Abstract
Cell migration is a critical process that underpins a number of physiological and pathological contexts such as the correct functioning of the immune system and the spread of metastatic cancer cells. Central to this process are the Rho family of GTPases, which act as core regulators of cell migration.
Rho GTPases are molecular switches that associate with lipid membranes and act to choreograph molecular events that underpin cell migration. Specifically, these GTPases play critical roles in coordinating force generation through driving the formation of cellular protrusions as well as cell–cell and cell–matrix adhesions.
Here we provide an update on the many roles of Rho-family GTPases in coordinating protrusion and adhesion formation in the context of cell migration, as well as describing how their activity is controlled to by a variety of complex signalling networks.
Current Opinion in Cell Biology 2019, 56:64–70
This review comes from a themed issue on Cell architecture
Edited by Johanna Ivaska and Manuel Théry
For a complete overview see the Issue and the Editorial
Available online 3rd October 2018
https://doi.org/10.1016/j.ceb.2018.09.003
0955-0674/© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Rho-family GTPases are molecular switches; most which cycle from an ‘on’ GTP bound state to an ‘off’ GDP bound state, driven by GEFs (guanine nucleotide exchange factors) and GAPs (GTPase-activating proteins) respectively. Association with lipid membranes through a lipid (farnesyl or geranylgeranyl) tail ensures Rho family GTPases signal at membrane-cytosol interfaces and exquisite control the ratio of cytosolic to membrane bound GTPase is achieved by the Rho-GDI (Rho GDP-dissociation inhibitor) family of proteins [1]. An atypical subgroup of Rho-family GTPases, known as the Rnd family are constitutively GTP bound, and instead are thought to be regulated by control of their association with lipid membranes, via 14-3-3 proteins which can bind to Rnd GTPase lipid tails [2]. Through the extensive regulation of Rho GTPase activation and localisation the cell can control the activation of Rho-family GTPases in a precise spatio-temporal manner [1]. In fact Rho-family GTPases have long been appreciated as signalling molecules that allow the cell to relay information to a variety of cellular machineries including the NADPH oxidase complex and vesicle trafficking components [3,4]. The role of Rho GTPases in controlling the actin cytoskeleton was highlighted by Alan Hall’s seminal work linking RhoA, Rac1 and Cdc42 to the formation of stress fibres, lamellipodia and filopodia, respectively [5, 6, 7]. Furthermore, the discovery that RhoA drives the formation of stress fibres highlighted the importance of Rho GTPase signalling during the formation of cell–matrix adhesions [6]. This review will focus on Rho GTPase signalling in the context of cell migration, examining how these molecular switches signal to cellular protrusions and cell–matrix adhesions. Here we summarise what is known about Rho-family GTPases in the context of leading edge protrusion formation, highlighting recent studies that have helped to uncover the complexity of these fascinating molecular switches. Specifically, this review will highlight four major aspects of Rho GTPase biology: the effectors of Rho GTPases, the regulators of Rho GTPases, the role of Rho GTPases in determining cellular directionality and the importance of Rho GTPases in the context of cell–matrix adhesions. All four aspects play major roles in understanding how Rho GTPases signal during migration and all four are far from being fully understood.
Rho-family GTPase effectors
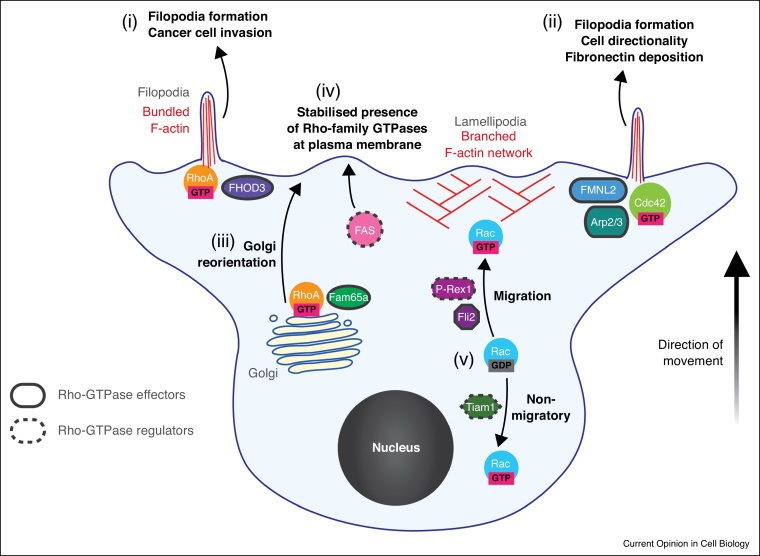
Following the discovery that Rac1 and Cdc42 stimulate the formation of lamellipodia and filopodia respectively, numerous factors were identified that enable these GTPases to build a protrusive leading edge. Of key importance are the proteins that enabled Rac1 and Cdc42 to drive actin nucleation. These included the Arp2/3 activators of the WAVE and WASP family for both Rac1 and Cdc42 respectively [8,9]. The discovery of these proteins led to the concept, based on 2D cell culture studies that Rac1 and Cdc42 signalling to the Arp2/3 complex is essential for the establishment of the leading edge. However this concept was extended and challenged by the direct observation of RhoA signalling at the leading edge of mouse fibroblasts and human cancer cells migrating in 2D cell culture [10, 11, 12, 13]. Furthermore knockout studies of Arp2/3 complex components in fibroblasts migrating in 2D demonstrated that Arp2/3 is not a universal requirement for movement on such surfaces, although defects in lamellipodia formation and directional migration in both haptotaxis and chemotaxis have been observed [14, 15, 16]. The universal requirement for Arp2/3 in migration was also challenged by the discovery of amoeboid migration which utilises RhoA signalling at the leading edge of the cell to disrupt cortical actin, allowing the cell to control the number and size of plasma membrane-based blebs that drive the cell’s movement through gaps in 3D extracellular matrix [17, 18, 19]. Therefore, it is not surprising that studies continue to identify proteins that act downstream of Rho-GTPases to facilitate protrusion formation and couple such formation to the motility of the rest of the cell. An example of such work includes the identification of FAM65A as a RhoA effector. By binding to Golgi associated FAM65A, RhoA is thought to re-orientate the Golgi network towards the leading edge, facilitating efficient migration of single cells in 2D [20]. FMNL2 has recently been identified as a formin that localises to the leading edge of cells in 2D and promotes filopodia formation downstream of Cdc42 [21]. Furthermore RhoA activation at the leading edge of cells in 3D matrix promotes filopodia formation and invasive migration through ROCK-mediated activation of the formin FHOD3 [22,23] (Figure 1).
Figure 1.
Rho GTPases in protrusion formation summary. (i) RhoA can signal to the formin FHOD3, via the ROCK family kinases, to promote the invasion of cancer cells into 3D fibronectin rich ECM. This form on invasive migration occurs downstream of the upregulated endocytic recycling of the α5β1 integrin, and does not require the action of the Arp2/3 complex. (ii) Cdc42 can drive the formation of filopodia by activating the formin FMNL2 and/or Arp2/3. (iii) RhoA-FAM65 interaction can re-orientate the Golgi apparatus towards the leading edge of the cell in 2D environments, facilitating efficient migration. (iv) Fatty acid synthesis alters the biochemical and biophysical properties of the plasma membrane, stabilising the presence of Rho GTPases in the membrane. This may have important implications for understanding how the metabolic state of a cell may affect its ability to migrate. (v) Different GEFs can promote differential Rac1 signalling, either promoting a migratory output by ensuring Rac1 binds to FLI2 (P-Rex1) or preventing a migratory output (Tiam1).
Given the complexity of the leading edge of migrating cells and the refinement of methodologies being developed to study it, it seems likely that the list of proteins known to act down stream of Rho GTPases will continue to grow. This should ultimately provide a more in-depth understanding of migration in both physiological and pathological contexts.
Cellular directionality
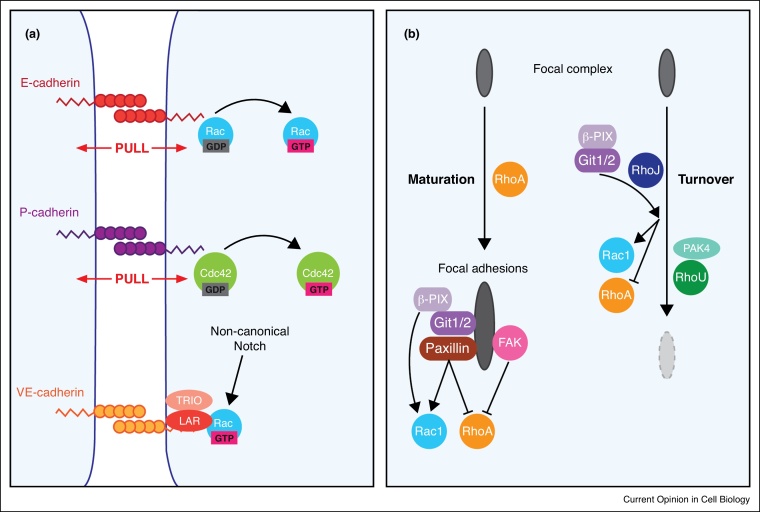
To obtain a comprehensive understanding of cell migration, it is essential to understand how cues from the cell’s external environment are relayed to the actin cytoskeleton, so the cell can migrate towards the cue; a process herein referred to as cellular directionality. Understanding cellular directionality is particularly important for the cell migration field as motile cells must be able to both prioritise external cues and rapidly change direction in response to an ever-changing external environment. Whilst the types of cue that can trigger cell migration (e.g. chemokines, matrix-derived etc.) have been well described, the search for an internal ‘compass’ has proven somewhat difficult [24,25]. Whilst for years this role was thought to be provided by PI3 kinase, the discovery that cells can migrate in the absence of this kinase re-opened this question [26, 27, 28]. Increasingly however, the Rho-family GTPases have been implicated in this role. For example Rac1 signalling can relay directional information between Drosophila border cells migrating as a cluster in vivo, via E-cadherin mediated mechano-sensing [29]. Similarly, P-cadherin mediated mechano-transduction can drive cell polarisation during collective mouse myoblast migration in a 2D culture system, by signalling to Cdc42 [30] (Figure 2a).
Figure 2.
Rho family GTPases in the context of adhesion. (a) Rac1 and CDC42 control the directionality of groups of migrating cells when activated via the mechanical stretch of E-cadherin and P-cadherin respectively. Non-canonical notch signalling leads to the formation of a VE-Cadherin-LAR-TRIO complex that leads to the activation of Rac1. (b) RhoA signalling can promote focal complex maturation, leading to the recruitment of integrin associated proteins. These proteins include the Git1/2-β-Pix complex that binds to paxillin and signals to promote Rac1 signalling and supress RhoA signalling. RhoJ can enhance focal complex turnover, by recruiting the β-Pix-GIT complex in order to block RhoA signalling, blocking RhoA mediated focal adhesion maturation. RhoU, when stabilised by PAK4 can also promote the turnover of focal adhesions.
Cdc42 has also been implicated as an internal compass during neutrophil migration. In order to successfully trap and destroy motile bacteria, neutrophils must rapidly respond to the ever changing position of the bacterium [31]. Rho-GTPase FRET sensors, in combination with photoactivatable chemokines demonstrated a role for Cdc42 in responding to the chemokine and controlling neutrophil steering (and suppression of RhoA), whereas a shallow gradient of Rac activity more distal to the leading edge might provide the ‘engine’ [32••]. This neutrophil study was performed in 2D culture, and thus it remains to resolved if Cdc42 performs this role during in vivo migration, whereby the neutrophil must integrate and prioritise numerous migratory cues.
It is interesting to note that Rac1 and Cdc42 have both been shown to control cellular directionality: Rac1 in the collective migration of Drosophila border cells [29] and in fibroblasts [33] and Cdc42 in both collective cell migration and in neutrophils [32••,30]. Cdc42 would seem to be the more obvious candidate as a universal compass, should such an entity exist, given its defined role in establishing cell polarity [34,35]. However, given the significant differences between the cell types used in these studies, and the difficulty of finding a universal compass that controls the directionality of a migrating cell, it is likely that different members of the Rho family of GTPases can serve as a compass in a context-dependent manner.
Signalling to Rho-family GTPases
Since the discovery of small GTPases, many questions have persisted as to the nature of the GEFs and GAPs that control the on/off cycle of these switches [1]. Rho-family GTPases are no exception, and despite numerous regulators having been identified, it is still not clear why there are so many or how much functional redundancy exists. Answering these questions is essential as GEFs and GAPs provide an interface through which the cell is able to communicate to Rho-family GTPases [1]. Recently, work from Marei et al. has addressed this question in a mammalian culture system, confirming the relevance of previous studies in yeast. In the NIH3T3 mouse embryonic fibroblast cell line, the Rac GEF P-Rex1 promoted cell migration in 2D and a more contractile phenotype in 3D, whilst TIAM1 signalled to block migration. The key to these differential outcomes seemed to be dependent on P-Rex1 enhancing the interaction of Rac1 with FLI2 [36,37,38•]. This work suggests that GEFs may serve to function as more than just ‘switch flippers’ and act to direct Rho GTPase signalling via specific effector pathways (Figure 1).
In addition to understanding the GEF-GAP cycle regulating Rho-family GTPases, studies have continued to address the role of the Rho-GDIs, which binds to the geranylgeranyl tail to sequester Rho-family GTPases from interacting with lipid membranes, preventing their activation. Whilst it would be tempting to speculate that Rho-GDIs act to maintain a pool of unused Rho-family GTPases, a recent study has shown that Rho-GDI serves a specific role in controlling the level of Rho-GTPase activation by coordinating GTPase activity and re-activation on a ∼10 s timescale [39]. Furthermore, a role for the lipid composition of the plasma membrane in regulating this association has been proposed. By inhibiting fatty acid synthetase (FAS) in migrating inflammatory macrophages, Wei et al. were able to demonstrate a role for fatty acids in stabilising the presence of Rho-family GTPases at the plasma membrane, in the context of diabetes [40•] (Figure 1).
Despite an in-depth understanding of the proteins that directly interact with Rho GTPases to switch them on and off, numerous questions persist as to which signalling pathways act upstream of these regulators, and where GEFs, GAPs and GDIs feature in terms of the complex signalling networks that are known to promote cell migration. Recent studies have sought to address this by both extending the list of proteins that are known to signal upstream of Rho-family GTPases, as well as utilising a variety of systems-based techniques based on mathematical modelling to predict how Rho-family GTPases will signal in response to perturbations of the signalling networks they interact with.
Rho-GTPases have long been known to signal downstream of a variety of receptors such as receptor tyrosine kinases (RTKs), G-protein-coupled receptors (GPCRs) and integrins to name a few. Recently, a study has extended this list, identifying a novel role for non-canonical Notch signalling in driving Rac1 activity via the GEF TRIO, which in turn reinforces the formation of adherens junctions [41••] in endothelial cells both in vitro and in vivo. It will therefore be interesting to observe if this pathway plays a role in collective cell migration, which is co-ordinated via cadherin-based adhesions (Figure 2a).
In terms of placing Rho-family GTPases within the context of a signalling network, Boolean modelling of Rac/RhoA signalling in invasive cancer cells has established a link between MAP kinase signalling downstream of RTKs, and the activation of RhoA, which we had previously shown to drive invasion into fibronectin-rich extracellular matrix [22,23]. Model simulations predicted MAPK signalling controls a negative feedback loop via the Sos1-Eps8-Abi1 complex that supresses Rac1 activity, enabling the activation of RhoA in cells migrating both in 2D plastic and 3D cell-derived matrix. Experimental inhibition of MAP kinase signalling enabled the re-activation of Rac1 at the leading edge of the cell, supressing filopodia formation and invasion into extracellular matrix and on cell-derived matrix. Critically, knockdown of Eps8 (a key component of the RacGEF complex in this system) rendered cells insensitive to MAPK inhibition, re-enabling cells to activate RhoA at the leading edge of the cell, driving invasive migration [42•]. Such feedback loops may provide plasticity to the migrating cell, enabling it to re-programme its leading edge in response to a changes in the surrounding environment [43].
Similar approaches using more sophisticated kinetic modelling identified a role for PAK signalling in mediating a bi-stable switch [44]. Exposing MDA-MB-231 breast carcinoma cells in 2D culture to increasing amounts of PAK inhibitor had different effects on Rac and RhoA signalling depending on whether cells had been pre-incubated with the same inhibitor, demonstrating the predicted hysteresis. Interestingly this bi-stability is conserved in actin dynamics, and suggests that cytoskeletal signalling pathways encode a memory of activation status [44].
In summary, it is becoming increasingly clear that as the list of Rho GTPase regulators increases, systems-based studies are needed to understand how these regulators function as a network. Furthermore, mathematical modelling enables the development of unique hypotheses that cannot be generated through qualitative analysis, and produces specific, testable predictions. Therefore, it is apparent that there is much to be learnt about Rho-family GTPases through quantitative mathematical modelling.
Rho GTPases in cell–matrix interactions
The importance of Rho-family GTPases in cell matrix interactions has been well appreciated ever since the initial identification of RhoA as a regulator of stress fibres, which showed that focal adhesions are unable to form in the absence of RhoA signalling [6]. Since then, a number of studies have shown extensive reciprocal signalling between matrix receptors and Rho-family GTPases, however for the purpose of this review, we shall focus on a handful of recent studies that have extended our understanding of direct signalling between focal adhesions and Rho GTPases.
Focal adhesions have long been known to control the activity of Rho-family GTPases via adaptor proteins that can signal to GEFs and GAPS, such as paxillin, which can signal to both activate Rac1 and suppress RhoA, and FAK which can signal to supress RhoA activity [45,46]. β-Pix is a Rac GEF recruited to adhesion complexes through interaction with Git1/2 recruitment to paxillin [47,48]. Interestingly Git1/2-β -Pix can also be recruited to adhesion complexes by RhoJ, which mediates adhesion turnover by sustaining Rac1 activity and preventing RhoA activation [49]. These types of interactions can govern the transition of nascent adhesion complex to focal complexes, but restrain the maturation to focal adhesion (which requires RhoA–driven contractility [6,50]). Interestingly, RhoU is stabilised by interaction with PAK4 in a Cdc42 and kinase-independent manner to regulate adhesion turnover [51]. This suggests that complex feedback networks exist between Rho GTPases and adhesion complexes that might determine the intricate and subtle morphological adaptations of adhering and migrating cells (Figure 2b). All these studies were performed principally in 2D cell culture and thus it remains to be understood how RhoJ and RhoU mediate crosstalk with focal complexes in 3D matrix environments.
Whilst it has long been appreciated that Rho-family GTPases are able to signal directly to focal adhesions, it is also becoming increasingly clear that they can achieve this indirectly, through their influence on the extracellular matrix to which the integrins bind. Cdc42 and RhoA have long been known to promote the trafficking of metalloproteinases to the tips of invadopodia to promote cancer cell metastasis by driving an interaction between IQGAP with the exocyst complex [52]. More recently a study has established a clear link between Cdc42 and fibronectin deposition allowing for the formation of focal adhesions within lamellipodia and permitting migration over the resulting matrix in a Rac1-dependent manner [53•]. Given that filopodia drive cancer cell invasion into fibronectin containing matrix [23,54], it will be fascinating to discover if filopodia can also drive fibronectin deposition in vivo.
Whilst cell–matrix interactions are relatively well understood in 2D, it is vital that these studies are translated to 3D in vivo systems given the stark biochemical and mechanical differences between such systems. Understanding how Rho-family GTPases coordinate cell–matrix interactions in vivo is particularly challenging given the technical difficulties that are associated with studying cell–matrix receptors, such as integrins, in 3D. However, studying how Rho-GTPases coordinate cell matrix interactions in vivo is essential to understanding cell migration, in contexts such as wound healing and cancer.
Conclusion
The complexity and intricacy of Rho-family GTPase signalling continues to increase as methodologies for studying them becomes more advanced. It is becoming clear that the plasticity and variety of structures that can be found at the leading edge is huge and more work is required to understand how Rho-GTPases signal. This will involve the identification of more Rho GTPase binding partners and an increased systems level understanding of their function that incorporates features of the extracellular environment. Furthermore, the diversity of contexts in which this family of proteins have been studied is large, and the results vary accordingly, highlighting the need to understand how Rho family GTPases and their associated proteins evolved to meet the varying requirements of different organisms.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The Wellcome Trust Centre for Cell-Matrix Research is supported by grant 203128/Z/16/Z. Cancer Research UK PhD studentship grant C147/A19404. BJW is supported by a Wellcome Trust PhD studentship grant 109330/Z/15/A.
References
- 1.Ridley A.J. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Riou P., Kjær S., Garg R., Purkiss A., George R., Cain R.J., Bineva G., Reymond N., McColl B., Thompson A.J. 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell. 2013;153:640–653. doi: 10.1016/j.cell.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abo A., Pick E., Hall A., Totty N., Teahan C.G., Segal A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 4.Ridley A.J. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- 5.Ridley A.J., Paterson H.F., Johnston C.L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 6.Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 7.Nobes C.D., Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 8.Aspenström P., Lindberg U., Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 9.Machesky L.M., Mullins R.D., Higgs H.N., Kaiser D.A., Blanchoin L., May R.C., Hall M.E., Pollard T.D. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci U S A. 1999;96:3739–3744. doi: 10.1073/pnas.96.7.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goulimari P., Kitzing T.M., Knieling H., Brandt D.T., Offermanns S., Grosse R. Galpha12/13 is essential for directed cell migration and localized Rho-Dia1 function. J Biol Chem. 2005;280:42242–42251. doi: 10.1074/jbc.M508690200. [DOI] [PubMed] [Google Scholar]
- 11.Kurokawa K., Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol Biol Cell. 2005;16:4294–4303. doi: 10.1091/mbc.E04-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pertz O., Hodgson L., Klemke R.L., Hahn K.M. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 13.Machacek M., Hodgson L., Welch C., Elliott H., Pertz O., Nalbant P., Abell A., Johnson G.L., Hahn K.M., Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C., Asokan S.B., Berginski M.E., Haynes E.M., Sharpless N.E., Griffith J.D., Gomez S.M., Bear J.E. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell. 2012;148:973–987. doi: 10.1016/j.cell.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suraneni P., Rubinstein B., Unruh J.R., Durnin M., Hanein D., Li R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol. 2012;197:239–251. doi: 10.1083/jcb.201112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suraneni P., Fogelson B., Rubinstein B., Noguera P., Volkmann N., Hanein D., Mogilner A., Li R. A mechanism of leading edge protrusion in the absence of Arp2/3 complex. Mol Biol Cell. 2015;26 doi: 10.1091/mbc.E14-07-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahai E., Marshall C.J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 18.te Boekhorst V., Preziosi L., Friedl P. Plasticity of cell migration in vivo and in silico. Annu Rev Cell Dev Biol. 2016;32:491–526. doi: 10.1146/annurev-cellbio-111315-125201. [DOI] [PubMed] [Google Scholar]
- 19.Charras G., Sahai E. Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol. 2014;15:813–824. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- 20.Mardakheh F.K., Self A., Marshall C.J. RHO binding to FAM65A regulates Golgi reorientation during cell migration. J Cell Sci. 2016;129:4466–4479. doi: 10.1242/jcs.198614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block J., Breitsprecher D., Kühn S., Winterhoff M., Kage F., Geffers R., Duwe P., Rohn J.L., Baum B., Brakebusch C. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22:1005–1012. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacquemet G., Green D.M., Bridgewater R.E., von Kriegsheim A., Humphries M.J., Norman J.C., Caswell P.T. RCP-driven α5β1 recycling suppresses Rac and promotes RhoA activity via the RacGAP1-IQGAP1 complex. J Cell Biol. 2013;202:917–935. doi: 10.1083/jcb.201302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul N.R., Allen J.L., Chapman A., Morlan-Mairal M., Zindy E., Jacquemet G., Fernandez del Ama L., Ferizovic N., Green D.M., Howe J.D. α5β1 integrin recycling promotes Arp2/3-independent cancer cell invasion via the formin FHOD3. J Cell Biol. 2015;210:1013–1031. doi: 10.1083/jcb.201502040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berzat A., Hall A. Cellular responses to extracellular guidance cues. EMBO J. 2010;29:2734–2745. doi: 10.1038/emboj.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bear J.E., Haugh J.M. Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr Opin Cell Biol. 2014;30:74–82. doi: 10.1016/j.ceb.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickert P., Weiner O.D., Wang F., Bourne H.R., Servant G. Leukocytes navigate by compass: roles of PI3Kgamma and its lipid products. Trends Cell Biol. 2000;10:466–473. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang F., Herzmark P., Weiner O.D., Srinivasan S., Servant G., Bourne H.R. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat Cell Biol. 2002;4:513–518. doi: 10.1038/ncb810. [DOI] [PubMed] [Google Scholar]
- 28.Hoeller O., Kay R.R. Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17:813–817. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Cai D., Chen S.-C., Prasad M., He L., Wang X., Choesmel-Cadamuro V., Sawyer J.K., Danuser G., Montell D.J. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plutoni C., Bazellieres E., Le Borgne-Rochet M., Comunale F., Brugues A., Séveno M., Planchon D., Thuault S., Morin N., Bodin S. P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J Cell Biol. 2016;212:199–217. doi: 10.1083/jcb.201505105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niggli V. Signaling to migration in neutrophils: importance of localized pathways. Int J Biochem Cell Biol. 2003;35:1619–1638. doi: 10.1016/s1357-2725(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 32••.Yang H.W., Collins S.R., Meyer T. Locally excitable Cdc42 signals steer cells during chemotaxis. Nat Cell Biol. 2016;18:191–201. doi: 10.1038/ncb3292. [DOI] [PMC free article] [PubMed] [Google Scholar]; An elegant paper that combines the use of a caged derivative of fMLF with FRET probes to demonstrate the role of CDC42 in controlling neutrophil directionality in 2D environments. The authors uncover a steep gradient of Cdc42 activity, which acts to antagonise RhoA activity and polarise the cell.
- 33.Pankov R., Endo Y., Even-Ram S., Araki M., Clark K., Cukierman E., Matsumoto K., Yamada K.M. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams A.E., Johnson D.I., Longnecker R.M., Sloat B.F., Pringle J.R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etienne-Manneville S. Cdc42 - the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 36.Zhou K., Wang Y., Gorski J.L., Nomura N., Collard J., Bokoch G.M. Guanine nucleotide exchange factors regulate specificity of downstream signaling from Rac and Cdc42. J Biol Chem. 1998;273:16782–16786. doi: 10.1074/jbc.273.27.16782. [DOI] [PubMed] [Google Scholar]
- 37.Krause S.A., Cundell M.J., Poon P.P., McGhie J., Johnston G.C., Price C., Gray J.V. Functional specialisation of yeast Rho1 GTP exchange factors. J Cell Sci. 2012;125:2721–2731. doi: 10.1242/jcs.100685. [DOI] [PubMed] [Google Scholar]
- 38•.Marei H., Carpy A., Woroniuk A., Vennin C., White G., Timpson P., Macek B., Malliri A. Differential Rac1 signalling by guanine nucleotide exchange factors implicates FLII in regulating Rac1-driven cell migration. Nat Commun. 2016;7:10664. doi: 10.1038/ncomms10664. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study combines proteomics with migration experiments in 2D and 3D assays to show that whilst the Rac GEF Tiam-1 opposes migration, the Rac GEF P-Rex1 promotes migration by enhancing the recruitment of the actin remodelling protein FLII. This suggests that GEFs direct the behaviour and downstream function of their substrate GTPases, rather than simply catalysing exchange of GDP for GTP.
- 39.Hodgson L., Spiering D., Sabouri-Ghomi M., Dagliyan O., DerMardirossian C., Danuser G., Hahn K.M. FRET binding antenna reports spatiotemporal dynamics of GDI–Cdc42 GTPase interactions. Nat Chem Biol. 2016;12:802–809. doi: 10.1038/nchembio.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Wei X., Song H., Yin L., Rizzo M.G., Sidhu R., Covey D.F., Ory D.S., Semenkovich C.F. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the importance of fatty acids in retaining Rho-family GTPase presence in the plasma membrane. Deletion of fatty acid synthase altered the levels of cholesterol and other lipids in the plasma membrane, which in turn disrupted the recruitment and activity of RhoA and Rac, establishing an unusual link between fatty acid metabolism and cell migration. This study specifically examined mouse macrophages both in tissue sections andin vivo.
- 41••.Polacheck W.J., Kutys M.L., Yang J., Eyckmans J., Wu Y., Vasavada H., Hirschi K.K., Chen C.S. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552:258. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper develops biomimetic engineered microvessels to demonstrate a novel, non-canonical notch signalling cascade that activates Rac1 to build adherens junctions in vascular endothelial cells. They show that Notch1 interacts with VE-cadherin and forms a mechanosensitive complex with Rac and upstream regulators LAR and Trio, to control endothelial integrityin vitro and in vivo.
- 42•.Hetmanski J.H.R., Zindy E., Schwartz J.-M., Caswell P.T. A MAPK-driven feedback loop suppresses Rac activity to promote RhoA-driven cancer cell invasion. PLOS Comput Biol. 2016;12 doi: 10.1371/journal.pcbi.1004909. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study combines mathematical modelling with 2D and physiologically relevant 3D cell-based assays to demonstrate the existence of feedback between Rac and RhoA that governs the switch between lamellipodial and pro-invasive filopodial cancer cell invasion.
- 43.Hetmanski J.H.R., Schwartz J.-M., Caswell P.T. Rationalizing Rac1 and RhoA GTPase signaling: a mathematical approach. Small GTPases. 2018;9:224–229. doi: 10.1080/21541248.2016.1218406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byrne K.M., Monsefi N., Dawson J.C., Degasperi A., Bukowski-Wills J.-C., Volinsky N., Dobrzyński M., Birtwistle M.R., Tsyganov M.A., Kiyatkin A. Bistability in the Rac1, PAK, and RhoA signaling network drives actin cytoskeleton dynamics and cell motility switches. Cell Syst. 2016;2:38–48. doi: 10.1016/j.cels.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallés A.M., Beuvin M., Boyer B. Activation of Rac1 by paxillin-Crk-DOCK180 signaling complex is antagonized by Rap1 in migrating NBT-II cells. J Biol Chem. 2004;279:44490–44496. doi: 10.1074/jbc.M405144200. [DOI] [PubMed] [Google Scholar]
- 46.Ren X.D., Kiosses W.B., Sieg D.J., Otey C.A., Schlaepfer D.D., Schwartz M.A. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 47.Manabe R., Kovalenko M., Webb D.J., Horwitz A.R. GIT1 functions in a motile, multi-molecular signaling complex that regulates protrusive activity and cell migration. J Cell Sci. 2002;115:1497–1510. doi: 10.1242/jcs.115.7.1497. [DOI] [PubMed] [Google Scholar]
- 48.Turner C.E., Miller J.T. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- 49.Wilson E., Leszczynska K., Poulter N.S., Edelmann F., Salisbury V.A., Noy P.J., Bacon A., Rappoport J.Z., Heath J.K., Bicknell R. RhoJ interacts with the GIT-PIX complex and regulates focal adhesion disassembly. J Cell Sci. 2014;127:3039–3051. doi: 10.1242/jcs.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dart A.E., Box G.M., Court W., Gale M.E., Brown J.P., Pinder S.E., Eccles S.A., Wells C.M. PAK4 promotes kinase-independent stabilization of RhoU to modulate cell adhesion. J Cell Biol. 2015;211:863–879. doi: 10.1083/jcb.201501072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakurai-Yageta M., Recchi C., Le Dez G., Sibarita J.-B., Daviet L., Camonis J., D’Souza-Schorey C., Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Zimmerman S.P., Asokan S.B., Kuhlman B., Bear J.E. Cells lay their own tracks - optogenetic Cdc42 activation stimulates fibronectin deposition supporting directed migration. J Cell Sci. 2017;130:2971–2983. doi: 10.1242/jcs.205948. [DOI] [PMC free article] [PubMed] [Google Scholar]; A fascinating study that shows fibronectin deposition can be driven by Cdc42, which in turn supports focal adhesion formationin vitro. This study utilised an optogenetic method to locally active Rho GTPases at sites of interest in cells migrating in 2D microenvironments.
- 54.Jacquemet G., Hamidi H., Ivaska J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr Opin Cell Biol. 2015;36:23–31. doi: 10.1016/j.ceb.2015.06.007. [DOI] [PubMed] [Google Scholar]