Abstract
New drugs that can resolve inflammation without immunosuppressive effects are at the medicinal chemistry frontier. Pro-resolving endogenously formed small molecules, i.e. the resolvins, are excellent candidates displaying such bioactions. The first total synthesis of the specialized pro-resolving mediator RvD1n-3 DPA has been achieved using the underutilized sp3-sp3 Negishi cross coupling reaction and an alkyne hydrosilylation-protodesilylation protocol. Biological evaluations revealed that this novel mediator displays low nanomolar pro-resolving properties and potently activates the human DRV1/GPR32 receptor. As such, this endogenous natural product is a lead compound for the development of novel immunoresolvents.
Keywords: Karstedt’s catalyst, sp3-sp3 cross-coupling, total synthesis, natural products, specialized pro-resolving mediators
Graphical Abstract
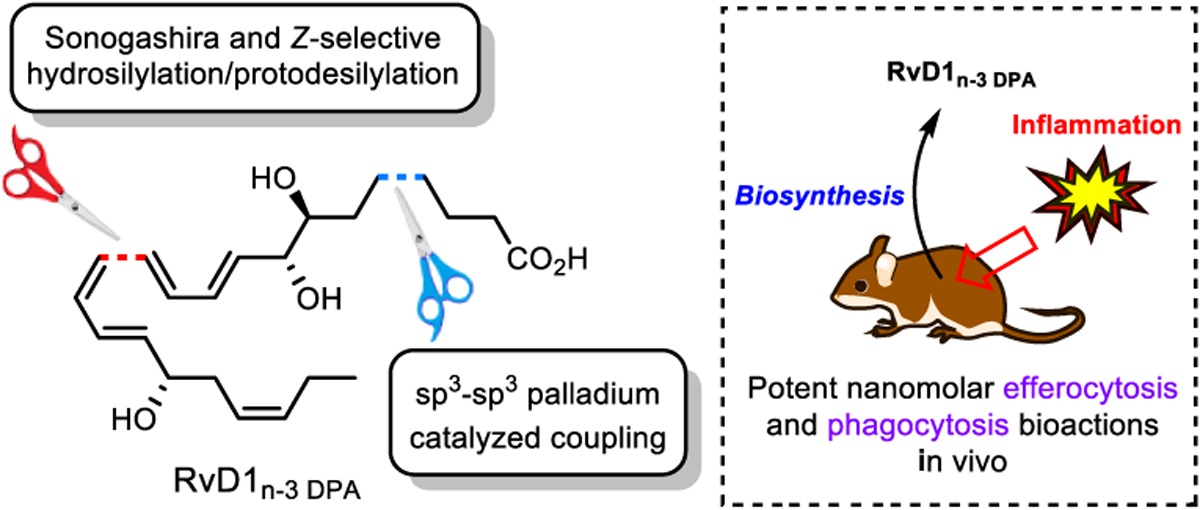
Two synthetic methods seldom used in total synthesis of natural products paved the way for the first total synthesis of RvD1n-3 DPA. The concise preparation of multi-mg quantities of this chemical sensitive lipid mediator allowed extensive in vivo and in vitro biological studies.
Inflammation is divided into acute and chronic inflammation. The acute inflammatory response is normally in health self-limited resolving on its own. It is traditionally divided into initiation and resolution phases.[1] Unresolved inflammation is associated with many human diseases such as cardiovascular disorders, cancer, rheumatoid arthritis, Parkinson and Alzheimer’s disease.[2] An increased understanding of the molecular, biochemical and cellular mechanisms involved in the resolution of inflammation have emerged recently.[3] Essential for these advancements have been the isolation, structural elucidation and total synthesis of specialized pro-resolving mediators (SPMs).[3] SPMs display potent anti-inflammatory and pro-resolving actions, often in the low nanomolar range, via G protein-coupled receptors (GPCRs).[4] The resolution of inflammation is now recognized to be an active process highly regulated by individual families of SPMs and is therefore seen as a biomedical paradigm shift.[5] SPMs are stereoselectively biosynthesized from ω−3 polyunsaturated fatty acids (PUFAs), i.e. docosahexaenoic acid (DHA), docosapentaenoic acid (n-3 DPA) and eicosapentaenoic acid.[3],[6] Since SPMs are formed only in nanogram quantities in vivo in the presence of lipoxygenases and cyclooxygenase-2, stereoselective total synthesis is essential for the assignment of the absolute configuration and even more important for detailed biological studies.
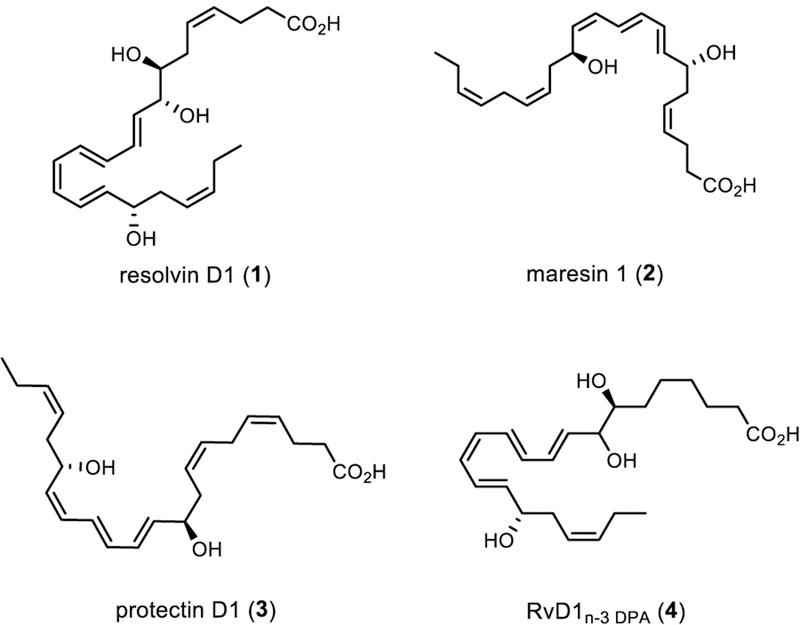
The resolvins, protectins and maresins (Figure 1) are examples of different families of SPMs that are attracting significant interest from the biomedical community.[7],[8] The DHA-derived SPM resolvin D1 (RvD1, 1) has been isolated from inflammatory exudates[8a],[8b] and its structure was later established by total synthesis and LC/MS-MS matching experiments.[9] RvD1 activates host defense mechanisms in bacterial infections,[10] enhance wound healing and ensures the return to homeostasis by initiating resolution pathways[11] after stereochemical activation of its receptors ALX/FPR2 and DRV1/GPR32.[12]
Figure 1.
Examples of SPMs.
The congener of 1, RvD1n-3 DPA (4), was recently reported and its structure partially elucidated based on UV and LC/MS-MS data.[13] The stereoselective total synthesis and biological investigations of SPMs has been in our focus over the years[14] and we present herein the first total synthesis of RvD1n-3 DPA (4) together with results from in vitro and in vivo studies. The assignment of the exact absolute configuration of 4 is established based on LC/MS-MS matching experiments. The results disclosed herein enable new strategies to control inflammatory processes using immunoresolvents in medical applications and should assist future structure-function studies useful for drug discovery processes.
Biosynthetic considerations and physical properties (i.e. MS- and UV-data) of the isolated biological produced molecule gave evidence for the proposed structure of 4 (Figure 2) with a highly sensitive E,E,Z,E-tetraene embedded by two chiral allylic alcohols; one of these is assumed to be anti-configured. In addition, the chemical sensitive Z-double bond in the tetraene part of 4 is extremely labile and prone to isomerization in the presence of light, heat or acids. Moreover, the allylic alcohols are susceptible to water elimination into the fully conjugated decomposition product.[3b],[9] This tetraenemoiety was envisioned to be formed by a Z-selective reduction of the alkyne of 4 that was supposed to be prepared from 5 and 6 (Figure 2). The alkyne 5 should be available from known alkyne 7. We also intended to utilize a sp3-sp3 cross coupling as one of the key steps in the formation of vinylic iodide 6. Of note, only a few examples exist of such cross coupling reactions applied in natural product synthesis.[15],[16] Hence, elaboration of the appropriate reaction conditions is vital for the success of the presented synthesis. Moreover, only one earlier report has utilized the Pd-catalyzed sp3-sp3 Negishi reaction in natural product synthesis.[14d] Engaging bromide 8 and zinc-reagent 9 would expand the scope of this useful transformation.[15] Functional group manipulations of the primary TBS-protected alcohol should provide key intermediate 6. Commercially available reagents 10 and 11 were chosen for large-scale synthesis of alkyne 7 and bromide 8, respectively (Figure 2).
Figure 2.
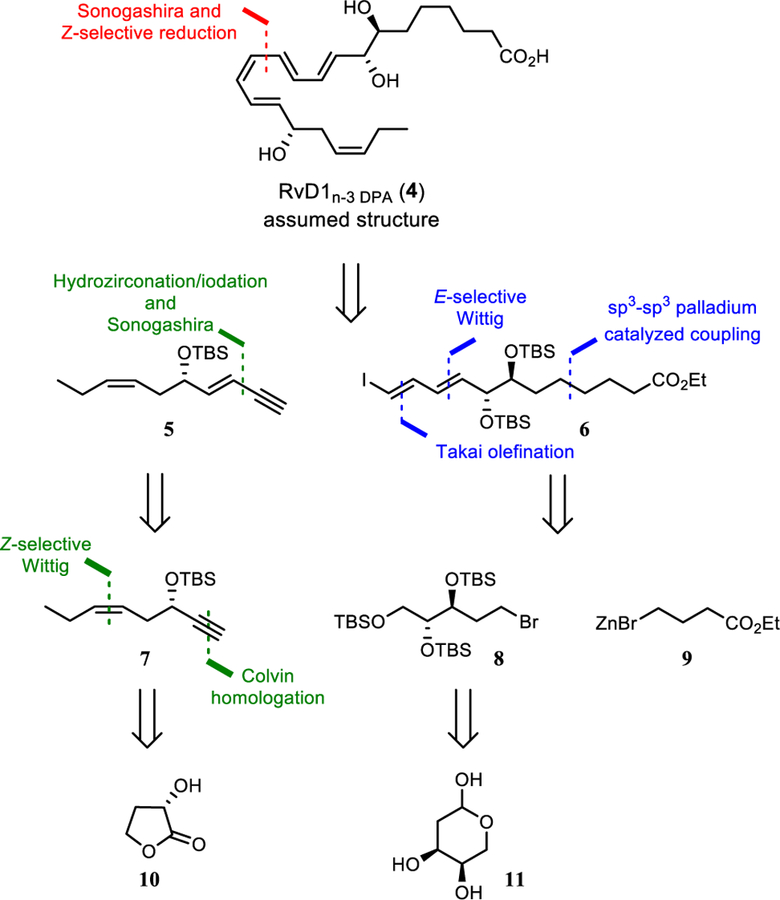
Retrosynthetic analysis.
Our total synthesis of RvD1n-3 DPA (4) commenced with preparing the known terminal alkyne 7[14d] that was reacted in a one-pot zirconation-iodination Sonogashira protocol[18] affording, via 13, geometrical pure 5 in 36% yield from 12 (Scheme 1).
Scheme 1.
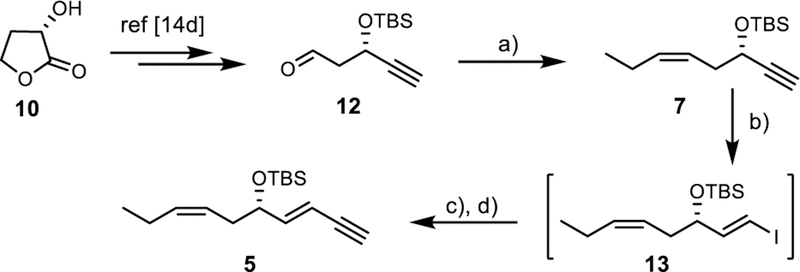
Reagents and conditions: a) BrPh3PPr, NaHMDS, HMPA, THF, - 78 °C, 20 h, 67%; b) Cp2ZrCl2, DIBAL-H, I2, THF, 0 °C, 30 min; c) TMSCCH, piperidine, CuI, Pd(PPh3)2Cl2, THF, rt., 3 h, 53%; d) K2CO3, MeOH, rt., 2h, quant.
The bromide 8 was prepared on a multi-gram scale from 2-deoxy-D-ribose (11); the latter was converted into aldehyde 15 via 14 (thioacetalization, TBS-protection, hydrolysis) in 69% yield (Scheme 2). Reduction of 15, followed by Appel halogenation, gave the bromide 8 that was reacted with 9 in a Pd-catalyzed sp3-sp3 Negishi reaction. In contrast to earlier success using this coupling reaction, only a disappointingly 10% isolated yield of desired ester 16 was obtained.[14d] All efforts to improve the yield were fruitless. The original protocol reported by Organ and co-workers use LiCl[16] as a Pd-activator. LiCl is also a source for halide exchange reactions. MS-analysis revealed that the bromide 8 had been converted into its congeneric chloride. Altering experimental conditions (Supporting information) revealed that reacting 8 and 9 in the presence of LiBr (1.1 equiv.) in THF/DMI (1:1) at 40 oC produced the wanted Negishi product 16 and increased the chromatographically isolated yield to 54%. Selective deprotection of the primary alcohol in 16 was found to work best with PTSA in MeOH yielding 17 in 57% isolated yield (81% based on recovered starting material), Scheme 2. Oxidation under Dess-Martin conditions gave the aldehyde of 17 that was subjected immediately to an E-selective Wittig reaction to yield 18. Treating 18 under standard Takai conditions[18] afforded a 16.7:1 ratio of chromatographically separable isomers enabling the required E,E-configured diene 6 to be isolated in 78% yield. The Sonogashira reaction between terminal alkyne 5 and vinylic iodide 6 was performed yielding the desired ethyl ester of the internal alkyne 19 in 85% isolated yield. Both the Lindlar and the Boland reduction on 19 and its triol failed to give the desired ethyl ester of 2, contrary to earlier reports on similar internal alkynes.[9],[19] The Karstedt alkyne hydrosilylation/protodesilylation protocol is an alternative to the Z-selective reduction of internal alkynes for making cis-olefins.[20] To our knowledge, this protocol has been utilized only once in a total synthesis of a natural product.[21] Gratifyingly, when we converted the internal alkyne in 19 in the presence of the Karstedt’s catalyst (2%) and excess dimethylethoxy silane, a regioisomeric mixture of mono-Z-substituted alkene dimethylsilyl-ethoxy ethers 20a and 20b were formed. Pleasingly, exposing this mixture to TBAF (8 equiv.) in THF gave the desired ethyl ester 21 of RvD1n-3 DPA (4) in 78% yield over the two steps. Both the UV (λmax = 301 nm (EtOH)) and NMR-data of 21 revealed stereoselective formation of the E,E,Z,E-tetraene (Supporting information). Basic hydrolysis (LiOH, H2O, THF, 0 °C) afforded the desired natural product 4 in 93% yield (Scheme 2). The overall yield was 4% (9% based on recovered starting material) over 14 steps (longest linear sequence). The spectroscopic data (UV, MS and NMR) were in accord with the assigned structure of 4.
Scheme 2.
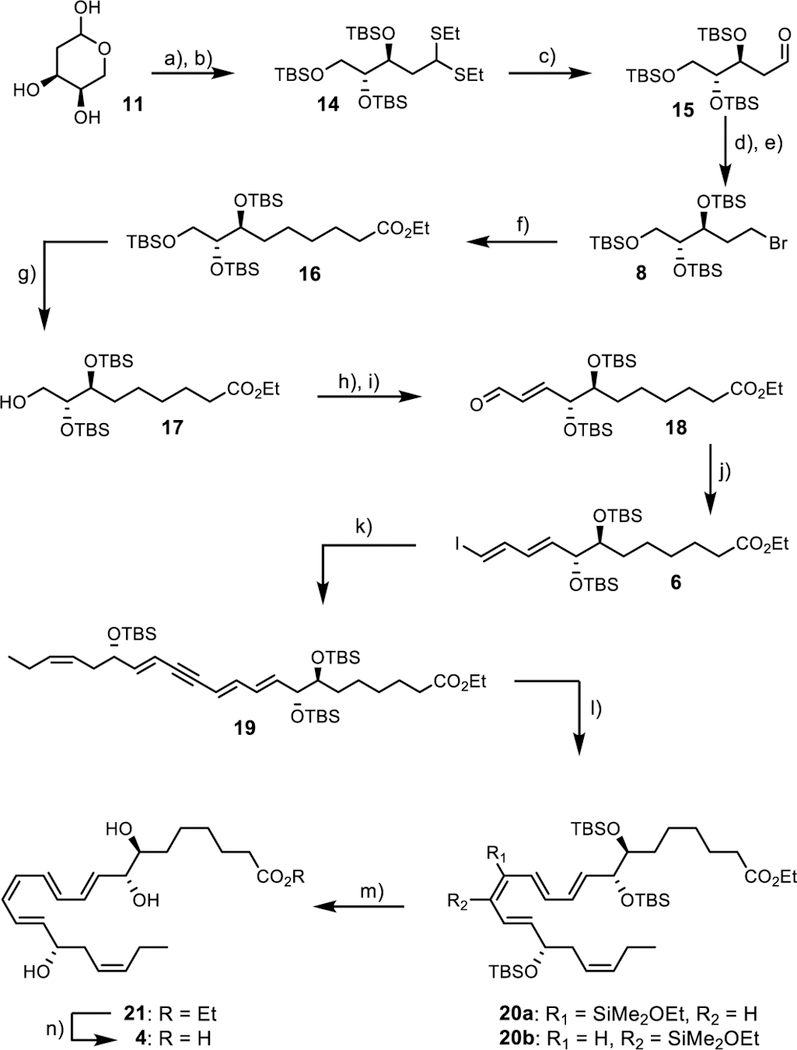
Reagents and conditions: a) EtSH, HCl, rt., 3 h, 71%; b) TBSOTf, 2,6-lutidine, 0 °C, 16 h, 97%; c) NBS, 2,6-lutidine, acetone/H2O, 0 °C, 1 h, quant; d) NaBH4, MeOH, 0 °C, 1 h, 85%; e) CBr4, PPh3, 2,6-lutidine, −10 °C, 4 h, 83%; f) BrZn(CH2)3CO2Et, Pd-PEPPSI™-IPr, LiBr, THF/DMI, 40 °C, 2 h, 54%; g) PTSA, MeOH, −20 °C, 1 h, 57% (81% brsm); h) DMP, NaHCO3, CH2Cl2, rt., 20 h, quant; i) Ph3P=CHCHO, toluene, 95 °C, 18 h, 58% (91% brsm); j) CrCl2, CHI3, THF/dioxane, 0 °C to rt., 1.5 h, 78%; k) Pd(PPh3)4, CuI, benzene, Et2NH, 5, rt. 18 h, 85%; l) Karstedt’s cat., Me2SiHOEt, toluene, rt, 16 h; m) TBAF, THF, 0 °C to rt., 20 h, 78%; n) LiOH, THF, MeOH, H2O, 0 °C, 4 h, 93%.
Since SPMs are biosynthesized in nanogram amounts in vivo matching authentic and synthetic material with results from LC/MS-MS MRM experiments are required for absolute configuration determinations. Metabololipidomics[22] LC/MS-MS experiments were used to determine to see if synthetic RvD1n-3 DPA (4) matched authentic 4. Co-injection of equal amounts of biological and synthetic materials gave a single sharp peak (RT = 11.3 min) in all experiments demonstrating co-elution of authentic and synthetic materials (Supporting information).
Evaluations of efferocytosis and phagocytosis bioactions were then performed using macrophage-based assays to determine whether synthetic 4 carried the biological actions characteristic of SPMs. Human macrophages were differentiated from peripheral blood monocytes and incubated with RvD1n-3 DPA (4) at 0.1 or 1.0 nM concentrations in order to verify the ability of synthetic 4 to promote efferocytosis, a key pro-resolving action. At both concentrations tested an up-regulation in the uptake of fluorescently labeled apoptotic cells was observed, compared to vehicle, (Figure 3A). Another hallmark pro-resolving property of SPMs is the up-regulation of bacterial phagocytosis.[23] In order to test if synthetic 4 possesses this bioaction, macrophages were incubated with fluorescently labelled E. coli in the presence or absence of RvD1n-3 DPA (4) . These experiments demonstrated that synthetic 4 increased the uptake of bacteria in a dose-dependent manner (Figure 3B). In vivo experiments with mice were also performed to test whether the biological actions with synthetic 4 observed in vitro were also carried in vivo. Mice were administered E. coli together with either vehicle or RvD1n-3 DPA (4) (50 ng/mouse) via intra peritoneal injection. After 4 h peritoneal lavages were collected and the neutrophil counts were determined. Using only 50 ng per mouse reduced the PMN number by ca 25% compared to vehicle (Figure 3C). Finally mice infected with E.coli were each administered 50 ng of 4 and phagocytosis in exudate leukocytes was determined. These results demonstrate that synthetic 4 displays protective actions characteristic of SPMs.[3]
Figure 3.
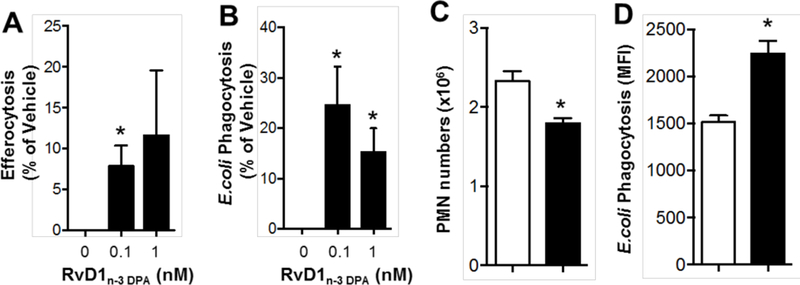
RvD1n-3 DPA (4) regulates human monocyte-derived macrophage and mouse neutrophil responses. See Supporting information for details.
SPMs act stereoselectively in pico- to nanomolar ranges as agonists for GPCRs.[4] Ca 40% of all approved drugs exhibit their activity towards this receptor class.[24] SPMs have attracted a great interest as novel lead compounds towards development of new immunoresolvent based therapeutics.[5] Among them, RvD1 (1) activates both ALX/FPR2 and human DRV1/GPR32 receptors to promote resolution of inflammation.[4], [12]
Hence, we examined whether RvD1n-3 DPA (4) and its ethyl ester also interact with recombinant human ALX/FPR2 and DRV1/GPR32 using beta-arrestin-based system.[12] With CHO-GPR32 cells, SPM 4 was the most potent compound activating beta-arrestin, followed by RvD1 (1) and RvD1n-3 DPA ethyl ester (Figure 4A). As mentioned, RvD1 (1) also activates the human ALX/FPR2 receptor.[12] With HEK-ALX cells, RvD1n-3 DPA (4) displayed similar activity as RvD1 (1), whereas the ethyl ester of RvD1n-3 DPA (4) gave much lower potency (Figure 4B). Together, these results show that 4 activates both recombinant human ALX/FPR2 and DRV1/GPR32 giving similar potencies as RvD1 (1), whereas RvD1n-3 DPA ethyl ester showed diminished activity interacting with both receptors. Overall, these results demonstrate nanomolar agonist potencies for RvD1n-3 DPA (4) and furnish valuable information on the structure-activity relationships towards developing new anti-inflammatory and pro-resolving drugs without immunosuppression.[5],[25]
Figure 4.
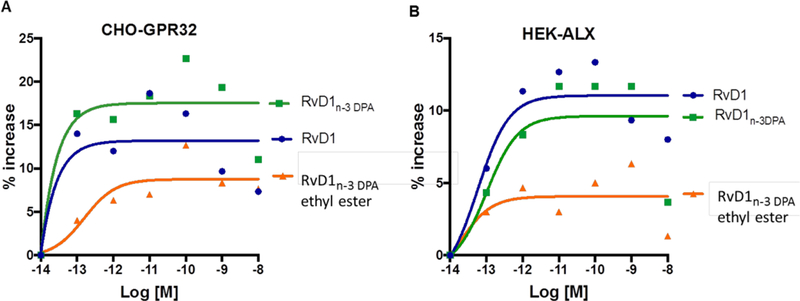
Ligand activation of human DRV1/GPR32 and ALX/FPR2. Results are dose responses analyzed by curve fitting. See Supporting information for details.
To conclude, RvD1n-3 DPA (4) has been stereoselectively prepared over 14 steps and featured the successful applications of two rarely used synthetic methods, i.e. the sp3-sp3 Pd-mediated Negishi cross-coupling reaction and the hydrosilylation/protodesilylation reaction with the Karstedt’s catalyst, in total synthesis of natural products. Our synthesis differs significantly to earlier reported synthesis of the congener RvD1 (1)[9],[19] and established the structure of 4 to be (7S,8R,9E,11E,13Z,15E,17S,19Z)-7,8,17-trihydroxydocosa-9,11,13,15,19-pentaenoic acid. The novel SPM RvD1n-3 DPA (4) exhibited nanomolar potencies in stimulating efferocytosis and phagocytosis bioactions in vitro and in vivo, as well as activated human ALX/FPR2 and DRV1/GPR32 receptors in the low nanomolar ranges. Such bioactions are of interest towards the development of new pro-resolving and anti-inflammatory remedies using SPMs as biotemplates.
Supplementary Material
Acknowledgements
The authors are grateful for collaborations and fruitful scientific interactions as well as funding for a Short Term Scientific Mission scholarship to L.G. from COST Action CM 1407. Funding to T.V.H (FRIPRO-FRINATEK 230470) from The Norwegian Research Council is gratefully appreciated. J.D. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant no: 677542) and the Barts Charity (grant no: MGU0343). J.D. is also supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 107613/Z/15/Z). C.N.S is supported by the National Institutes of Health GM Grant PO1GM095467 and continuous financial support is gratefully acknowledged.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under:
This is the peer-reviewed version of the following article: Chemistry 2019; 25:1476–80, which has been published in final form at [https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.201806029]. This article may be used for non-commercial purposes in accordance with Wiley-VCH Terms and Conditions for Self-Archiving.
How to cite:
- [1].a) Tabas I, Glass CK, Science 2013, 339, 166; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Medzhitov R, Ed. Innate Immunity and Inflammation Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2015. [Google Scholar]
- [2].Gordon S, Immunity 2016, 44, 463. [DOI] [PubMed] [Google Scholar]
- [3].a) Serhan CN, Nature, 2014, 510, 92; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Serhan CN, Petasis NA, Chem. Rev 2011, 111, 5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chiang N, Serhan CN, Mol. Aspects Med 2017, 58, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fullerton JN, Gilroy DW, Nat. Rev. Drug. Discov 2016, 15, 551. [DOI] [PubMed] [Google Scholar]
- [6].a) Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA, J. Immunol 2006, 176, 1848; [DOI] [PubMed] [Google Scholar]; b) Tungen JE, Aursnes A, Vlasakov I, Colas RA, Dalli J, Serhan CN, Hansen TV, J. Nat. Prod 2015, 78, 2924; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Primdahl KG, Aursnes M, Walker ME, Colas RA, Serhan CN, Dalli J, Hansen TV, Vik A, J. Nat. Prod 2016, 79, 2693; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrøm JZ, Petasis NA, Serhan CN, FASEB J 2013, 27, 2573; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Primdahl KG, Tungen JE, de Souza PRS, Colas RA, Dalli J, Hansen TV, Vik A, Org. Biomol. Chem 2017, 15, 8606; [DOI] [PubMed] [Google Scholar]; f) Pistorius KDF, de Souza PRS, Primdahl KG, Colas RA, Vik A, Hansen TV, Dalli J, Cell Chem. Biol, 2018, 25, 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Serhan CN, Levy BD, J. Clin. Invest 2018, 128, 2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].For isolation and structural elucidations, see a) Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL, J. Exp. Med 2002, 196, 1025; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN, J. Biol. Chem 2003, 278, 14677; [DOI] [PubMed] [Google Scholar]; c) Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG, Proc. Natl. Acad. Sci. USA 2004, 101, 8491; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ariel A, Pin-Lan L, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN, J. Biol. Chem 2005, 280, 43079; [DOI] [PubMed] [Google Scholar]; e) Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M, J. Exp. Med 2009, 206, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN, J. Biol. Chem 2007, 282, 9323; [DOI] [PubMed] [Google Scholar]; b) Uddin J, Design and synthesis of novel anti-inflammatory lipid mediators and anticancer small molecules, Ph.D. Thesis, University of Southern California, Los Angeles, CA, 2008. [Google Scholar]
- [10].Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN, Nature 2012, 484, 524; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schwab JM, Chiang N, Arita M, Serhan CN, Nature 2007, 447, 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ariel A, Serhan CN, Trends Immunol 2007, 28, 176. [DOI] [PubMed] [Google Scholar]
- [12].Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN, Proc. Natl. Acad. Sci. USA, 2010, 107, 1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dalli J, Colas RA, Serhan CN, Sci. Rep 2013, 3, 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Aursnes M, Tungen JE, Vik A, Dalli J, Hansen TV, Org. Biomol. Chem 2014, 12, 432; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Aursnes M, Tungen JE, Vik A, Colas RA, Cheng C-Y, Dalli J, Serhan CN, Hansen TV, J. Nat. Prod. Chem 2014, 77, 910; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tungen JE, Aursnes M, Vik A, Ramon S, Colas RA, Dalli J, Serhan CN, Hansen TV, J. Nat. Prod 2014, 77, 2241; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Tungen JE, Aursnes M, Dalli J, Arnardottir H, Serhan CN, Hansen TV, Chem. Eur. J, 2014, 20, 14575; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Tungen JE, Aursnes M, Hansen TV, Tetrahedron Lett 2015, 56, 1843; [Google Scholar]; f) Ramon S, Dalli J, Sanger JM, Winkler JW, Aursnes M, Tungen JE, Hansen TV, Serhan CN, Am. J. Phatol 2016, 186, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Geist E, Kirschning A, Schmidt T, Nat. Prod. Rep 2014, 31, 441; [DOI] [PubMed] [Google Scholar]; b) Haas D, Hammann JM, Greiner R, Knochel P, ACS Catal 2016, 6, 1540. [Google Scholar]
- [16].Hadei N, Kantchev EAB, O’Brien CJ, Organ MG, Org. Lett 2005, 7, 3805.. [DOI] [PubMed] [Google Scholar]
- [17].Xu C, Negishi E.-i, Tetrahedron Lett 1999, 40, 431. [Google Scholar]
- [18].Takai K, Nitta K, Utimoto K, J. Am. Chem. Soc 1986, 108, 7408. [DOI] [PubMed] [Google Scholar]
- [19].Rodriguez AR, Spur BW, Tetrahedron Lett 2012, 53, 6990. [Google Scholar]
- [20].Rooke DA, Ferreira EM, Angew. Chem. Int. Ed 2012, 51, 3225, [DOI] [PubMed] [Google Scholar]
- [21].Yang P, Yao M, Li J, Li Y, Li A, Angew.Chem. Int. Ed 2016, 55, 6964, [DOI] [PubMed] [Google Scholar]
- [22].Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN, Am. J. Physiol. Cell. Physiol 2014, 307, C39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buckley CD, Gilroy DW, Serhan CN, Immunity 2014, 40, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hauser AS, Attwood MM, Rask-Andersen M, Schiøth HB, Gloriam D, Nat. Rev. Drug Discov 2017, 16, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].a) Serhan CN, FASEB J 2017, 31, 1273; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vik A, Dalli J, Hansen TV, Bioorg. Med. Chem. Lett 2017, 27, 2259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.