Abstract
Myocardial infarction (MI), caused by coronary heart disease (CHD), remains one of the most common causes of death in the United States. Over the last few decades, scientists have invested considerable resources on the study and development of cell therapies for myocardial regeneration after MI. However, due to a number of limitations, they are not yet readily available for clinical applications. Mounting evidence supports the theory that paracrine products are the main contributors to the regenerative effects attributed to these cell therapies. The next generation of cell-based MI therapies will identify and isolate cell products and derivatives, integrate them with biocompatible materials and technologies, and use them for the regeneration of damaged myocardial tissue. This review discusses the progress made thus far in pursuit of this new generation of cell therapies. Their fundamental regenerative mechanisms, their potential to combine with other therapeutic products, and their role in shaping new clinical approaches for heart tissue engineering, are addressed.
Keywords: cardiac patches, cell therapy, exosomes, injury targeting, microRNA, myocardial infarction, synthetic stem cells
1. Introduction
Coronary heart disease (CHD) is one of the leading causes of death in the United States.[1] It is characterized by stenosis, or obstruction of the coronary artery, and leads to myocardial infarction (MI). Each year, ≈735 000 Americans suffer from a heart attack. Two thirds of those experience an infarction for the first time.[1] Although the age-adjusted rates of recurrent CHD have declined in the past decade, the recurrence and mortality rate within five years after a first MI are still high, due to the tendency for ensuing heart failure (HF).[2] Cardiac function is carried out through the rhythmic contractions of the myocardium, which is composed of cardiomyocytes, extracellular matrix (ECM), and capillary microcirculation.[3] The incidence of MI results in myocardial ischemic necrosis, which enervates cardiac function and induces the remodeling of both the infarcted and noninfarcted zones of the myocardium. During this remodeling process, the infarcted myocardium begins to scar over and expands with time. The maturing scar restricts proper contraction biomechanics, leading to myocardial hypertrophy, left ventricular dilation, HF, and death.[4] The key to preserving heart function after MI lies in saving more of the viable myocardium while dialing back the disruptive role the myocardial scar plays in the dilation of the ventricle.
The regeneration of tissues is a complex and well-choreographed biological phenomenon that restores tissue architecture, morphology, and function through the replacement of unhealthy/damaged components via cell proliferation and differentiation. The heart myocardium, unlike naturally regenerative tissues, was once considered to be terminally differentiated, without regenerative abilities after injury.[5,6] However, this common assertion has been challenged by a number of studies. The heart of zebrafish, for example, was found to regenerate after serious injury,[7,8] due to the specificity of the myocardium environment and proliferative cardiomyocytes.[9] Additionally, neonatal mice cardiomyocytes were found to regenerate mainly through pre-existing cardiomyocytes.[4] Scientists now believe that the human heart is capable of some level of regeneration with varying degrees of myocardium renewal.[10,11] Recently, researchers achieved the regeneration of adult cardiomyocytes from mice and humans by regulating their cell cycle.[12] Researchers generated induced-pluripotent stem cells (iPSCs) from fibroblasts or bone marrow cells for heart repair using various reprogramming techniques.[13,14] Also, embryonic stem cell (ESC)-derived cardiomyocytes demonstrated their potential for cardiac tissue engineering in preclinical animal models.[15] As an important heart regenerative therapy method, adult stem cell therapy has become one of the most eye-catching research topics, generating a large array of preclinical and clinical studies. In a clinical trial using cardiosphere-derived autologous stem cells for the reversal of ventricular dysfunction (CADUCEUS), the MI patients who were intracoronarily infused with cardiosphere-derived cells (CDCs), experienced reduced infarct-scar mass, increased viable heart tissue, a thickened infarction-relative wall, and increased regional contractility.[16,17] Additionally, bone marrow-derived mesenchymal stem cells (MSCs)[18,19] and adipose tissue-derived stem cells[20] have been shown to improve cardiac function for MI injured hearts. There is mounting evidence demonstrating that most adult stem cells impart their therapeutic benefits primarily through paracrine factors, which alter the microenvironment of the cardiac ECM and regulate the remodeling process after MI injury.[21–26] Although cell therapies have the potential to significantly advance the field of cardiac regenerative medicine, there are many challenges hampering the utility of cell therapies in clinical applications. Our review will address the opportunities and challenges involved with cardiac cell therapy, as well as the things we can learn from biomaterials and bioengineering approaches to drive cell therapies to the next generation. Our review will also summarize both cellular and noncellular approaches (or the combination of the two) for cardiac regenerative medicine purposes.
2. Paracrine Mechanisms
2.1. Paracrine Mechanisms of CDCs
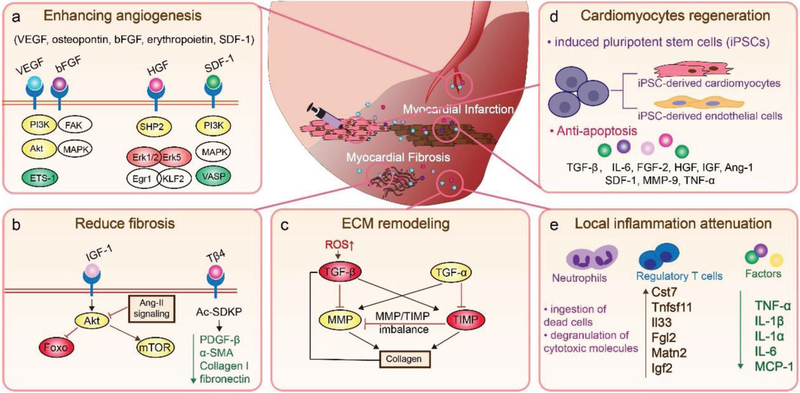
CDCs are derived from myocardial tissue samples and represent a natural mixture of intrinsic cardiac stromal cells (CSCs). They consistently express CD105, partially express CD90, and are negative for hematopoietic biomarkers such as CD45, CD31, and CD34. The fraction of ckit-positive cells in CDCs is negligible and they do not contribute to the overall therapeutic benefits of CDCs. Studies have reported the ability of CDCs to protect the heart by secreting vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), stromal cell-derived factor 1 (SDF-1), insulin-like growth factor 1 (IGF-1), and basic fibroblast growth factor (bFGF)[27–29] (Figure 1). VEGF is a signaling protein that binds to the surface of endothelial cells, ECM proteins, and other molecules. It induces angiogenesis in the injured heart by encouraging the differentiation of vascular endothelial cells through the calcium signaling pathway and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway.[30] Other angiogenic factors, such as bFGF, which binds to heparan sulfates in the ECM, help mediate endothelial cell migration, proliferation, and tube structure formation.[31] HGF, which is found in elevated levels in the heart, helps to prevent oxidative stress after MI and promotes self-repair through the HGF/Met signaling pathway. Studies have shown that transplanted CDCs release HGF, which enhances the HGF/Met system, improving angiogenesis, repressing immunomodulation, and reducing fibrosis.[32,33] By stimulating the SDF-1/CXCR4 axis, SDF-1 preserved viable cardiomyocytes and increased vascular density in a mouse model of acute MI.[34] In addition, as a strong chemokine, SDF-1 is an effective recruiter of endothelial progenitor cells (EPCs) from bone marrow.[35] IGF-1 stimulates the Akt/Foxo pathway and plays an essential role in preventing angiotensin II-induced cardiac inflammation and fibrosis.[36,37]
Figure 1.
Regenerative factors from transplanted cells mediate communication with the surrounding cardiac tissue and change the extracellular microenvironment to attenuate local inflammation. a) Stem cell secreted factors enhance angiogenesis by mediating endothelial cell migration and proliferation. b) Factors also prevent cardiac inflammation and fibrosis after infarction. c) Factors play an important role in post-MI ECM remodeling, such as balancing the expression of MMP and TIMP. d) Factors contribute to cardiomyocyte regeneration by promoting the recruitment and differentiation of stem cells, and direct cardiomyocyte preservation and regulation. e) Factors attenuate local inflammation through microenvironment alteration.
2.2. Paracrine Mechanisms of MSCs
MSCs in animal MI models have been demonstrated to attenuate the expression of collagen types I and III in the cardiac ECM via paracrine signaling.[21] They have also been reported to decrease tissue inhibitors of metalloproteinase (TIMP)-1 and increase matrix metalloproteinases (MMPs), which have a distinct spatial and temporal role in cardiac remodeling.[38] After MI injury, MMPs expressed by infiltrated macrophages and fibroblasts, especially MMP-2 and MMP-9, trigger regenerative signals through the MMP/TIMP axis, and mediate ECM protein degradation, cell proliferation, and migration.[38,39] Furthermore, adrenomedullin overexpression in MSCs significantly improved heart function and decreased the heart fibrosis area.[40] Thymosin-β4 (Tβ4), which is also secreted by MSCs, restores cardiac function and contributes to cardiac repair after MI injury.[41,42] Tβ4 proteins suppress the epigenetic repressor methyl-CpG-binding protein 2. In doing so, they reverse the expression of peroxisome proliferator-activated receptor-γ and downregulate fibrogenic genes, platelet-derived growth factor (PDGF)-β receptor, α-smooth muscle actin, collagen I, and fibronectin, resulting in reduced fibrosis.[43] Thus, these paracrine signals, released by MSCs to communicate with surrounding cardiac cells, stimulate the production of regenerative factors that have the potential to heal damaged tissues (Figure 1).
2.3. Paracrine Mechanisms of Cardiomyocytes Derived from iPSCs
The successful reprogramming of somatic cells, such as fibroblasts, into iPSCs, is a cornerstone for regenerative medicine therapies. iPSCs from a cardiac patient could be differentiated into cardiomyocytes (or endothelial cells in vitro), and transplanted back into the patient as a way of reducing the risks of immune rejection and tumor formation. A safer way to apply iPSC therapy is to use its secretome instead of live cells. One of the mechanisms that iPSCs use to exert their cardiac protection is paracrine signaling.[44] For example, after the intramyocardial injection of iPSC-derived cardiomyocytes in the hearts of MI mouse models, researchers found that these cells protected the injured myocardium more effectively than undifferentiated stem cells because of their upregulated production of paracrine cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-8, granulocyte colony stimulating factor, and VEGF. In addition, the iPSC-derived cardiomyocytes also promoted cell migration by releasing the paracrine signaling molecules plasminogen activator inhibitor 1 and vascular cell adhesion molecule 1. These factors not only enhanced cell engraftment and promoted angiogenesis, but also increased cell proliferation and inhibited apoptosis, leading to the repair of the MI injured myocardium (Figure 1).[45] Moreover, the extracellular vesicles secreted from iPSC-derived cardiomyocytes reduced arrhythmic burden and promoted cardiac function recovery.[46]
2.4. Modulation of Local Cardiac Inflammation by Paracrine Factors
Local inflammation after MI plays an important role in cardiac remodeling. The infarct triggers the increased production of inflammatory cytokines through the upregulation of NF-kB expression.[47] The inflammatory cytokines include TNF-α, IL-1β, IL-1α, IL-6, and free radicals.[48,49] Neutrophils, prompted by IL-8, C5a, N-formyl-methionyl-leucyl-phenylalanine, and the leukotriene B4 inflammatory cascade, migrate to the infarcted myocardium, where they ingest dead cells and degranulate cytotoxic molecules.[50] Additionally, IL-1 drives resident and infiltrated macrophages to synthesize proteases and chemokines, and consume about 40% of the apoptotic cardiac cells.[50] Post-MI remodeling also activates MMPs that degrade cardiac ECM rapidly. This degradation releases matrix fragments that drive inflammation.[51] It has been reported that transplanted MSCs have attenuated local inflammation by secreting paracrine signaling molecules into the microenvironment, leading to the decreased expression of TNF-α, IL-1α, IL-6, and monocyte chemoattractant protein-1.[21] Furthermore, the administration of IL-10-enriched MSCs reduced apoptosis of cardiac cells via upregulated PI3K/Akt pathways.[52] The over expressed IL-10 also stimulated CD11b+Ly6G− macrophage polarization toward osteopontin-producing macrophages (galectin-3hi CD206+) for cardiac tissue repair and HF prevention after MI.[53] This macrophage polarization process was caused by an enhanced IL-10-STAT3-galactin-3 axis.[53] The overexpression of six factors (Cst7, Tnfsf11, Il33, Fgl2, Matn2, and Igf2) secreted by regulatory T cells promoted cardiomyocyte proliferation via paracrine signaling (Figure 1).[54]
3. Opportunities and Challenges for Clinical Application
Stem cell therapies targeting the heart myocardium are clinically applicable. However, the efficacy of cardiac cell therapy is hampered by a number of limitations.[55] Their ability to differentiate and replicate with ease makes stem cells attractive therapeutic agents, but also increases the risk of aberrant, uncontrolled replication, which can induce tumorigenicity.[56] This risk should always be taken into consideration in clinical practice.[55] In addition, the heterogenic or allogeneic transplantation of stem cells may result in immunological issues.[55] Furthermore, the manufacturing, storage, and transportation of stem cells is complex, expensive, and time-consuming, making it difficult to meet batch quality specifications and pharmaceutical regulations. Moreover, since the stem cells migrate from blood vessels to the heart via active vascular expulsion[57] or angiopellosis,[58] the clinical application of stem cells through vein is restrained by low cell retention/engraftment rates and poor survival rates, which minimizes their long-term treatment efficacy.[55,59] The intramyocardial injection of stem cells usually requires open-chest or thoracoscopic surgery, increasing the changes of aggravating MI patients with secondary injuries and of introducing infections. Furthermore, cell therapy increases proarrhythmic risks through three major mechanisms: reentry, automaticity, and triggered activity.[60,61] Thus, cardiac stem cell therapies need to be improved before they can be successfully applied in a clinical setting.
4. Harnessing Paracrine Mechanisms to Improve Stem Cell Therapy
The role of stem cell paracrine signaling in myocardial regeneration has been studied for decades and has advanced our understanding of stem cell regenerative therapeutics.[22] However, the identification of paracrine factors and their functions remains a challenge due to the overwhelming range of multifunctional molecules and the cross-interactive signaling pathways involved. In addition, the diversity in the temporal and spatial expression patterns of the molecular factors and signaling pathways make the process even more complicated. Moreover, paracrine signaling not only impacts cardiac regenerative therapy, but also cardiac excitation–contraction coupling, the orchestrated process of initial myocyte electrical excitation.[62] Thus, paracrine factors contribute to the stem cells’ regenerative efficacy as an orchestrated system. Relevant future studies will strengthen our knowledge of the complex processes, possibly providing us with methods and directions that will help optimize stem cell therapy for MI and take it to the next level.
5. A New Era for Cardiac Cell Therapy: Integrating Biomaterials and Nanomedicine
Biomaterials, such as polymers and native tissue derivatives, are materials that can be used for diagnosing, treating, repairing, or replacing diseased tissues and organs. Unlike medications, the function of biomaterials is structure-related and hardly affected by pharmacological or immunological activities. Usually, biomaterials are integrated with different drugs to promote therapeutic availability and efficiency. The study of synthetic stem cells, cardiac patches, and nanomedicine, has ushered stem cell therapy into a new era.
To solve the aforementioned limitations and exploit the paracrine effects of live cell therapy, scientists have been focused on the interdisciplinary development of nonlive, synthetic stem cells,[63,64] nanorobots,[65–67] and cardiac patches[68] for MI treatment (Figure 2). So far, many of these studies have produced exciting results in both rodent and relevant large animal models. In the following sections, we will summarize the fundamental mechanisms used by these new cell-derived products, as well as the progress made in their development to date.
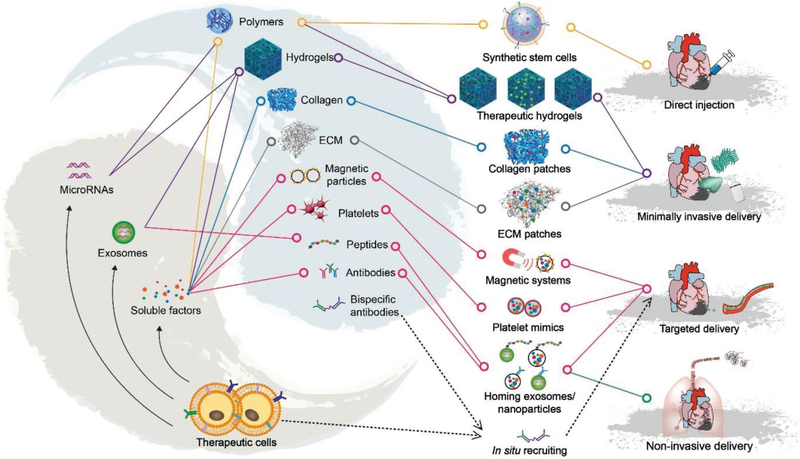
Figure 2.
Toolbox elements for a new era of cardiac regenerative medicine. Stem cell-driven regenerative therapeutics will usually involve the integration of stem cells with artificial/natural materials. Such integration leads to the fabrication of new regenerative medicine products, including synthetic stem cells, acellular cardiac patches, and targeting nanoparticles. The research on drug delivery methods has evolved from open-chest surgical procedures to minimally invasive procedures, noninvasive inhalation, and the intravenous delivery of drugs with specific targeting abilities.
5.1. Synthetic Stem Cells: Opportunities
The primary goal of engineering a synthetic stem cell is to selectively preserve their beneficial therapeutic functions and remove their drawbacks according to treatment needs. This is a practice that seeks to pharmacoengineer stem cell therapies using drug composition and delivery principles. Previously, nanoparticles coated with cancer cell membranes were created for cancer immunotherapy.[69] Recently, synthetic stem cells were fabricated by encapsulating stem cell secretome with the biodegradable and biocompatible polymer poly lactic-co-glycolic acid (PLGA). The encapsulation was achieved with the water/oil/water emulsion technique.[63] The PLGA capsule was then coated with stem cell membranes using well-established methods.[67–71] These synthetic stem cells are similar in size to their natural homologues, and encapsulate regenerative factors normally secreted by live cells. In addition, PLGA provides a safe, nontoxic, biodegradable polymer that has been used in various control-release systems.[72] These synthetic stem cells mimic the paracrine processes of live stem cells[63] and effectively eliminate the danger of aberrant stem cell differentiation, which may cause tumorigenicity (Figure 3).[55] For example, the intramyocardial administration of synthetic stem cells made from human CSCs[63] and human bone marrow-derived MSCs[64] was shown to preserve cardiac function in a mouse model of MI via paracrine mechanisms.
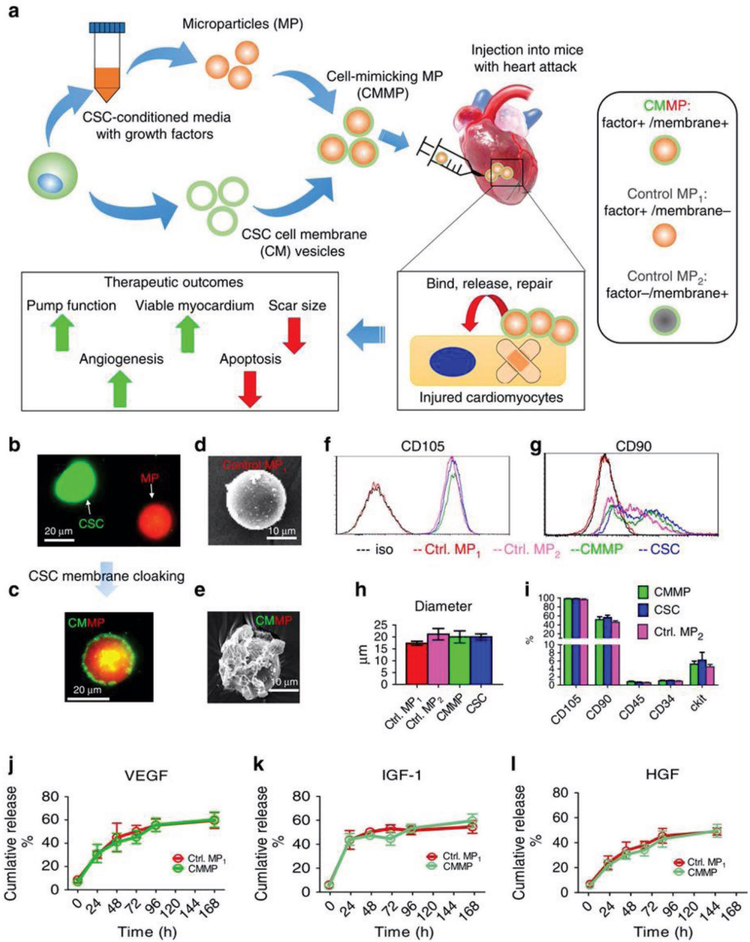
Figure 3.
Fabrication of synthetic stem cells, or cell-mimicking microparticle (CMMP). a) Overall biochemical design and study model of CMMPs. MPs (that is, Control MP1) were fabricated from PLGA and conditioned media from human CSCs. Then, MPs were cloaked with membrane fragments of CSCs to form CMMPs. Control MP2 was fabricated by cloaking empty PLGA particles with CSC membranes. The therapeutic potential of CMMPs was tested in a mouse model of MI. b,c) Texas red succinimidyl ester-labeled MPs (b, red) were cloaked with the membrane fragments of green fluorescent DiO-labeled CSCs (b, green) to form CMMP (c, red particle with green coat). Scale bar = 20 μm. d,e) Scanning electron microscope revealed the CSC membrane fragments on CMMPs (e) but not on Control MP1 (noncloaked MP) (d). Scale bar = 10 μm. f,g) CMMPs and Control MP2 were positive for major human CSC markers CD105 (f) and CD90 (g), indicating the successful membrane cloaking on CMMPs. Noncloaked Control MP1 was not positive for either. h) CMMPs, Control MP1, and Control MP2 have similar in size to CSCs. n = 3 for each group. i) CMMPs and Control MP2 carried surface antigens that were similar to CSCs. n = 3 for each group. j–l) Similar release profiles of CSC factors (namely, VEGF, IGF-1, and HGF) were observed in CMMPs and Control MP1, indicating membrane cloaking did not affect the release of CSC factors from CMMPs and Control MP1. n = 3 for each time point. All data are mean ± s.d. Comparisons between any two groups were performed using a two-tailed unpaired Student’s t-test. Comparisons between more than two groups were performed using one-way ANOVA, followed by the post hoc Bonferroni test. This work is licensed under (a). Reproduced with permission under the terms of the Creative Commons Attribution 4.0 International License.[63] Copyright 2018, Springer Nature.
5.2. Synthetic Stem Cells: Challenges
These studies not only proved the synthetic stem cell concept successfully but also demonstrated its potential for clinical application. However, there are still significant challenges keeping synthetic stem cells from transitioning to clinical practice. The first involves their optimization. The protein composition of the encapsulated regenerative secretome needs to be better defined.[73] Additionally, the treatment efficacy of each batch is hard to determine before administration, as there are no established standards. In addition, the use of different cell lines confers batch variability to the synthetic stem cells because each line may produce secretomes with slightly different protein signatures.[74] To insure consistency, it will be important to optimize/characterize the secretome stock and reduce variables during the manufacturing process. Although the secretome profile may be changed by controlling the in vitro conditions,[75,76] changing the secretome profile in vivo is not currently possible with synthetic stem cells. Furthermore, it will be essential to find a delivery method or to modify the synthetic cells in such a way that the problem of post administration retention is minimized. Currently, <10% of injected synthetic stem cells remain in the heart myocardium 7 days after injections.[63] Moreover, to make the clinical procedure safer, the synthetic stem cell administration paradigm should avoid, or minimize, the use of invasive surgical procedures that can further injure the heart myocardium.
5.3. Exosomes and microRNAs (miRNAs): Opportunities
To study the therapeutic effects of exosomes, scientists have isolated them from various types of stem cells (i.e., MSCs, cardiac progenitor cells (CPCs), ESCs, iPSCs, and CD34+ EPCs)[77–79] through different isolation techniques, including ultracentrifugation, size isolation, and immunoaffinity capture. In vivo, exosomes are secreted by most cell types through the fusion of multivesicular bodies with the plasma membrane.[80,81] Once secreted, they are imbibed by recipient cells through receptor–ligand interactions, direct membrane fusion, and endocytosis/phagocytosis.[82] Therefore, exosomes may potentially play a role as intercellular communicators.[83] Also, as bilipid membrane vesicles, with diameters between 30 and 150 nm,[77,84] exosomes usually carry membrane-bound or encapsulated proteins and miRNAs with the capability to trigger various complex molecular processes and pathways.[85] Studies show that stem cell-derived exosomes exert their cardiac therapeutic benefits mainly through the gene products and miRNAs they carry, which stimulate angiogenesis,[78] decrease cell apoptosis,[86] and reverse injury caused by inflammation.[87] Studies by Arslan et al. and Ibrahim et al., for example, showed that stem cell-derived exosomes can treat post-MI heart dysfunctions.[81,88] The following is a recapitulation of the molecular signals and pathways that drive the exosomal-mediated regenerative/protective therapies for MI heart.
MSC-derived exosomes contain glyceraldehyde 3-phosphate dehydrogenase, enolase, pyruvate kinase m2, phosphoglycerate kinase, and phosphoglucomutase. These are important enzymes that increase ATP production by upregulating phosphofructose kinase levels.[81] The MSC-derived exosomes also contain peroxiredoxins and glutathione S-transferases that can depress oxidative stress. Furthermore, the MSC-derived exosomes activate adenosine receptors and phosphorylate the PI3K/Akt signaling pathway.[81,89] Additionally, the intramyocardial injection of CSC-derived exosomes has been shown to decrease infarct size and preserve left ventricular ejection fraction after MI injury.[88,90] When the release of exosomes in CSCs was suppressed by GW4869, a reversible inhibitor of neutral sphingomyelinase, the therapeutic efficacy of CSCs decreased.[91] It is important to note that the cells that receive and imbibe the released exosomes also play an important role. Fibroblasts uptake CSC-derived exosomes and modify their own secreted exosomes. These modified exosomes can increase collagen degradation by MMPs and decrease collagen production through transforming growth factor beta 1 (TGF-β1) inhibition, helping to minimize the size of the infarct scar.[92] Moreover, exosomes derived from ESCs have been shown to enhance a number of cells in ways that induce cardiac repair. Namely, they enhance the pluripotent markers octamer-binding transcription factor 4, sex determining region Y-box 2, and Nanog in embryonic fibroblasts, reduce caspase-3 cleavage in H2O2-stressed H9c2 myoblasts, and increase tube formation in human umbilical vein endothelial cells (HUVECs) in vitro.[93] Also, ESC-derived exosomes play a key role in promoting endogenous repair by regulating endogenous stem cell functions.[93]
The therapeutic efficacy of exosomes is also mediated by the miRNAs inside of the exosomes. These noncoding miRNAs can regulate many important cellular pathways involved in cardiac regeneration. For example, miR-146a, from CDC-derived exosomes, is known to suppress MI injury via the targeting of Irak-1 and Traf6, and plays a major role in inflammation attenuation by dampening the toll-like receptor signaling pathway.[94] In addition, miR-146a reduces oxidative stress from ischemic injury by suppressing the reduced nicotinamide adenine dinucleotide phosphate oxidase Nox-4, a molecule involved in producing oxygen radicals in cardiovascular pathophysiology.[95] Exosomal miR-21–5p, from MSCs, increases cardiac contractile force and calcium handling by regulating PI3K signaling.[96] The importance of miRNA content in the therapeutic profile of exosomes has motivated some scientists to use an miRNA-mimicking approach to promote cardiomyocyte proliferation and cardiac regeneration.[97] Table 1 provides a detailed summary of miRNAs related to cell therapy for MI treatment.
Table 1.
The object and function of miRNAs in cardiac therapy.
| miRNA | Object | Function | Ref | |
|---|---|---|---|---|
| miR-1 | Inhibition | myoD | Reprogram fibroblasts into cardiomyocytes muscle growth and differentiation | [98] |
| miR-1–2 | Inhibition | Irx4, Hrt2, Hand1, Gata6 | Regulation of cardiac growth and differentiation, electrical conduction, and cell-cycle control | [99] |
| miR-126 | Inhibition | Spred1, PIK3R2/p85-β | Directly repressing negative regulators of the VEGF pathway; regulates angiogenic signaling and vascular integrity; stem/progenitor cells differentiation | [100] |
| miR-132 | Inhibition | RasGAP-p120 | Enhancing tube formation in endothelial cells | [101] |
| miR-133a | Inhibition | SRF, cyclin D2, | Regulation of cardiac growth and differentiation, electrical conduction, and cell-cycle control | [102] |
| miR-146 | Inhibition | IRAK and TRAF6 | Preventing NF-κB activation, inflammatory cell infiltration | [103] |
| miR-15a, miR-195 | Inhibition | Chek1 | Cardiac regeneration; associated with an increased number of mitotic cardiomyocytes |
[104] |
| miR-17–92 | Inhibition | STAT3, PTEN | Cardiac development and regeneration; cardiomyocyte proliferation in postnatal and adult hearts | [105] |
| miR-19a | Inhibition | PTEN; SPRY2 |
Antiapoptotic; stem/progenitor cells differentiation; the activation of the Akt and ERK signaling pathways |
[106] |
| miR-204 | Inhibition | Jarid2 | The proliferation and differentiation of human cardiomyo-cyte progenitor cells (hCMPCs) into cardiomyocytes | [107] |
| miR-21 | Inhibition | PTEN PI3K |
Induced angiogenesis; AKT and ERK activation | [108] |
| miR-210 | Inhibition | ephrin A3 and PTP1b | Inhibiting apoptosis in cardiomyocytic cells | [109] |
| miR-22 | Inhibition | Mecp2 | Reduced cardiac fibrosis; reduced apoptosis in ischemic cardiomyocytes, ameliorated fibrosis, and improved cardiac function post-MI |
[110] |
| miR-221 | Inhibition | BIM/BCL2L11; p53 | Vascular remodeling; increasing efficiency of cardiac cell transplantation and heart regeneration |
[111] |
| miR-208 | Inhibition | β-MHC | Enhance the reprogramming of fibroblasts into cardiomyocytes | [112] |
| miR-294 | Promotion | c-myc; Klf4 | Plays a central role in regulating CPC cell cycle in association with promoting proliferation, survival | [93] |
| miR-302 | Inhibition | Hippo pathway | Cardiac regeneration persistent dedifferentiation in cardiomyocytes | [97] |
| miR-451 | Inhibition | GATA4 | Inhibiting caspase 3/7 activation and cardiomyocyte apoptosis | [113] |
| miR-499 | Inhibition | Sox6, Rod1 | Enhance the reprogramming of fibroblasts into cardiomyocytes | [114] |
5.4. Exosomes and miRNAs: Challenges
There is emerging evidence suggesting that exosomes released from various stem cells exert their therapeutic potential via the mediation of intercellular communications, the regulation of signaling pathways, and cell reprograming. However, the complexities of the massive and multifunctional exosomal proteins and miRNAs[115,116] have, thus far, prevented us from understanding the therapeutic mechanisms in detail. Thus, we still have many questions left to explore, such as where the signaling initiation begins, what the long-term therapeutic side effects are, how we can distinguish between exosome-induced cardiac regeneration, repair, or preservation, what the mechanism of exosome-mediated reprograming is, how to standardize the efficiencies and dosages for MI hearts, and the biogenesis of massive miRNAs, among others. From a quantitative, systems biology perspective, future research needs to better define the role of exosomes derived from different cells, the content of exosomes, and exosome targeting.[117] It is also important to understand the stimulation dynamics/protocols that lead to miRNA signature differences in exosomes within and between cell lines.[117] For example, unlike the exosomes from regular MSCs, the exosomes from SuxiaoJiuxin-treated MSCs can specifically upregulate the protein expression of a key epigenetic chromatin marker, histone 3 lysine 27 (H3K27), in HL-1 cardiomyocytes. The upregulation of H3K27 is achieved via the repression of its ubiquitously transcribed tetratricopeptide repeat, X chromosome.[118] Additional challenges that must be overcome before exosomes can be transplantable drugs include their targeting to sites of injury, retention problems, the lack of long-term efficacy data, and the need for long term cytotoxicity studies. Nonetheless, despite these challenges, exosomes and miRNAs have the potential to be the next generation of therapeutics, moving us away from the stem cell approach and into the molecular level, using their byproducts.
5.5. Cardiac Patch: Opportunities
In the past two decades, tissue engineered cardiac patches have been designed to improve cardiac function after MI, with positive results seen in both small and large animal models.[119,120] To improve this therapeutic approach, scientists have sought optimal cell and material combinations as part of their efforts to refine patch creation and delivery.[121,122] Single layer cell patches were created previously. These earlier iterations suffered from cell death due to lack of blood supply after transplantation.[123] To solve this issue, researchers began to add other cell types into the patch, including ESCs, iPSCs, endothelial cells, and so forth.[124,125] These additions led to multiple-cell layered cardiac patches with microvessels composed of self-assembled human vesicular endothelial cells that successfully integrated with the host four weeks after implantation.[126] More recent iterations offer a larger array of scaffolding material. For example, some scaffolds are made by suspending cells in a matrix of biomaterials. This scaffolding technique is better at achieving vascular integration compared to using cell sheets. Thus, these patches are more suitable for surgical applications.[121] Among the materials typically used to create cardiac patch scaffolds, the most common are collagen, fibrin, and an array of polymers. These are typically infused with synthetic agents or cellularized with therapeutic cells. Some patches, derived from animal products, can be decellularized and then recellularized with therapeutic cells, or infused with other beneficial agents.[127] Since ECM has a tissue-specific protein composition, researchers enhanced the bioactivity of decellularized amniotic membranes for future cardiac applications by intergrading cardiac ECM hydrogel.[128] Recently, an ECM patch, 3D-printed with bioinks composed of cardiac ECM, human CPCs, and gelatin methacrylate, has been developed for heart repair, establishing the possibility of using bioprinting as a cardiac patch fabrication method.[129] Also, poly(N-isopropylac rylamine-co-acrylic acid) or P(NIPAM-AA) nanogel was used to encapsulate cells for MI treatment.[130] Moreover, a new biomimetic microvessel (BMV)-integrated cardiac patch was created by leveraging microfluidic hydrodynamic focusing to construct biomimetic microvessels. The BMV lumens are lined with HUVECs and encapsulated in a fibrin gel spiked with human CSCs.[131] This endothelialized BMV patch mimicked the natural vascular structure of the heart, helping it integrate with the host myocardium after implantation.[131] Also, a human cardiac muscle patch was generated by suspending iPSCs, including cardiomyocytes, smooth muscle cells, and endothelial cells, in a fibrin scaffold. This muscle patch can beat synchronously in vitro during 7 days of culture. It significantly reduced infarct scar size and left ventricular wall stress after transplantation in swine MI models.[132] Unfortunately, cardiac patches have had a slow path to clinical applications due to aforementioned live-cell therapy limitations.[133] Thus, we will introduce cardiac patches without live cells (but with acellular substances such as proteins, RNAs, or ECM alone) for MI treatment.
5.5.1. A Microparticle-Incorporated Collagen Cardiac Patch
Collagen is one of the most prevalent extracellular components of the myocardium and can be molded into a variety of shapes.[121] Recently, researchers developed collagen-alginate cross-linked scaffolds with IGF-1- or HGF-loaded alginate microparticles using a spray-drying technique.[126] In vitro testing of the patch on CSC substrates suggested that it was capable of releasing both proteins for 15 days. The sustained release of IFG-1 and HGF increased CSC mitogenic and proliferative effects.[127] Although this cardiac patch has not been used in vivo yet, it provided a successful in vitro model that can be used to compare the regenerative potentials of different protein factors that can be utilized to optimize the control release properties of patches.
5.5.2. A Spray Painted Platelet-Fibrin Cardiac Patch
To solve the problem of open-chest surgery for cardiac patch placement, researchers created a spray-paintable, polymerizable biomaterial that can be applied to the heart myocardium using a minimally invasive procedure (Figure 4).[134] Due to its rapid clot-forming properties, platelet-fibrin gel was used as a new biomaterial for cardiac repair in animal studies.[135] To increase the regenerative character of platelet-fibrin gel, researchers integrated several regenerative stem cell factors, including VEGF, IGF-1, HGF, TGF-β, and PDGF, into the gel during the fabrication process. To create the spray patch, calcium-containing media solution and platelet rich plasma were placed in separate lumens of a double-lumen syringe. A spraying device used compressed CO2 gas to drive down the pistons on the dual-lumen syringe, spraying the evenly mixed product directly onto the injured heart. Under scanning electron microscopy, the cardiac patch spray formed a fibrous structure quickly after implantation. This study showed that this cardiac patch can release the regenerative growth factors efficiently in the first two weeks after being sprayed. The spray patch increased the viability of cultured neonatal rat cardiomyocytes in vitro. It also effectively preserved cardiac function and reduced scar fibrosis in vivo. This spray patch not only provides us with a concept for using stem cell factor cocktails for cardiac regenerative therapy, but also demonstrates a new drug delivery approach for potential minimally invasive patch transplantation.
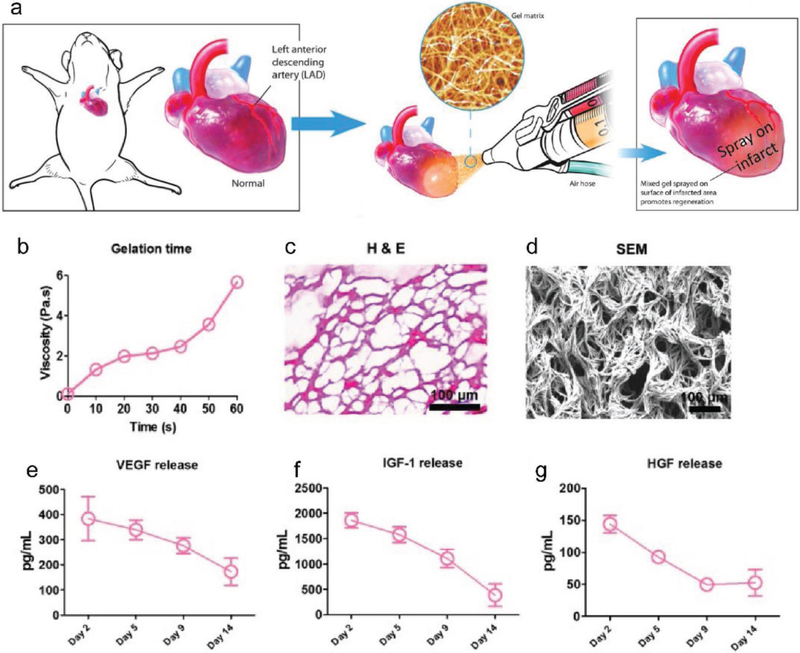
Figure 4.
Spray painting of gel matrix on infarcted myocardial areas. a) Schematic showing the spray painting of in situ polymerizable biomaterials on the heart after MI with a minimally invasive procedure. b) Using compressed air to mix platelet-rich plasma with calcium-containing media resulted in a stable gel formation in less than 1 min. c) H&E staining revealed the fibrous structure of the sprayed platelet fibrin gel. d) Representative scanning electron microscopy images of the sprayed platelet fibrin gel. e–i) Enzyme-linked immunosorbent assay of the concentrations of VEGF, IGF-1, and HGF from the sprayed platelet fibrin gel conditioned media at different time points (n = 3 per time point). Scale bar = 100 μm. H&E, hematoxylin and eosin. Reproduced with permission.[134] Copyright 2017, Mary Ann Liebert, Inc.
5.5.3. A miRNA-Assembled Hydrogel Cardiac Patch
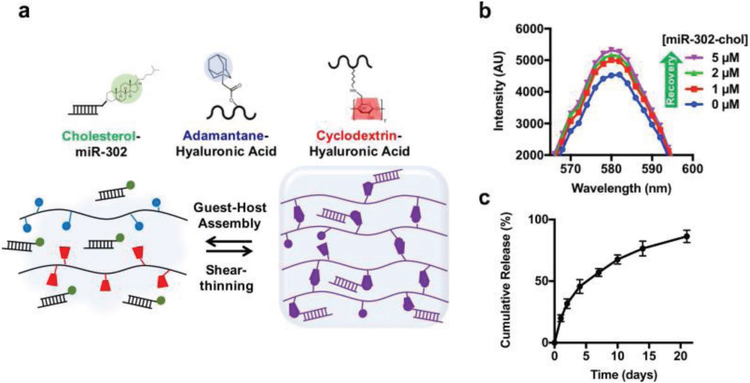
In order to reap the benefits of miRNA treatment, researchers created a miRNA-assembled hydrogel cardiac patch to diminish the rate of potential miRNA off-target. First, researchers designed an injectable hydrogel that exhibits shear-thinning and rapid self-healing behavior by assembling β-cyclodextrin-modified hyaluronic acid (CD-HA) with adamantane-modified hyaluronic acid (AD-HA).[136] Since miRNA-302 has a proliferative effect on neonatal mouse cardiomyocytes through the inhibition of Hippo signaling,[97] they then chose miRNA-302 and modified it with cholesterol to enhance cellular uptake and improve miRNA-gel affinity without changing the characteristics of the aforementioned injectable hydrogel. Finally, they integrated the modified miRNA-302 into the injectable hydrogel and formed a complex miRNA-302-enriched hydrogel, which can release miRNA-302 persistently for about three weeks (Figure 5).[136] The study demonstrated the regeneration of neonatal mouse cardiomyocytes, in vitro, and the preservation of cardiac function, in vivo,[136] using the patch. This study innovatively used a biochemical method to assemble therapeutic miRNAs into hydrogel patches with the capacity for sustainable release.
Figure 5.
miR-302 control-releasing gel. a) Schematic showing gel assembly and miR-302 interactions. HA was modified with AD or CD to create shear-thinning and self-healing gels, respectively. Likewise, cholesterol on miR-302-chol interacts with CD to induce the sustained release of the gel. b) Rhodamine/CD-HA interactions lead to the quenching of rhodamine fluorescence, but the fluorescence is recovered by the titration of cholesterol-modified miR-302 into the system and displacement of rhodamine complexes, indicating the integration between cholesterol and CD. c) Release of cholesterol-modified miR-302b and miR-302c (210 × 19−6 M of each) from gels (5 wt%) in 1.5 mL microcentrifuge tubes over three weeks, quantified by RiboGreen, a commercially available RNA quantification kit (mean ± s.d., n = 3). Reproduced with permission.[136] Copyright 2017, Macmillan Publisher Limited, part of Springer Nature.
5.5.4. Decellularized Cardiac-ECM Cardiac Patch
ECM is composed of interstitial matrix and basement membrane materials. Decellularized pig heart tissue can be used as a bioactive ECM cardiac patch with the capacity to stimulate a complex regenerative response without the addition of cells or growth factors. This decellularized cardiac extracellular matrix (dECM) patch induces de novo immature, striated-like muscle patterns (myosin light chain (MLC+), cardiac troponin I (cTnI+), connexin43+) by polarizing macrophages toward a constructive remodeling phenotype, and by driving the recruitment of cardiomyocyte progenitor cells.[137] Also, the dECM protein, agrin, was shown to promote heart regeneration after MI in mice[138] through Dag1, ERK, and Yap signaling pathways. Recently, dECM was mixed with hydrogel to increase its versatility.[139] Altogether, the bioactive dECM patch model provides us with a new candidate for the acellular regenerative treatment of MI hearts.
5.6. Cardiac Patch: Challenges
As a promising approach for cardiac repair and regeneration after MI, the cardiac patch strategy continues to be improved and engineered to better deliver its therapeutic benefits. However, there are still many challenges left to address before it can be implemented clinically. Although a shape-memory scaffold, capable of supporting multiple stretch cycles without deformation or impeding cardiac contractions, was successfully transplanted onto the heart in a minimal-invasive procedure,[140] most cardiac patches designed for epicardial delivery[133] require an open-chest surgery. Such invasive procedures cause innate surgical damage, increase the patient’s risk of death during surgery, induce inflammation after surgery, and subject the patient to a long recovery period. In addition, the possibility of forming pericardial adhesions during cardiothoracic function is an underlying weakness of the surgical cardiac patch transplantation which may cause disfunction of the heart or even increase morbidity and mortality.[141] Thus, the value of the cardiac patch strategy and its worth to the patient are debatable. In order to improve on the status quo, there has been increased emphasis on using minimally invasive implantation procedures, the pursuit of which has emerged as one of the most important goals in surgical transplantation.[134,140] In addition, in cardiac patch transplantations, sutures are often used to prevent patch shedding,[142] which is a heavy burden for an already injured heart. Many methods, however, have been proposed and validated as effective alternatives to sutures, including the use of gold nanorods,[143] biocompatible glues,[144] and techniques which may require patch modifications but may not be applicable for all patches. Moreover, arrhythmia caused by large heart engraftments is still a serious, possibly fatal problem[145] that will require further study to fully understand and avoid. Although cardiac patch materials are biocompatible and biodegradable, long-term safety studies are still required to test for chronic immune rejection. Indeed, all patches are external foreign substances which may cause relevant immune rejection, either by virtue of the materials used or their metabolic derivatives.[146] Moreover, it is important to further optimize the cardiac patches’ therapeutic potential, manufacture consistency, and cryostability before they can be fully developed clinical products.
5.7. Cardiac Injury Targeting: Opportunities
Due to low therapeutic retention, and to avoid the risk of losing therapeutic efficacy as a result of off-target delivery, heart stem cell therapies are usually administered intramyocardially. However, the invasive nature of this delivery method is clinically unappealing. Thus, research in recent years has focused on developing off site delivery strategies, coupled with myocardial injury targeting techniques, to shuttle the therapeutic to the injured myocardium. Two common injury biomarkers targets, for example, are MLC and cTnI. The concept of targeted drug delivery was originally developed in the field of cancer medicine. There, intravenously injected chemotherapy drugs are guided to the tumor tissues through a variety of methodologies. The cardiac targeting concept allows the drugs (therapeutic cells in our case) to deliberately interact with the heart’s infarcted area and impart their therapeutic benefits.
5.7.1. Peptide Targeting
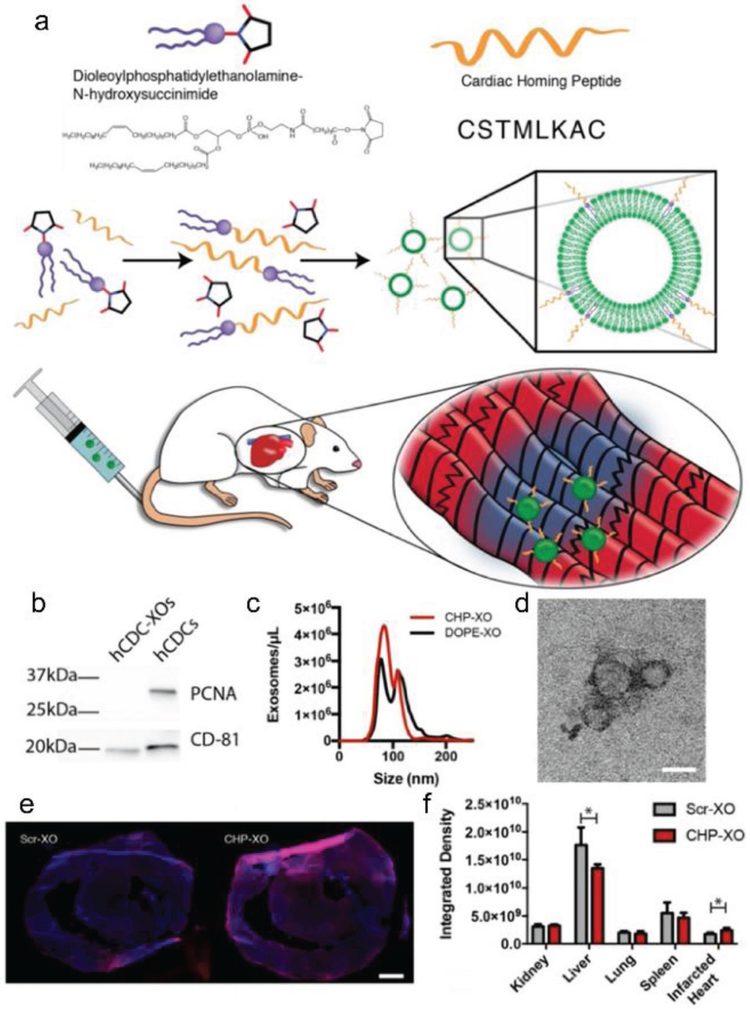
After screening out the three peptide sequences (CSTSMLKAC, CKPGTSSYC, and CPDRSVNNC) with the highest occurrence rates from ischemic heart tissue, researchers tested their homing ability in vivo by using conjugated synthetic peptides with fluorescein. Of these, the CSTSMLKAC peptide demonstrated the strongest affinity for the ischemic heart myocardium, as suggested by its strong fluorescein signature.[147] This cardiac homing peptide was conjugated with CSC-derived exosomes through a dioleoylphosphatidylethanolamine N-hydroxysuccinimide (DOPE-NHS) linker for cardiac regenerative therapy (Figure 6).[66] This technique dramatically increased the retention of therapeutic exosomes, even though the homing exosomes were delivered intravenously. From in vitro studies, the exosomes conjugated with homing peptides improved the viability and exosome-uptake of cultured neonatal rat cardiomyocytes, while reducing cell apoptosis. An in vivo study on a rat model of MI suggested that cardiac functions were effectively preserved due to the proangiogenic, promyogenic, antiapoptotic, and anti-inflammatory roles of exosomes.[66] This research provides us with a new technique to modify membrane-based nanoparticles, such as exosomes, to target MI.
Figure 6.
Cardiac homing exosomes. a) Myocardium-targeting exosomes were produced by reacting DOPE-NHS to Cardiac Homing Peptide (CHP). The lipophilic tails of the DOPE-CHP then spontaneously insert into the exosomal membrane, coating the exosome in CHP peptides. The exosomes were then intravenously injected into rats following I/R injury. b) Western blot for PCNA verifies absence of cell particulates in purified exosomes and CD-81 shows presence of exosomes. c) Nanoparticle tracking analysis shows that tagging the exosomes with CHP resulted in no significant changes in exosome size, with a modal exosome size of ≈95 nm. d) Transmission electron microscopy confirms the exosome structure. e,f) Ex vivo labeling of infarcted rat heart sections showed increased retention of both CHP-tagged exosomes compared to Scr-tagged exosomes (DAPI in blue and DiI-labeled exosomes in red). Scale bars: d = 50 nm, e = 1 mm. Reproduced with permission under the terms of the Creative Commons Attribution (CC BY-NC) 4.0 license.[66] Copyright 2018, the Authors. Published by Ivyspring International Publisher.
Post-MI induced myocardial disfunction and myocardial injury may require chronic treatment for years. Thus, it is necessary to maintain an effective local drug concentration and prolonged drug retention period. For this purpose, researchers engineered therapeutic peptide-loaded calcium phosphate nanoparticles that can achieve cardiac targeting through inhalation. Inhaled nanoparticles can rapidly diffuse into the alveoli, cross the alveolar capillary membrane, and be transported to the heart through the pulmonary veins. The efficacy of this targeting method was confirmed by tracking the nanoparticle uptake efficiency by cardiomyocytes in vivo.[148]
5.7.2. Magnetic Targeting
Magnetic stem cell targeting relies on the application of a magnetic field gradient to guide bioactive molecules to the site of injury.[149,150] Superparamagnetic nanoparticles composed of a magnetite (Fe3O4) or maghemite (γ-Fe2O3) core. [149] have many attractive features, such as a large, constant magnetic moment, giant-paramagnetic-atom-like behavior, fast magnetic field response, and low aggregation at room temperature.[151] Due to these features, several FDA-approved superparamagnetic nanoparticles have been applied in the biomedical field for a number of purposes, including drug delivery and magnetic resonance imaging (MRI).[149,152] Intravenously administered magnetic nanoparticles coated with polyethylene glycol and anti-CD34 successfully guided mononuclear cells (CD34+) to the targeted area.[153] In addition, Ferumoxytol, an FDA approved magnetic nanoparticle, has been used successfully to label human and rat CSCs, enhancing stem cell retention/engraftment in the injured area, and multiplying the therapeutic benefit.[154] It has been widely reported that the magnetic targeting strategy is nontoxic and can be applied universally to many cell types.[154]
5.7.3. Antibody Targeting
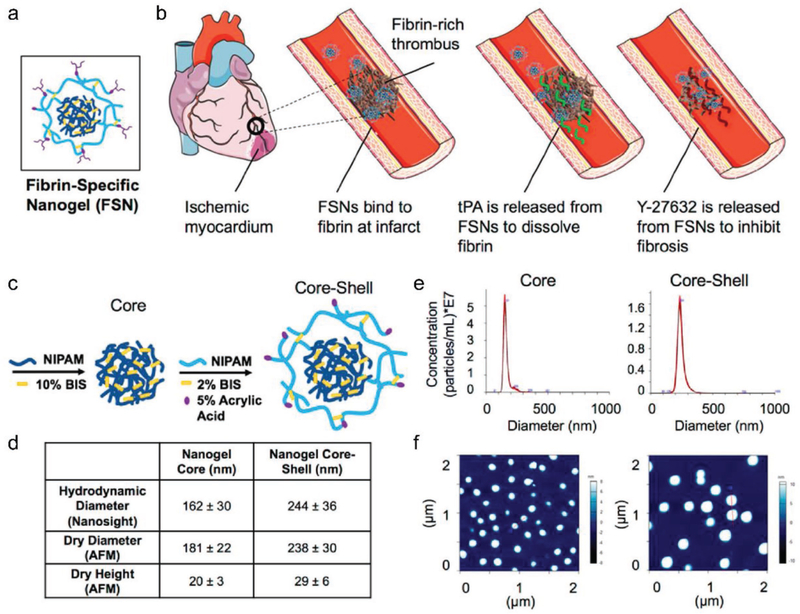
Another important development in the past decade is the use of heart injury biomarker-specific antibodies to target therapeutic cells to the infarcted heart.[155] MI events usually occur when coronary arteries become occluded as a result of fibrin-rich thrombi that form inside the lumen.[156] During MI, ECM will be deposited with plethoric fibrin due to an imbalanced thrombin/thrombomodulin ratio.[157] Nanogels are nanosized colloidal hydrogels with loading and releasing kinetics that are related to particle size, cross-linking density, and network homogeneity. They are notable for their potentials as delivery vehicles of small molecules and proteins (Figure 7).[158] Poly(N-isopropylacrylamide) nanogels were engineered as part of a dual-delivery system to reopen the clotted blood vessels and inhibit fibrosis during post-MI heart remodeling by targeting the deposited fibrin.[65] In the study, tissue plasminogen activator and a small-molecule cell contractility inhibitor (Y-27632) were encapsulated inside the nanogels. An antifibrin antibody was conjugated to the outside of the nanogel by the EDC/Sulfo-NHS coupling of the AAc functional handles in the nanogel shell.[65] Through the conjugated antifibrin antibody on the surface, the intracoronary injected nanoparticles specifically recognized and bound the fibrin-enriched area, allowing for accurate drug releasing and improved cardiac function following MI, in vivo.[65] These antibody-conjugated nanogels can also be used to encapsulate various proteins or molecules. The conjugation of different antibodies can be used to specifically target different markers.
Figure 7.
Fibrin-specific nanogel. a) The design of fibrin-specific nanogel. b) Drug-loaded nanoparticles combat MI, which occurs due to a fibrin-rich thrombus blocking blood flow and creating ischemic myocardium, and subsequent cardiac fibrosis upon reperfusion. Drug-loaded FSNs will bind to fibrin at the infarct site, release a fibrinolytic drug, and release a small-molecule cell contractility-inhibitor to mitigate cardiac fibrosis due to reperfusion injury. c) Core and C/S nanogel design. d) Size characterization (n = ≥30 per group). e) Nanosight particle tracking for hydrodynamic diameter measurements. f) Dry AFM images on a glass surface. Reproduced with permission.[65] Copyright 2018, American Chemical Society.
5.7.4. Bispecific Antibody Targeting
Bispecific antibodies (BsAb) are artificial proteins usually composed of fragments from two different monoclonal antibodies that, consequently, recognize two different antigens present on different cells.[159] They have been engineered and applied for cancer immunotherapy for decades. For example, a BsAb molecule can simultaneously bind a cytotoxic T cell and a targeted tumor cell with the two Fab regions. The T cell on one Fab site releases the cytotoxins perforin, granzymes, and granulysin to trigger a series of apoptotic events through the action of perforin. Granzymes then enter the cytoplasm of the tumor cell on the other Fab site. Additionally, the Fc region can bind to Fc receptors on macrophages, natural killer cells, or dendritic cells, facilitating antibody-dependent cell-mediated cytotoxicity and killing the attached tumor cell.[160] Nowadays, increasing knowledge in effector cell biology and the implementation of antibody engineering technologies has improved bispecific antibody strategies for immunotherapy of cardiac MI injury.[161] The binding of BsAbs with stem cells has been applied to enhance stem cell targeting and tissue regeneration.[162] BsAbs can also be conjugated with magnetic nanoparticles to help exogenous bone marrow-derived stem cells (expressing CD45) or endogenous CD34+ cells target injured neonatal rat cardiomyocytes.[163] A decade ago, researchers began to design BsAbs to target human CD34+ cells to the specific antigens expressed by ischemic myocardium.[155] They linked 2 whole antibodies (anti-CD45 and anti-MLC) on Fcs, using a sulfosuccinimidyl 4-(N-maleimidomethyl cyclohexane-1-carboxylate] crosslinker, the success of which was verified through flow cytometry. This study provides an example of how BsAbs can be used to target hematopoietic stem cells to injured myocardium, specifically.[155]
5.7.5. Platelet-Inspired MI Targeting
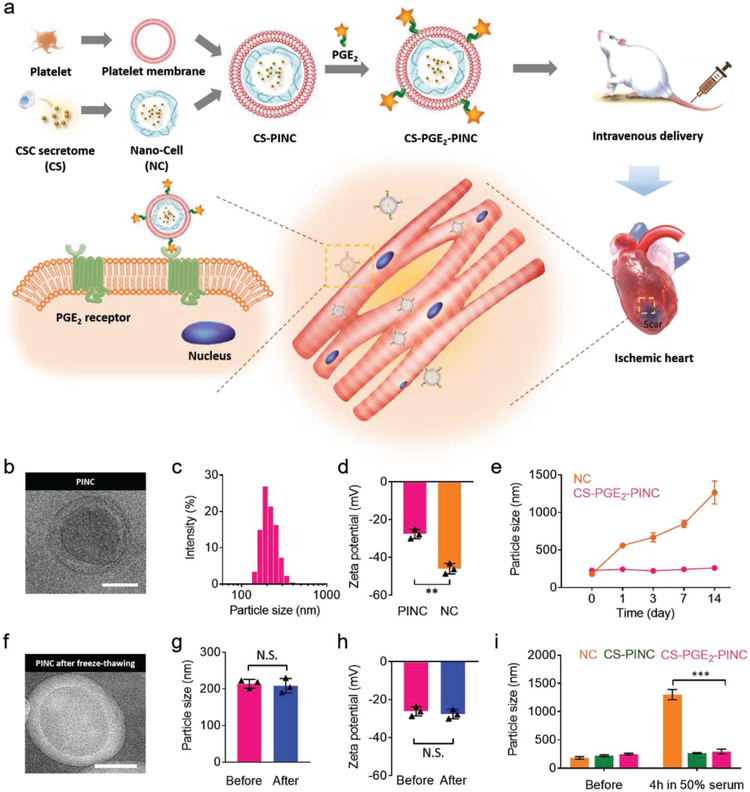
Before ischemic heart injuries, the major blood vessels usually suffer from vascular damage and clot formation. During this process, the damaged subendothelial matrix components will be exposed, including collagen, fibronectin, and von Wille-brand factor (vWF). The exposed ECM helps stimulate the aggregation of platelets via many receptor–ligand interactions, including GPlb/V/IX-vWF, αIIbβ3-fibronectin, and αIIbβ3-fibrinogen.[164,165] Under flow conditions, discoid platelets also form membrane tethers, which play an important role in platelet–matrix and platelet–platelet adhesive interactions.[166] Importantly, platelets have an innate ability to flow in the vasculature until they bind to the targeted injury sites.[67] Thus, researchers are developing platelet membrane coatings that effectively target the ischemic heart. In one of the studies,[67] platelet membrane-decorated CSCs were formed by coating CSCs with platelet membranes using a fusion method.[67,167] Rodent and porcine ischemia/reperfusion models were used to prove the targeting proficiency and therapeutic efficacy of these bioengineered cells.[67] To further improve this strategy, platelet-inspired nanocell (PINC), a nanoparticle composed of a therapeutic core and a prostaglandin E2 (PGE2) decorated platelet membrane shell, was fabricated for ischemic heart tissue targeting. PINC achieved dually targeting to the ischemic myocardium via both platelet inspiration and the PGE2 decoration (Figure 8a). In addition, PINCs showed a better cryostorage stability and have an innate ability to flow inside the vasculature until binding to the targeted injury sites (Figure 8b–i).[168]
Figure 8.
Fabrication and characterization of PINCs. a) Schematic illustration of the fabrication process of PINCs. The therapeutic effects of PINC injection were tested in mice with myocardial I/R injury. b) Transmission electron microscopy (TEM) image showing the ultrastructure of CSC secretome-loaded and PGE2-platelet membrane-coated nanocells (CS-PGE2-PINC). c) Size distribution of CS-PGE2-PINC measured by DLS. d) Zeta potentials of CS-PGE2-PINC and nanocells (NC). e) Particle sizes of bare NC and CS-PGE2-PINC over two weeks in PBS. f) TEM image showing the ultrastructure of CS-PGE2-PINC after freeze-thawing. g) The comparison of particle size and h) zeta potential of CS-PGE2-PINC before and after freeze-thawing. i) In vitro stability of NC, CSC secretome-loaded and platelet membrane-coated nanocells (CS-PINC), and CS-PGE2-PINC before and after incubation in 50% fetal bovine serum. Scale bars = 100 nm. All data are mean ± s.d. * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001; N.S., no statistical significance. Comparisons between any two groups were performed using a two-tailed unpaired Student’s t-test. Comparisons between more than two groups were performed using one-way ANOVA, followed by the post hoc Bonferroni test. Reproduced with permission.[168] Copyright 2018, Wiley-VCH.
5.8. Cardiac Injury Targeting: Challenges
Although the emergence of targeting methods has pushed myocardial regenerative therapies to brand-new heights, there are many deficiencies that need to be worked on. First, it is necessary to establish well-accepted standards in the field for evaluating the cardiac targeting efficiency. Second, in both antibody targeting and bispecific antibody targeting cases, the antibody added may induce immune reactions due to the intact Fc region, which can bind to Fc receptors on macrophages, natural killer cells, or dendritic cells. Binding by any of these cells to the Fc site would activate an antibody-dependent, cell-mediated cytotoxic response and harm the attached stem cells.[160] Furthermore, nanoparticles conjugated with antibodies may be phagocytosed and, consequently, have their therapeutic effects diminished.[169] Thus, antibodies used for therapeutic purposes should be better designed to avoid undesired immune reactions. Additionally, some targeting methods still require local (intracoronary) injections for effective delivery.[67] However, the intravenous injection of cardiac targeting drugs, which is more attractive and less invasive, may cause increased off-target drug delivery due to the long circulation route from the vein to the infarcted myocardium. For example, the cell-sized carriers may be mechanically retained in the capillary networks of the lungs after intravenous injection.[170] Moreover, the magnetic cardiac targeting strategy has to be thoroughly investigated in regards to particle size, surface chemistry, magnetic properties, and the ability of an external magnetic field to guide the injected stem cells to the heart.
6. Conclusion
This review began with a summary of the pathological repair process that occurs after MI. The paracrine mechanisms and the molecular pathways involved in stem cell therapies are discussed and summarized in detail. Based on the current literature, we classified and analyzed stem cell derivatives and byproducts that make up the new generation of stem cell therapeutics. These were divided into four categories: synthetic stem cells, exosomes, cardiac patches, and cardiac targeting modalities. The goals of these new concepts are to: (1) bypass the limitations of living-cell therapies by using nonlive cell products; (2) optimize the therapeutic efficacy by using molecular-level therapeutics; (3) diminish the invasiveness of current drug delivery routes by developing minimally invasive procedures or targeting concepts; and (4) get closer to clinical application. However, there are still many unsolved issues for future research, including the need for the improved understanding of molecular mechanisms, the application of new materials, the update of drug administration routes, pharmacokinetics, drug stability, side effects, and toxicity. If we can address these issues, the future of stem cell products will be bright, and both the quality and longevity of life for MI patients will improve.
Acknowledgements
This work was supported by the National Institutes of Health (Grant Nos. HL123920 and HL137093), awarded to K.C. English grammar was edited by Jhon Cores, Ph.D.
Biographies

Ke Huang is a Ph.D. student in the Department of Comparative Biomedical Sciences at NC State University. He received his Medical Degree in 2008 from Shanxi Medical University, and then earned a master’s degree in exercise physiology and adult fitness from the University of Akron. His research focuses on stem cell therapy for myocardial regeneration through cardiac patches and cardiac targeting. His research also involves uncovering the mechanisms stem cells use to regenerate damaged heart tissue. He enjoys reading, watching films, and swimming.

Shiqi Hu received her Ph.D. from the College of Chemical and Biological Engineering, in Zhejiang University, China, in 2017. She is experienced in anticancer drug delivery systems and tumor microenvironments, including polymer synthesis, nanoparticles preparation, and cancer models establishment. She enjoys reading, crafting, and playing the game of Go.

Ke Cheng is a Professor in the Department of Molecular Biomedical Sciences atNC State University anda Professor in the UNC/NCSU joint Department of Biomedical Engineering. He is also an adjunct professor at the UNC Eshelman School of Pharmacy. He also serves as the codirector of the functional tissue engineering program at the Comparative Medicine Institute. His formal education began with a B.S. in pharmaceutical engineering from the Zhejiang University, followed by a Ph.D. degree in biological engineering from the University of Georgia, and postgraduate training/junior faculty experiences at UCLA School of Medicine and Cedars-Sinai Medical Center.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
The ORCID identification number(s) for the author(s) of this article can be found under https://doi.org/10.1002/adhm.201801011.
Contributor Information
Ke Huang, Department of Molecular Biomedical Sciences and Comparative Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, Raleigh, NC 27607, USA.
Shiqi Hu, Department of Molecular Biomedical Sciences and Comparative Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, Raleigh, NC 27607, USA.
K. Cheng, Department of Molecular Biomedical Sciences and Comparative Medicine Institute, North Carolina State University, Raleigh, NC 27607, USA Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, Raleigh, NC 27607, USA; Pharmacoengineeirng and Molecular Pharmaceutics Division, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
References
- [1].Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, De Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee, Circulation 2017, 135, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jhund PS, McMurray JJV, Circulation 2008, 118, 2019. [DOI] [PubMed] [Google Scholar]
- [3].St MG. John Sutton, N. Sharpe, Circulation 2000, 101, 2981. [DOI] [PubMed] [Google Scholar]
- [4].Porrello ER, Olson EN, Stem Cell Res. 2014, 13, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steinhauser ML, Lee RT, EMBO Mol. Med 2011, 3, 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Uygur A, Lee RT, Dev. Cell 2016, 36, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gamba L, Harrison M, Lien C-L, Curr. Treat. Options Cardiovasc. Med 2014, 16, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ito K, Morioka M, Kimura S, Tasaki M, Inohaya K, Kudo A, Dev. Dyn 2014, 243, 1106. [DOI] [PubMed] [Google Scholar]
- [9].Ma D, Tu C, Sheng Q, Yang Y, Kan Z, Guo Y, Shyr Y, Scott IC, Lou X, Proteome Res J. 2018, 17, 1300. [DOI] [PubMed] [Google Scholar]
- [10].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J, Science 2009, 324, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Beltrami AP, Urbanek K, Kajstura J, Yan S-M, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P, N. Engl. J. Med 2001, 344, 1750. [DOI] [PubMed] [Google Scholar]
- [12].Mohamed TMA, Ang Y-S, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D, Cell 2018, 173, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Song G, Li X, Shen Y, Qian L, Kong X, Chen M, Cao K, Zhang F, Cell Biochem. Biophys 2015, 71, 1463. [DOI] [PubMed] [Google Scholar]
- [14].Shadrin IY, Allen BW, Qian Y, Jackman CP, Carlson AL, Juhas ME, Bursac N, Nat. Commun 2017, 8, 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moccia F, Diofano F, Rebuzzini P, Zuccolo E, Stem cells and cardiac regeneration, (Ed: Madonna R), Springer International Publishing, Cham, Switzerland: 2016, pp. 9–29. [Google Scholar]
- [16].Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E, Lancet 2012, 379, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Konstantinos Malliaras P, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Am J. College Cardiol 2014, 63, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elmadbouh I, Ashraf M, Physiol. Rep 2017, 5, e13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Amado LC, Saliaris AP, Schuleri KH, St John M, Xie J-S, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM, Proc. Natl. Acad. Sci. USA 2005, 102, 11474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miana VV, González EAP, eCancer Med. Sci 2018, 12, 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ, J. Mol. Cell. Cardiol 2011, 50, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gnecchi M, Zhang Z, Ni A, Dzau VJ, Circ. Res 2008, 103, 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burchfield JS, Dimmeler S, Fibrog. Tissue Repair 2008, 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ, Nat. Med 2005, 11, 367. [DOI] [PubMed] [Google Scholar]
- [25].Crisostomo PR, Abarbanell AM, Wang M, Lahm T, Wang Y, Meldrum DR, Am. J. Physiol.: Heart Circ. Physiol 2008, 295, H1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hensley MT, de Andrade J, Keene B, Meurs K, Tang J, Wang Z, Caranasos TG, Piedrahita J, Li TS, Cheng K, J. Cell. Mol. Med 2015, 19, 1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cheng K, Malliaras K, Smith RR, Shen D, Sun B, Blusztajn A, Xie Y, Ibrahim A, Aminzadeh MA, Liu W, Li TS, De Robertis MA, Marbán L, Czer LSC, Trento A, Marbán E, JACC: Heart Fail 2014, 2, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shen D, Tang J, Hensley MT, Li TS, Caranasos TG, Zhang T, Zhang J, Cheng K, Stem Cells Transl. Med 2016, 5, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ, Circ. Res 2016, 118, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koch S, Claesson-Welsh L, Cold Spring Harbor Perspect. Med 2012, 2, a006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Talwar T, Srivastava MVP, Ann. Indian Acad. Neurol 2014, 17, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gallo S, Sala V, Gatti S, Crepaldi T, Biomedicines 2014, 2, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sala V, Crepaldi T, Cell. Mol. Life Sci 2011, 68, 1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mayorga ME, Kiedrowski M, McCallinhart P, Forudi F, Ockunzzi J, Weber K, Chilian W, Penn MS, Dong F, Stem Cells Transl. Med 2018, 7, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M, Blood 2004, 104, 3472. [DOI] [PubMed] [Google Scholar]
- [36].Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P, Am. J. Physiol.: Heart Circ. Physiol 2010, 298, H1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gan W, Ren J, Li T, Lv S, Li C, Liu Z, Yang M, Biochim. Biophys. Acta, Mol. Basis Dis 2018, 1864, 1. [DOI] [PubMed] [Google Scholar]
- [38].Mishra PK, Givvimani S, Chavali V, Tyagi SC, Biochim. Biophys. Acta, Mol. Basis Dis 2013, 1832, 2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sandoval-Guzmán T, Currie JD, Curr. Opin. Cell Biol 2018, 55, 36. [DOI] [PubMed] [Google Scholar]
- [40].Li LL, Peng C, Zhang M, Liu Y, Li H, Chen H, Sun Y, Zhu C, Zhang Y, Mol. Med. Rep 2018, 17, 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Philp D, Kleinman HK, Ann. N. Y. Acad. Sci 2010, 1194, 81. [DOI] [PubMed] [Google Scholar]
- [42].Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR, Nature 2011, 474, 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shah R, Reyes-Gordillo K, Cheng Y, Varatharajalu R, Ibrahim J, Lakshman MR, Oxid. Med. Cell. Longevity 2018, 2018, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lalit PA, Hei DJ, Raval AN, Kamp TJ, Circ. Res 2014, 114, 1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, Yang PC, Circ. Res 2017, 121, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J, Kim J, Kanai M, Bi L, Brown K, Di Paolo G, Homma S, Sims PA, Topkara VK, Vunjak-Novakovic G, Nat. Biomed. Eng 2018, 2, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Frangogiannis NG, Smith CW, Entman ML, Cardiovasc. Res 2002, 53, 31. [DOI] [PubMed] [Google Scholar]
- [48].Ye Y, Wang J, Hu Q, Hochu GM, Xin H, Wang C, Gu Z, ACS Nano 2016, 10, 8956. [DOI] [PubMed] [Google Scholar]
- [49].Guo J, Lin G, Bao C, Hu Z, Hu M, Inflammation 2007, 30, 97. [DOI] [PubMed] [Google Scholar]
- [50].Frangogiannis NG, Curr. Opin. Physiol 2018, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG, J. Mol. Cell. Cardiol 2010, 48, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, Nitahara-Kasahara Y, Imagawa K, Hirato T, Ueda M, Kimura K, Okada T, Mol. Ther.–Methods Clin. Dev 2017, 6, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shirakawa K, Endo J, Kataoka M, Katsumata Y, Yoshida N, Yamamoto T, Isobe S, Moriyama H, Goto S, Kitakata H, Hiraide T, Fukuda K, Sano M, Circulation 2018, 138, 2021. [DOI] [PubMed] [Google Scholar]
- [54].Zacchigna S, Martinelli V, Moimas S, Colliva A, Anzini M, Nordio A, Costa A, Rehman M, Vodret S, Pierro C, Colussi G, Zentilin L, Gutierrez MI, Dirkx E, Long C, Sinagra G, Klatzmann D, Giacca M, Nat. Commun 2018, 9, 2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tang J, Cores J, Huang K, Cui XL, Luo L, Zhang JY, Li TS, Qian L, Cheng K, Stem Cells Transl. Med 2018, 7, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Heslop JA, Hammond TG, Santeramo I, Tort Piella A, Hopp I, Zhou J, Baty R, Graziano EI, Proto Marco B, Caron A, Sköld P, Andrews PW, Baxter MA, Hay DC, Hamdam J, Sharpe ME, Patel S, Jones DR, Reinhardt J, Danen EHJ, Ben-David U, Stacey G, Björquist P, Piner J, Mills J, Rowe C, Pellegrini G, Sethu S, Antoine DJ, Cross MJ, Murray P, Williams DP, Kitteringham NR, Goldring CEP, Kevin Park B, Stem Cells Transl. Med 2015, 4, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cheng K, Shen D, Xie Y, Cingolani E, Malliaras K, Marbán E, Stem Cells 2012, 30, 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Allen TA, Gracieux D, Talib M, Tokarz DA, Hensley MT, Cores J, Vandergriff A, Tang J, De Andrade JB, Dinh P-U, Yoder JA, Cheng K, Stem Cells 2017, 35, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Duelen R, Sampaolesi M, EBioMedicine 2017, 16, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Philippe M, Circulation 2009, 119, 2735.19470902 [Google Scholar]
- [61].Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E, Heart 2018, 104, 8. [DOI] [PubMed] [Google Scholar]
- [62].Mayourian J, Ceholski DK, Gonzalez DM, Cashman TJ, Sahoo S, Hajjar RJ, Costa KD, Circ. Res 2018, 122, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tang J, Shen D, Caranasos TG, Wang Z, Vandergriff AC, Allen TA, Hensley MT, Dinh PU, Cores J, Li TS, Zhang J, Kan Q, Cheng K, Nat. Commun 2017, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Luo L, Tang J, Nishi K, Yan C, Dinh PU, Cores J, Kudo T, Zhang J, Li TS, Cheng K, Circ. Res 2017, 120, 1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mihalko E, Huang K, Sproul E, Cheng K, Brown AC, ACS Nano 2018, 12, 7826. [DOI] [PubMed] [Google Scholar]
- [66].Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K, Theranostics 2018, 8, 1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tang J, Su T, Huang K, Dinh PU, Wang Z, Vandergriff A, Hensley MT, Cores J, Allen T, Li T, Sproul E, Mihalko E, Lobo LJ, Ruterbories L, Lynch A, Brown A, Caranasos TG, Shen D, Stouffer GA, Gu Z, Zhang J, Cheng K, Nat. Biomed. Eng 2018, 2, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sarig U, Sarig H, de-Berardinis E, Chaw S-Y, Nguyen EBV, Ramanujam VS, Thang VD, Al-Haddawi M, Liao S, Seliktar D, Kofidis T, Boey FYC, Venkatraman SS, Machluf M, Acta Biomater 2016, 44, 209. [DOI] [PubMed] [Google Scholar]
- [69].Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L, Nano Lett 2014, 14, 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liang H, Huang K, Su T, Li Z, Hu S, Dinh P-U, Wrona EA, Shao C, Qiao L, Vandergriff AC, Hensley MT, Cores J, Allen T, Zhang H, Zeng Q, Xing J, Freytes DO, Shen D, Yu Z, Cheng K, ACS Nano 2018, 12, 6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pei Q, Hu X, Zheng X, Liu S, Li Y, Jing X, Xie Z, ACS Nano 2018, 12, 1630. [DOI] [PubMed] [Google Scholar]
- [72].Hu C-MJ, Fang RH, Luk BT, Zhang L, Nanoscale 2014, 6, 65. [DOI] [PubMed] [Google Scholar]
- [73].Tran C, Damaser MS, Adv. Drug Delivery Rev 2015, 82–83, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liu P, Weng Y, Sui Z, Wu Y, Meng X, Wu M, Jin H, Tan X, Zhang L, Zhang Y, Sci. Rep 2016, 6, 37606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Teixeira FG, Panchalingam KM, Anjo SI, Manadas B, Pereira R, Sousa N, Salgado AJ, Behie LA, Stem Cell Res. Ther 2015, 6, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ahn C-S, Kim J-G, Shin MH, Lee YA, Kong Y, Proteomics 2018, 18, 1700341. [DOI] [PubMed] [Google Scholar]
- [77].Li P, Kaslan M, Lee SH, Yao J, Gao Z, Theranostics 2017, 7, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sahoo S, Klychko E, Thorne T, Misener S, Shinnick K, Millay M, Ito A, Liu T, Kamide C, Agarwal H, Perlman H, Qin G, Kishore R, Losordo DW, Circ. Res 2011, 109, 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ong S-G, Lee WH, Zhou Y, Wu JC, MicroRNA protocols, 3rd ed, (Ed: Ying S-Y), Springer, New York: 2018, pp. 127–136. [Google Scholar]
- [80].Kowal J, Tkach M, Théry C, Curr. Opin. Cell Biol 2014, 29, 116. [DOI] [PubMed] [Google Scholar]
- [81].Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP, Stem Cell Res 2013, 10, 301. [DOI] [PubMed] [Google Scholar]
- [82].He C, Zheng S, Luo Y, Wang B, Theranostics 2018, 8, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sahoo S, Losordo DW, Circ. Res 2014, 114, 333. [DOI] [PubMed] [Google Scholar]
- [84].Borges FT, Reis LA, Schor N, Braz. J. Med. Biol. Res 2013, 46, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yan S, Han B, Gao S, Wang X, Wang Z, Wang F, Zhang J, Xu D, Sun B, Oncotarget 2017, 8, 60149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S, Circulation 2012, 126, 2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kishore R, Khan M, Circ. Res 2016, 118, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ibrahim AG-E, Cheng K, Marbán E, Stem Cell Rep 2014, 2, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Li X, Arslan F, Ren Y, Adav SS, Poh KK, Sorokin V, Lee CN, de Kleijn D, Lim SK, Sze SK, J. Proteome Res 2012, 11, 2331. [DOI] [PubMed] [Google Scholar]
- [90].Barile L, Milano G, Vassalli G, Stem Cell Invest 2017, 4, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, Wang Y, Zingarelli B, Peng T, Fan G-C, Biochim. Biophys. Acta, Mol. Basis Dis 2015, 1852, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E, Eur. Heart J 2017, 38, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VNS, Benedict C, Nickoloff E, Johnson J, Gao E, Losordo DW, Houser SR, Koch WJ, Circ. Res 2015, 117, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Cheng K, Ibrahim A, Hensley MT, Shen D, Sun B, Middleton R, Liu W, Smith RR, Marban E, Am J. Heart Assoc 2014, 3, e001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vasa-Nicotera M, Chen H, Tucci P, Yang AL, Saintigny G, Menghini R, Mahè C, Agostini M, Knight RA, Melino G, Federici M, Atherosclerosis 2011, 217, 326. [DOI] [PubMed] [Google Scholar]
- [96].Mayourian J, Ceholski DK, Gorski P, Mathiyalagan P, Murphy JF, Salazar SI, Stillitano F, Hare JM, Sahoo S, Hajjar RJ, Costa KD, Circ. Res 2018, 122, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM, Morrisey EE, Sci. Transl. Med 2015, 7, 279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liu N, Williams AH, Kim Y, McAnally J, Bezprozvannaya S, Sutherland LB, Richardson JA, Bassel-Duby R, Olson EN, Proc. Natl. Acad. Sci. USA 2007, 104, 20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D, Cell 2007, 129, 303. [DOI] [PubMed] [Google Scholar]
- [100].Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DYR, Srivastava D, Dev. Cell 2008, 15, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G, Cardiovasc. Res 2014, 103, 530. [DOI] [PubMed] [Google Scholar]
- [102].Liu N, Bezprozvannaya S, Genes Dev. 2008, 22, 3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C, Circ Res. 2011, 109, 670.21778430 [Google Scholar]
- [104].Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam Y, Matkovich SJ, Ii GWD, Van Rooij E, Olson EN, 2011, 670. [DOI] [PMC free article] [PubMed]
- [105].Gu H, Liu Z, Zhou L, Biomed Res int. 2017, 2017, 9102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M, Int. J. Cardiol 2015, 182, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Liang D, Li J, Wu Y, Zhen L, Li C, Qi M, Wang L, Deng F, Huang J, Lv F, Liu Y, Ma X, Yu Z, Zhang Y, Chen Y-H, Int. J. Cardiol 2015, 201, 38. [DOI] [PubMed] [Google Scholar]
- [108].Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, Li Q, Xiang C, Zhang L, Wu R, Zhu W, Yu H, Hu X, Wang J, Stem Cells Transl. Med 2017, 6, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, Zhou F, Yang X, Yang J, Zeng C, Wang WE, Biochim. Biophys. Acta, Mol. Basis Dis 2017, 1863, 2085. [DOI] [PubMed] [Google Scholar]
- [110].Feng Y, Huang W, Wani M, Yu X, Ashraf M, PLoS One 2014, 9, e88685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV, Biomed Res Int 2015, 2015, 354517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Callis TE, Pandya K, Seok HY, Tang R-H, Tatsuguchi M, Huang Z-P, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ, J. Clin. Invest 2009, 119, 2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y, Biochem. Biophys. Res. Commun 2013, 431, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hosoda T, Zheng H, Cabral-de-Silva M, Sanada F, Ide-Iwata N, Ogórek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A, Circulation 2011, 123, 1287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [115].Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK, Int. J. Proteomics 2012, 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK, Nucleic Acids Res 2010, 38, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Davis ME, Circ. Res 2016, 119, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ruan X, Li Y, Ju C, Shen Y, Lei W, Chen C, Li Y, Yu H, Liu Y, Kim I, Wang X.-l., Weintraub NL, Tang Y, Acta Pharmacol. Sin 2018, 39, 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Zimmermann W-H, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T, Nat. Med 2006, 12, 452. [DOI] [PubMed] [Google Scholar]
- [120].Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y, Circulation 2012, 126, S29. [DOI] [PubMed] [Google Scholar]
- [121].Zhang J, Curr. Treat. Options Cardiovasc. Med 2015, 17, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang J, Zhu W, Radisic M, Vunjak-Novakovic G, Circ. Res 2018, 123, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Suzuki R, Hattori F, Itabashi Y, Yoshioka M, Yuasa S, Manabe-Kawaguchi H, Murata M, Makino S, Kokaji K, Yozu R, Fukuda K, Biochem. Biophys. Res. Commun 2009, 387, 353. [DOI] [PubMed] [Google Scholar]
- [124].Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE, Proc. Natl. Acad. Sci. USA 2009, 106, 16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kreutziger KL, Muskheli V, Johnson P, Braun K, Wight TN, Murry CE, Tissue Eng., Part A 2011, 17, 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Schaefer JA, Guzman PA, Riemenschneider SB, Kamp TJ, Tranquillo RT, Tissue Eng J. Regener. Med 2018, 12, 546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].O’Neill HS, O’Sullivan NJ. Ruiz-Hernandez Porteous, E., Kelly HM, O’Brien FJ, Duffy GP, J. Tissue Eng. Regener. Med 2016, 12, e384. [DOI] [PubMed] [Google Scholar]
- [128].Becker M, Maring AJ, Schneider M, Herrera Martin XA, Seifert M, Klein O, Braun T, Falk V, Stamm C, Int. J. Mol. Sci 2018, 19, 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Bejleri D, Streeter BW, Nachlas ALY, Brown ME, Gaetani R, Christman KL, Davis ME, Adv. Healthcare Mater 2018, 0, 1800672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Tang J, Cui X, Caranasos TG, Hensley MT, Vandergriff AC, Hartanto Y, Shen D, Zhang H, Zhang J, Cheng K, ACS Nano 2017, 11, 9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Su T, Huang K, Daniele MA, Hensley MT, Young AT, Tang J, Allen TA, Vandergriff AC, Erb PD, Ligler FS, Cheng K, ACS Appl. Mater. Interfaces 2018, 10, 33088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J, Circulation 2018, 137, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ogle BM, Bursac N, Domian I, Huang NF, Menasché P, Murry CE, Pruitt B, Radisic M, Wu JC, Wu SM, Zhang J, Zimmermann W-H, Vunjak-Novakovic G, Sci. Transl. Med 2016, 8, 342ps13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Tang J, Vandergriff A, Wang Z, Hensley MT, Cores J, Allen TA, Dinh P-U, Zhang J, Caranasos TG, Cheng K, Tissue Eng., Part C 2017, 23, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shen D, Tang J, Hensley MT, Li T, Caranasos TG, Zhang T, Zhang J, Cheng K, Stem Cells Transl. Med 2016, 5, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, Cavanaugh CA, Zhou S, Kanade R, Atluri P, Morrisey EE, Burdick JA, Nat. Biomed. Eng 2017, 1, 983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sarig U, Sarig H, de-Berardinis E, Chaw SY, Nguyen EB, Ramanujam VS, Thang VD, Al-Haddawi M, Liao S, Seliktar D, Kofidis T, Boey FY, Venkatraman SS, Machluf M, Acta Biomater. 2016, 44, 209. [DOI] [PubMed] [Google Scholar]
- [138].Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E, Nature 2017, 547, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Spang MT, Christman KL, Acta Biomater. 2018, 68, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Pahnke A, Li RK, Caldarone CA, Radisic M, Nat. Mater 2017, 16, 1038. [DOI] [PubMed] [Google Scholar]
- [141].Cannata A, Petrella D, Russo CF, Bruschi G, Fratto P, Gambacorta M, Martinelli L, Ann. Thorac. Surg 2013, 95, 1818. [DOI] [PubMed] [Google Scholar]
- [142].Sugiura T, Hibino N, Breuer CK, Shinoka T, J. Cardiothorac. Surg 2016, 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Malki M, Fleischer S, Shapira A, Dvir T, Nano Lett. 2018, 18, 4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fujimatsu T, Oosawa H, Takai F, Aruga M, Ogiwara F, Mawatari E, Sakurai S, Gen. Thorac. Cardiovasc. Surg 2007, 55, 345. [DOI] [PubMed] [Google Scholar]
- [145].Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE, Nature 2014, 510, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Morais JM, Papadimitrakopoulos F, Burgess DJ, AAPS J 2010, 12, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT, J. Mol. Cell. Cardiol 2011, 50, 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, Carullo P, Ramirez-Rodríguez GB, Patrício T, Esposti LD, Rossi F, Ravanetti F, Pinelli S, Alinovi R, Erreni M, Rossi S, Condorelli G, Post H, Tampieri A, Catalucci D, Sci. Transl. Med 2018, 10, eaan6205. [DOI] [PubMed] [Google Scholar]
- [149].Cores J, Caranasos GT, Cheng K, J. Funct. Biomater 2015, 6, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Sensenig R, Sapir Y, MacDonald C, Cohen S, Polyak B, Nanomedicine 2012, 7, 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Lu A-H, Salabas EL, Schüth F, Angew. Chem., Int. Ed 2007, 46, 1222. [DOI] [PubMed] [Google Scholar]
- [152].Arbab AS, Bashaw LA, Miller BR, Jordan EK, Bulte JWM, Frank JA, Transplantation 2003, 76, 1123. [DOI] [PubMed] [Google Scholar]
- [153].Chen J, Huang N, Ma B, Maitz MF, Wang J, Li J, Li Q, Zhao Y, Xiong K, Liu X, ACS Appl. Mater. Interfaces 2013, 5, 5976. [DOI] [PubMed] [Google Scholar]
- [154].Vandergriff AC, Hensley TM, Henry ET, Shen D, Anthony S, Zhang J, Cheng K, Biomaterials 2014, 35, 8528. [DOI] [PubMed] [Google Scholar]
- [155].Lee RJ, Fang Q, Davol PA, Gu Y, Sievers RE, Grabert RC, Gall JM, Tsang E, Yee MS, Fok H, Huang NF, Padbury JF, Larrick JW, Lum LG, Stem Cells 2009, 25, 712. [DOI] [PubMed] [Google Scholar]
- [156].Srikanth S, Ambrose JA, Curr. Cardiol. Rev 2012, 8, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Nesheim M, Ital. Hear. J 2001, 2, 641. [PubMed] [Google Scholar]
- [158].Malmsten M, Microgels in Drug Delivery, (Eds: Fernandez-Nieves A, Wyss HM, Johanttsson, Weitz DA), Wiley-VCH Verlag GmbH & Co; KGaA, Weinheim: 2011, pp 375–405. [Google Scholar]
- [159].Byrne H, Conroy PJ, Whisstock JC, O’Kennedy RJ, Trends Biotechnol. 2013, 31, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Mellman I, Coukos G, Dranoff G, Nature 2011, 480, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Tang J, Shen D, Zhang J, Ligler FS, Cheng K, Expert Opin. Biol. Ther 2015, 15, 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lum LG, Fok H, Sievers R, Abedi M, Quesenberry PJ, Lee RJ, Blood Cells, Mol., Dis 2004, 32, 82. [DOI] [PubMed] [Google Scholar]
- [163].Cheng K, Shen D, Hensley MT, Middleton R, Sun B, Liu W, De Couto G, Marbán E, Nat. Commun 2014, 5, 4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lippi G, Franchini M, Targher G, Nat. Rev. Cardiol 2011, 8, 502. [DOI] [PubMed] [Google Scholar]
- [165].Modery-Pawlowski CL, Kuo H-H, Baldwin WM, Sen Gupta A, Nanomedicine 2013, 8, 1709. [DOI] [PubMed] [Google Scholar]
- [166].Jackson SP, Blood 2007, 109, 5087. [DOI] [PubMed] [Google Scholar]
- [167].Hu C-M, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L, Nature 2015, 526, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Su T, Huang K, Ma H, Liang H, Dinh P, Chen J, Shen D, Allen T, Qiao L, Li Z, Hu S, Cores J, Frame BN, Young AT, Yin Q, Liu J, Qian L, Caranasos TG, Brudno Y, Ligler FS, Cheng K, Adv. Funct. Mater 2018, 0, 1803567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Overdijk MB, Verploegen S, Bögels M, van Egmond M, van Bueren JJL, Mutis T, Groen RWJ, Breij E, Martens ACM, Bleeker WK, Parren PW, mAbs 2015, 7, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Muzykantov V, Muro S, Int. J. Transp. Phenom 2011, 12, 41. [PMC free article] [PubMed] [Google Scholar]