]
- 42.Clarke A, Johnston NM. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999;68:893–905. doi: 10.1046/j.1365-2656.1999.00337.x. [DOI] [Google Scholar]
- 43.Aspillaga E, et al. Ordinary and extraordinary movement behaviour of small resident fish within a Mediterranean marine protected area. Plos One. 2016;11:e0159813. doi: 10.1371/journal.pone.0159813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloret O, Planes S. Condition, feeding and reproductive potential of white seabream Diplodus sargus as indicators of habitat quality and the effect of reserve protection in the northwestern Mediterranean. Mar. Ecol. Prog. Ser. 2003;248:197–208. doi: 10.3354/meps248197. [DOI] [Google Scholar]
- 45.Di Lorenzo M, et al. Fitting the size of no-take zones to species movement patterns: a case study on a Mediterranean seabream. Mar. Ecol. Prog. Ser. 2014;502:245–255. doi: 10.3354/meps10723. [DOI] [Google Scholar]
- 46.Di Franco A, et al. Linking home ranges to protected area size: The case study of the Mediterranean Sea. Biol. Conserv. 2018;221:175–181. doi: 10.1016/j.biocon.2018.03.012. [DOI] [Google Scholar]
- 47.D’Anna G, Giacalone VM, Pipitone C, Badalamenti F. Movement pattern of white seabream, Diplodus sargus (L., 1758) (Osteichthyes, Sparidae) acoustically tracked in an artificial reef area. Ital. J. Zool. 2011;78:255–263. doi: 10.1080/11250000903464059. [DOI] [Google Scholar]
- 48.Leitão F, Santos MN, Monteiro CC. Contribution of artificial reefs to the diet of the white sea bream (Diplodus sargus) ICES J. Mar. Sci. 2007;64:473–478. doi: 10.1093/icesjms/fsm027. [DOI] [Google Scholar]
- 49.Alós J, Cabanellas-Reboredo M, March D. Spatial and temporal patterns in the movement of adult twobanded sea bream Diplodus vulgaris (Saint-Hilaire, 1817) Fish. Res. 2012;115–116:82–88. doi: 10.1016/j.fishres.2011.11.025. [DOI] [Google Scholar]
- 50.Gonçalves JMS, et al. Age and growth, maturity, mortality and yield-per-recruit for two banded bream (Diplodus vulgaris Geoffr.) from the south coast of Portugal. Fish. Res. 2003;62:349–359. doi: 10.1016/S0165-7836(02)00280-1. [DOI] [Google Scholar]
- 51.Hadj Taieb A, Ghorbel M, Hamida NBH, Jarboui O. Reproductive biology, age and growth of the twobanded seabream Diplodus vulgaris (Pisces: Sparidae) in the Gulf of Gabès, Tunisia. J. Mar. Biol. Assoc. U.K. 2013;93:1415–1421. doi: 10.1017/S0025315412001737. [DOI] [Google Scholar]
- 52.Lawrence JM, Kafri J. Numbers, biomass, and caloric content of the echinoderm fauna of the rocky shores of Barbados. Mar. Biol. 1979;52:87–91. doi: 10.1007/BF00386861. [DOI] [Google Scholar]
- 53.Fanelli E, Cartes JE. Temporal variations in the feeding habits and trophic levels of three deep-sea demersal fishes from the western Mediterranean Sea, based on stomach contents and stable isotope analyses. Mar. Ecol. Prog. Ser. 2010;402:213–232. doi: 10.3354/meps08421. [DOI] [Google Scholar]
- 54.Fanelli E, et al. Food partitioning and diet temporal variation in two coexisting sparids, Pagellus erythrinus and Pagellus acarne. J. Fish Biol. 2011;78:869–900. doi: 10.1111/j.1095-8649.2011.02915.x. [DOI] [PubMed] [Google Scholar]
- 55.Gordoa A, Molí B. Age and growth of the sparids Diplodus vulgaris, D. sargus and D. annularis in adult populations and the differences in their juvenile growth patterns in the north-western Mediterranean Sea. Fish. Res. 1997;33:123–129. doi: 10.1016/S0165-7836(97)00074-X. [DOI] [Google Scholar]
- 56.March D, Alós J, Grau A, Palmer M. Short-term residence and movement patterns of the annular seabream Diplodus annularis in a temperate marine reserve. Estuar. Coast. Shelf Sci. 2011;92:581–587. doi: 10.1016/j.ecss.2011.02.015. [DOI] [Google Scholar]
- 57.Relini G, Relini M, Torchia G, de Angelis G. Trophic relationships between fishes and an artificial reef. ICES J. Mar. Sci. 2002;59:S36–S42. doi: 10.1006/jmsc.2002.1212. [DOI] [Google Scholar]
- 58.Sánchez-Jerez P, Gillanders BM, Rodríguez-Ruiz S, Ramos-Esplá AA. Effect of an artificial reef in Posidonia meadows on fish assemblage and diet of Diplodus annularis. ICES J. Mar. Sci. 2002;59:S59–S68. doi: 10.1006/jmsc.2002.1213. [DOI] [Google Scholar]
- 59.Mouine N, Francour P, Ktari MH, Chakroun-Marzouk N. Reproductive biology of four Diplodus species Diplodus vulgaris, D. annularis, D. sargus sargus and D. puntazzo (Sparidae) in the Gulf of Tunis (central Mediterranean) J. Mar. Biol. Assoc. U.K. 2012;92:623–631. doi: 10.1017/S0025315411000798. [DOI] [Google Scholar]
- 60.Chaouch H, Hamida OBA-BH, Ghorbel M, Jarboui O. Diet composition and food habits of Diplodus puntazzo (Sparidae) from the Gulf of Gabès (Central Mediterranean) J. Mar. Biol. Assoc. U.K. 2013;93:2257–2264. doi: 10.1017/S0025315413000805. [DOI] [Google Scholar]
- 61.Koeck B, et al. Functional differences between fish communities on artificial and natural reefs: a case study along the French Catalan coast. Aquat. Biol. 2014;20:219–234. doi: 10.3354/ab00561. [DOI] [Google Scholar]
- 62.Davies, T. W., Coleman, M., Griffith, K. M. & Jenkins, S. R. Night-time lighting alters the composition of marine epifaunal communities. Biol. Lett. 11, 10.1098/rsbl.2015.0080 (2015). [DOI] [PMC free article] [PubMed]
- 63.Macpherson E. Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes. J. Exp. Mar. Biol. Ecol. 1998;220:127–150. doi: 10.1016/S0022-0981(97)00086-5. [DOI] [Google Scholar]
- 64.Marini S, et al. Automated estimate of fish abundance through the autonomous imaging device GUARD1. Measurement. 2018;126:72–75. doi: 10.1016/j.measurement.2018.05.035. [DOI] [Google Scholar]
- 65.Campos-Candela A, Palmer M, Balle S, Alós J. A camera-based method for estimating absolute density in animals displaying home range behaviour. J. Anim. Ecol. 2018;87:825–837. doi: 10.1111/1365-2656.12787. [DOI] [PubMed] [Google Scholar]
- 66.Arlinghaus R, et al. Passive gear-induced timidity syndrome in wild fish populations and its potential ecological and managerial implications. Fish Fish. 2017;18:360–373. doi: 10.1111/faf.12176. [DOI] [Google Scholar]
- 67.Guidetti P, Vierucci E, Bussotti S. Differences in escape response of fish in protected and fished Mediterranean rocky reefs. J. Mar. Biol. Assoc. U.K. 2008;88:625–627. doi: 10.1017/S0025315408000933. [DOI] [Google Scholar]
- 68.Sbragaglia V, et al. Spearfishing modulates flight initiation distance of fishes: the effects of protection, individual size, and bearing a speargun. ICES J. Mar. Sci. 2018;75:1779–1789. doi: 10.1093/icesjms/fsy059. [DOI] [Google Scholar]
- 69.Stevenson, T. J. et al. Disrupted seasonal biology impacts health, food security and ecosystems. Proc. R. Soc. Lond. B282, 10.1098/rspb.2015.1453 (2015). [DOI] [PMC free article] [PubMed]
- 70.Thackeray SJ, et al. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Global Change Biol. 2010;16:3304–3313. doi: 10.1111/j.1365-2486.2010.02165.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data are available as supplementary file.