Abstract
Background:
We studied the effect of early antihypertensive treatment on death, major disability, and vascular events among patients with acute ischemic stroke according to their baseline systolic blood pressure (SBP).
Methods:
We randomly assigned 4,071 acute ischemic stroke patients with SBP between 140 and <220 mmHg to receive antihypertensive treatment or to discontinue all antihypertensive medications during hospitalization. A composite primary outcome of death and major disability and secondary outcomes were compared between treatment and control stratified by baseline SBP levels of <160, 160-179, and ≥180 mmHg.
Results:
At 24 hours after randomization, differences in SBP reductions were 8.8, 8.6 and 7.8 mmHg between the antihypertensive treatment and control groups among patients with baseline SBP <160, 160-179, and ≥180 mmHg, respectively (p<0.001 among subgroups). At day 14 or hospital discharge, the primary and secondary outcomes were not significantly different between the treatment and control groups among subgroups. However, there was a significant interaction between antihypertensive treatment and baseline SBP subgroups on death (p=0.02): odds ratio (95% CI) of 2.42 (0.74-7.89) in patients with baseline SBP <160 mmHg and 0.34 (0.11-1.09) in those with baseline SBP ≥180 mmHg. At the 3-month follow-up, the primary and secondary clinical outcomes were not significantly different between the treatment and control groups by baseline SBP levels.
Conclusion:
Early antihypertensive treatment had a neutral effect on clinical outcomes among acute ischemic stroke patients with various baseline SBP levels. Future clinical trials are warranted to test BP-lowering effects in acute ischemic stroke patients by baseline SBP levels.
Keywords: acute ischemic stroke, antihypertensive treatment, major disability, mortality, blood pressure
Introduction
Stroke is the second most common cause of death and the leading cause of major, long-term disability worldwide.1 Prospective cohort studies and clinical trials have demonstrated that hypertension is the most important modifiable risk factor for incident and recurrent stroke.2–5 Elevated blood pressure (BP) is common in the acute phase of ischemic stroke, occurring in about 75% of all patients.6 This increase in BP may be due to uncontrolled or undiagnosed chronic hypertension, or a response to physical and psychological stresses from brain ischemia.7,8 This initial hypertensive response is self-limiting and typically resolves over several days, with BP spontaneously declining in most patients.7,8 Recently, several clinical trials have shown that individual antihypertensive medications or BP-lowering strategies in patients with acute ischemic stroke have a neutral effect on death or major disability outcomes.9–12
Observational epidemiologic studies have reported “U-shaped” or “J-shaped” associations of admission BP with subsequent mortality, major disability, and recurrent stroke among patients with acute ischemic stroke.13–15 Extreme high BP is clearly detrimental, because it leads to hypertensive encephalopathy, hemorrhagic transformation, cardiac complications, and renal insufficiency.16 However, an optimal BP range in acute ischemic stroke is still not known. Current clinical guidelines recommend that antihypertensive medications should be withheld unless the systolic BP is >220 mmHg or the diastolic BP is >120 mmHg among patients with acute ischemic stroke.16 It is common in clinical practice, nevertheless, to lower BP among patients with acute ischemic stroke if systolic BP is >180 mmHg.17
The China Antihypertensive Trail in Acute Ischemic Stroke (CATIS) was a multicenter randomized controlled trial designed to test the effect of BP reduction within the first 48 hours after the onset of an acute ischemic stroke on death and major disability10. CATIS provides an opportunity to assess the effect of early antihypertensive treatment on death, major disability, recurrent stroke, and vascular events among patients with acute ischemic stroke according to their baseline systolic BP levels (<160, 160-79, and ≥180 mmHg).
Methods
Trial Design and Participants
CATIS was a multicenter, single-blind, blinded end-points randomized controlled clinical trial performed in 26 hospitals across China. Details on the trial design and methods have been described in a previous paper.10 In brief, 4071 patients aged 22 years or older who had an acute ischemic stroke within 48 hours of symptom onset and an elevated systolic BP between 140 mmHg and <220 mmHg were recruited. Ischemic stroke was confirmed by either computed tomography or magnetic resonance imaging of the brain. Patients who had a systolic BP ≥220 mmHg, diastolic BP ≥120 mmHg, severe heart failure, acute myocardial infarction, unstable angina, atrial fibrillation, aortic dissection, cerebrovascular stenosis, or resistant hypertension were excluded. Additionally, those in a deep coma and those treated with intravenous thrombolytic therapy were excluded.
This study was approved by the institutional review boards at Tulane University in the US and Soochow University in China, in addition to the ethical committees at all 26 participating hospitals. Written consent was obtained from all study participants or their immediate family members. A data and safety monitoring board met annually to review the accumulating data for safety and to monitor the trial for either superiority or inferiority of BP reduction on the clinical outcomes.
Intervention
In the CATIS study, patients were randomly assigned to either a control or treatment group. Randomization was conducted centrally and the study population was stratified by hospital and use of hypertension medications. Randomization schedules were generated using SAS PROC PLAN and concealed until an eligible participant was ready for enrollment. Although treating study physicians and nurses were not blinded to group assignments, the patients and research staff who collected study outcome data were masked to treatment allocation.
The treatment group received antihypertensive treatment aimed at lowering systolic BP by 10%-25% within the first 24 hours after randomization, reducing systolic BP to ≤140 mmHg and diastolic BP to ≤90 mmHg within 7 days, and maintaining this level of BP control for the rest of the patient’s hospitalization. The treatment was designed to test for BP strategy rather than the efficacy of individual antihypertensive drugs. As such, multiple antihypertensive agents, including intravenous angiotensin-converting enzyme inhibitors (enalapril, first-line), calcium channel blockers (second-line), and diuretics (third-line), were used individually or in combination to achieve the targeted BP reduction according to a pre-specified treatment algorithm that was detailed in a previous paper.10
At the patient’s hospital discharge, trial participants in both groups were prescribed to antihypertensive medications according to clinical guidelines.10
Measurements
Participant demographic characteristics and medical histories were collected at enrollment. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS; scores range from 0 to 42, with higher scores indicating more severe neurologic deficits) at baseline, 14 days or hospital discharge, and 3 months after treatment.18 Computed tomography or magnetic resonance imaging of the brain was performed according to standard techniques to confirm the diagnosis of ischemic stroke in all trial participants. Three BP measurements were obtained at baseline by trained nurses according to a common protocol adapted from procedures recommended by the American Heart Association, and the average of the measurements was used for analysis.19 BP was measured with the patient in a supine position using a standard mercury sphygmomanometer and one of four cuff sizes (pediatric, regular adult, large adult, or thigh) based on participant’s arm circumference. After randomization, 3 BP measurements were obtained every two hours for the first 24 hours, every four hours during the second and third days, and 3 times a day thereafter until hospital discharge or death.
Outcome Assessment
The primary outcome was a combination of death and major disability, defined as a modified Ranking Scale score of 3-6 (modified Ranking Scale scores range from 0 to 6, with a score of 0 indicating no symptoms, a score of 5 indicating severe disability, and a score of 6 indicating death).20 Secondary outcomes included an ordered 7-level categorical score of the modified Rankin Scale for neurological functional status, vascular disease events (e.g. vascular deaths, nonfatal stroke, nonfatal myocardial infarction, and hospitalized and treated angina, heart failure, or peripheral arterial disease), recurrent fatal and nonfatal stroke, and all-cause mortality assessed at the 3-month posttreatment follow-up visit.
Study outcomes were assessed at 14 days or at hospital discharge, whichever was first, and at 3 months posttreatment by trained neurologists and nurses who were unaware of treatment assignment. Data on medical history, deaths, vascular events, BP, NIHSS score, and modified Rankin Scale score were obtained. Death certificates were obtained for deceased participants. Hospital data was abstracted for all vascular events. A trial-wide outcome assessment committee, blinded to treatment assignment, reviewed and adjudicated vascular events based on the criteria established in the Antihypertensive and lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT).21
Statistical Analysis
Data were analyzed according to participants’ randomized treatment assignments, regardless of their subsequent medication status (intention-to-treat). Participants were divided into three pre-specified subgroups according to their baseline systolic BP (<160, 160-179, and ≥180 mmHg). Within each BP subgroup, the proportion of participants with primary and secondary outcomes at 14 days or discharge and at the 3-month posttreatment follow-up visit were compared based on their treatment group using a χ2 test at a two-sided α level of 5% without correction for multiple comparisons because the subgroup analysis was used to generate study hypotheses rather than test a hypothesis. The medians and interquartile range of Rankin score and hospitalization were calculated and compared using the Wilcoxon rank-sum test.22 Logistic regression analysis was used to estimate unadjusted odds ratios and 95% confidence intervals associated with comparing treatment groups. Additionally, the odd ratios of ordinal Rankin scores were computed using ordinal logistic regression in order to estimate the effect of BP reduction on the full range of the modified Rankin Scale.23 Heterogeneity of the treatment effect on primary and secondary outcomes according to BP subgroups was asses by adding an interaction term in logistic regression models. In a sensitivity analysis, age, sex, baseline systolic BP, body-mass index, diabetes, cigarette smoking, stroke subtype, and NIHSS score at baseline as well as time from onset to randomization were adjusted. These prognostic variables were selected as confounders because they were significantly or borderline-significantly different (p<0.10) between treatment and control groups by baseline BP or they were reported to influence study outcomes.24,25 Data analyses were performed using SAS version 9.4 (SAS Institute Inc.; Cary, North Carolina, USA) and Stata version 12 (StataCorp; College Station, Texas, USA).
Results
Study participants
Of the 4071 patients included in this study, 2038 were randomly assigned to receive antihypertensive treatment and 2033 were assigned to control. Seven participants in the treatment group and six in the control group withdrew from the trial during hospitalization. In addition, 43 participants in treatment and 40 in control groups were lost to follow-up at 3 months (Figure 1). All participants were included in analysis at 14 days or hospital discharge and 3,975 (1,988 in intervention and 1,987 in control) participants were included in analysis at 3 months.
Figure 1.
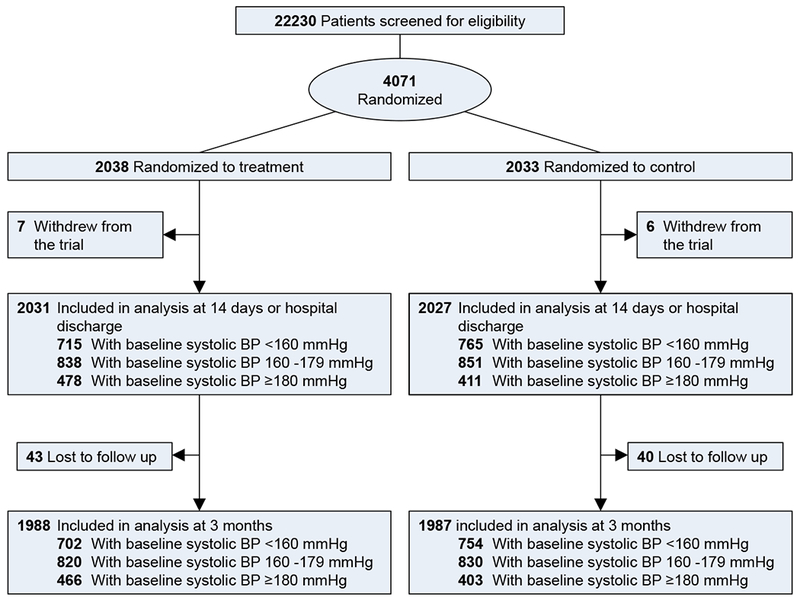
Flow chart of trial participants.
At baseline there were 1486 patients (719 in the treatment group and 767 in control) in the systolic BP <160 mmHg subgroup, 1696 (841 in treatment and 855 in control) in the systolic BP 160-179 mmHg subgroup, and 889 (478 in treatment and 411 in control) in the systolic BP ≥180 mmHg subgroup. Baseline characteristic were balanced between antihypertensive treatment and control comparison in each of the systolic BP subgroups (Table 1).
Table 1.
Baseline characteristics of trial participants according to baseline systolic blood pressure levels.
| Systolic BP <160 mm Hg | Systolic BP 160-179 mm Hg | Systolic BP ≥180 mm Hg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment (n=719) | Control (n=767) | P value | Treatment (n=841) | Control (n=855) | P value | Treatment (n=478) | Control (n=411) | P value | |
| Male, n (%) | 484 (67.3) | 482 (62.8) | 0.07 | 539 (64.1) | 546 (63.9) | 0.92 | 294 (61.5) | 259 (63.0) | 0.64 |
| Age, mean (SD) (years) | 62.2 (10.8) | 61.0 (11.1) | 0.03 | 62.1 (10.8) | 62.5 (10.5) | 0.43 | 61.8 (10.6) | 62.1 (11.6) | 0.69 |
| Time from onset to randomization, mean (SD) (hrs) | 16.2 (13.3) | 15.8 (13.5) | 0.64 | 15.2 (12.6) | 14.7 (12.7) | 0.42 | 14.2 (12.5) | 13.5 (12.4) | 0.41 |
| Blood pressure at entry, mean (SD) (mmHg) | |||||||||
| Systolic | 149.8 (5.8) | 150.4 (5.6) | 0.05 | 167.2 (6.0) | 167.1 (6.4) | 0.60 | 191.0 (11.7) | 190.9 (11.4) | 0.92 |
| Diastolic | 92.0 (8.7) | 91.8 (9.3) | 0.63 | 96.8 (9.4) | 96.6 (9.6) | 0.61 | 104.0 (12.1) | 105.3 (12.9) | 0.11 |
| BMI, mean (SD) (kg/m2) | 24.8 (2.9) | 25.1 (3.2) | 0.08 | 25.0 (3.1) | 24.8 (3.1) | 0.24 | 25.0 (3.5) | 25.2 (3.2) | 0.33 |
| History of hypertension, n (%) | 553 (76.9) | 597 (77.8) | 0.67 | 668 (79.4) | 663 (77.5) | 0.34 | 389 (81.4) | 339 (82.5) | 0.67 |
| Use of antihypertensive medications, n (%) | 353 (49.1) | 374 (48.8) | 0.90 | 422 (50.2) | 400 (46.8) | 0.16 | 239 (50.0) | 209 (50.9) | 0.80 |
| Hyperlipidemia, n (%) | 52 (7.2) | 62 (8.1) | 0.54 | 60 (7.1) | 55 (6.4) | 0.57 | 25 (5.2) | 23 (5.6) | 0.81 |
| Diabetes, n (%) | 149 (20.7) | 132 (17.2) | 0.08 | 132 (15.7) | 143 (16.7) | 0.57 | 88 (18.4) | 75 (18.3) | 0.95 |
| History of coronary heart disease, n (%) | 82 (11.4) | 96 (12.5) | 0.51 | 93 (11.1) | 92 (10.8) | 0.84 | 41 (8.6) | 40 (9.7) | 0.55 |
| Current cigarette smoking, n (%) | 248 (34.5) | 267 (34.8) | 0.90 | 291 (34.6) | 338 (39.5) | 0.04 | 186 (38.9) | 155 (37.7) | 0.71 |
| Current alcohol drinking, n (%) | 221 (30.7) | 239 (31.2) | 0.86 | 256 (30.4) | 265 (31.0) | 0.80 | 137 (28.7) | 135 (32.9) | 0.18 |
| NIHSS score at baselinea, median (IQR) | 4.0 (2.0 to 7.0) | 4.0 (2.0 to 7.0) | 0.95 | 4.0 (2.0 to 8.0) | 5.0 (3.0 to 8.0) | 0.11 | 5.0 (2.0 to 8.0) | 5.0 (3.0 to 8.0) | 0.16 |
| Ischemic stroke subtypeb, n (%) | |||||||||
| Thrombotic | 546 (75.9) | 616 (80.3) | 0.04 | 668 (79.4) | 665 (77.8) | 0.41 | 361 (75.5) | 314 (76.4) | 0.76 |
| Embolic | 44 (6.1) | 45 (5.9) | 0.84 | 33 (3.9) | 38 (4.4) | 0.59 | 22 (4.6) | 20 (4.9) | 0.85 |
| Lacunar | 153 (21.3) | 126 (16.4) | 0.02 | 157 (18.7) | 172 (20.1) | 0.45 | 107 (22.4) | 87 (21.2) | 0.66 |
Difference in means between the antihypertensive treatment and control groups was tested using a Student’s t test, percentages using a χ2 test, and medians using the Wilcoxon rank-sum test. IQR, interquartile range.
Scores range from 0 (normal neurologic status) to 42 (coma with quadriplegia).
Eleven patients with both thrombotic and embolic, 92 with thrombotic and lacunar, five with embolic and lacunar, and one with all three subtypes.
Blood Pressure Reduction
At 24 hours after randomization, mean systolic BP was reduced by 12.9 (8.5%), 22.2 (13.2%), and 34.8 mmHg (18.1%) in the treatment group, and 4.1 (2.7%), 13.6 (8.1%), and 27.0 mmHg (13.9%) in the control group (p<0.001 for all group differences between treatment and control) for patients with a baseline systolic BP <160, 160-179, and ≥180 mmHg, respectively (Figure 2). Mean systolic BP was 133.2, 137.3, and 143.2 mmHg in the treatment group and 142.5, 147.5, and 152.3 mmHg in the control group at day 7 after randomization (p<0.001 for all group differences between treatment and control) for patients with a baseline systolic BP <160, 160-179, and ≥180 mmHg, respectively. The corresponding systolic BP was 132.5, 135.0, and 139.0 mmHg in the treatment group and 140.2, 144.9, and 147.5 mmHg in the control group at day 14 or hospital discharge (p<0.001 for all group differences between treatment and control), respectively.
Figure 2.
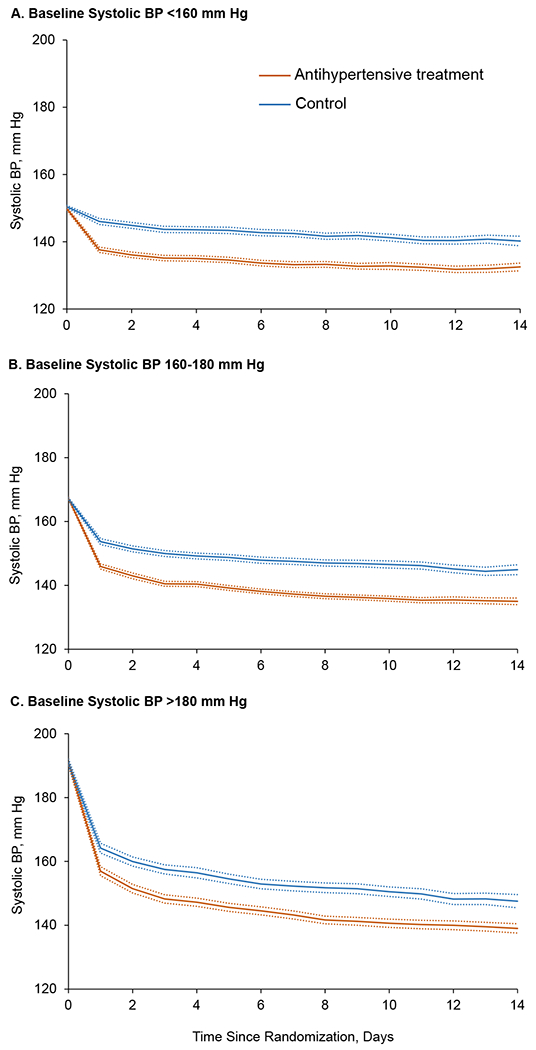
Mean and 95% confidence interval of systolic BP since randomization by treatment group.
Upper panel: subgroup with baseline systolic BP <160 mmHg; middle panel: baseline systolic BP 160-179 mmHg; and lower panel: baseline systolic BP ≥180 mmHg.
Clinical Outcomes at 14 Days or Hospital Discharge
At 14 days or hospital discharge, the proportion of patients that experienced the primary outcomes of death or major disability was not significantly different between treatment and control groups based on systolic BP subgroups (Table 2). Likewise, there was no significant difference in modified Rankin scale scores between treatment and control among systolic BP subgroups. However, there was a significant interaction between antihypertensive treatment and baseline systolic BP subgroups on death (p=0.02): odds ratio (95% CI) of 2.42 (0.74 to 7.89) in patients with baseline systolic BP <160 mmHg and 0.34 (0.11 to 1.09) in patients with baseline systolic BP ≥180 mmHg).
Table 2.
Clinical outcomes at 14 days or hospital discharge according to baseline systolic blood pressure levels.
| Outcome variables | <160 mm Hg | 160-179 mm Hg | ≥180 mm Hg | P value for homogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (n=715) | Control (n=765) | OR (95% CI) | P value | Treatment (n=838) | Control (n=851) | OR (95% CI) | P value | Treatment (n=478) | Control (n=411) | OR (95% CI) | P value | ||
| Primary outcome | |||||||||||||
| Death or major disabilitya, n (%) | 225 (31.5) | 228 (29.8) | 1.08 (0.87 to 1.35) | 0.49 | 288 (34.4) | 297 (34.9) | 0.98 (0.80 to 1.19) | 0.82 | 170 (35.6) | 156 (38.0) | 0.90 (0.69 to 1.19) | 0.46 | 0.29 |
| Secondary outcomes | |||||||||||||
| Score on modified Rankin scaleb, median (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.34 | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.47 | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.25 | 0.12 | |||
| Participants, n (%) | |||||||||||||
| 0 (no symptoms) | 66 (9.2) | 55 (7.2) | 1.09 (0.91 to 1.31)c | 0.34 | 93 (11.1) | 70 (8.2) | 0.94 (0.79 to 1.11) c | 0.47 | 45 (9.4) | 29 (7.1) | 0.87 (0.69 to 1.10) c | 0.25 | 0.29 |
| 1 (no significant disability despite symptoms) | 228 (31.9) | 287 (37.5) | 266 (31.7) | 278 (32.7) | 159 (33.3) | 136 (33.1) | |||||||
| 2 (slight disability) | 196 (27.4) | 195 (25.5) | 191 (22.8) | 206 (24.2) | 104 (21.8) | 90 (21.9) | |||||||
| 3 (moderate disability) | 97 (13.6) | 110 (14.4) | 120 (14.3) | 129 (15.2) | 75 (15.7) | 58 (14.1) | |||||||
| 4 (moderately severe disability) | 88 (12.3) | 81 (10.6) | 110 (13.1) | 129 (15.2) | 60 (12.6) | 72 (17.5) | |||||||
| 5 (severe disability) | 31 (4.3) | 33 (4.3) | 46 (5.5) | 28 (3.3) | 31 (6.5) | 16 (3.9) | |||||||
| 6 (dead) | 9 (1.3) | 4 (0.5) | 12 (1.4) | 11 (1.3) | 4 (0.8) | 10 (2.4) | |||||||
| Death, n (%) | 9 (1.3) | 4 (0.5) | 2.42 (0.74 to 7.89) | 0.14 | 12 (1.4) | 11 (1.3) | 1.11 (0.49 to 2.53) | 0.80 | 4 (0.8) | 10 (2.4) | 0.34 (0.11 to 1.09) | 0.07 | 0.02 |
| Duration of initial hospitalization, median (IQR), days | 12.0 (9.0-14.0) | 13.0 (9.0-14.0) | 0.94 | 13.0 (9.0-14.0) | 13.0 (9.0-14.0) | 0.13 | 13.0 (9.0-14.0) | 13.0 (9.0-14.0) | 0.99 | 0.82 | |||
Difference in the percentages of composite death or major disability and all-cause mortality between the antihypertensive treatment and control groups was tested using a χ2 test, the medians of Rankin score and hospitalization using the Wilcoxon rank-sum test, and odds ratios of ordinal Rankin scores using ordinal logistic regression.
Modified Rankin Score of 3 or greater.
Scores on the modified Rankin Scale, for which a score of 0 indicates no symptoms, a score of 5 indicates severe disability, and a score of 6 indicates death.
Odds of a 1-unit higher modified Rankin score.
Clinical Outcomes at 3-Month Posttreatment Follow-up Visit
At the 3-month posttreatment follow-up visit, systolic and diastolic BP was significantly lower in the treatment group compared to the control group, with systolic BP differences (95% CI) of −2.6 (−3.8 to −1.5), −3.3 (−4.5 to −2.1), and −3.4 (−5.2 to −1.6) and diastolic BP differences (95% CI) of −1.6 (−2.4 to −0.8), −1.5 (−2.3 to −0.8) and −1.1 (−2.2 to 0.0) among patients with baseline systolic BP <160, 160-179, and ≥180 mmHg, respectively (Table 3). The proportion of participants who experienced death or major disability was not significantly different between the treatment and control groups among systolic BP subgroups (Table 3). Similarly, there was no significant difference in modified Rankin scale scores, recurrent stroke, vascular events, or composite event of vascular events and death between the treatment and control groups by systolic BP subgroups.
Table 3.
Clinical outcomes at 3-month posttreatment follow-up visit according to baseline systolic blood pressure levels.
| Outcome variables | Systolic BP <160 mm Hg | Systolic BP 160-179 mm Hg | Systolic BP ≥180 mm Hg | P value for homogeneity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (n=702) | Control (n=754) | BP difference or OR (95% CI) | P value | Treatment (n=820) | Control (n=830) | BP difference or OR (95% CI) | P value | Treatment (n=466) | Control (n=403) | BP difference or OR (95% CI) | P value | ||
| Blood pressure at 3 months after randomization, mm Hg, mean ± SD | |||||||||||||
| Systolic | 137.4 (11.0) | 140.0 (11.0) | −2.6 (−3.8 to −1.5) | <0.001 | 139.3 (11.5) | 142.7 (12.8) | −3.3 (−4.5 to −2.1) | <0.001 | 142.1 (12.5) | 145.5 (13.8) | −3.4 (−5.2 to −1.6) | <0.001 | 0.46 |
| Diastolic | 85.0 (7.7) | 86.7 (7.7) | −1.6 (−2.4 to −0.8) | <0.001 | 86.0 (7.9) | 87.5 (8.0) | −1.5 (−2.3 to −0.8) | <0.001 | 87.4 (8.0) | 88.5 (8.3) | −1.1 (−2.2 to 0.0) | 0.05 | 0.45 |
| Use of antihypertensive medication, n (%) | 574 (82.5) | 531 (70.7) | 1.95 (1.52 to 2.51) | <0.001 | 683 (83.9) | 626 (76.1) | 1.64 (1.28 to 2.10) | <0.001 | 410 (88.7) | 330 (82.9) | 1.63 (1.10-2.40) | 0.01 | 0.38 |
| Death or major disabilitya, n (%) | 155 (22.1) | 162 (21.5) | 1.04 (0.81 to 1.33) | 0.78 | 223 (27.2) | 215 (25.9) | 1.07 (0.86 to 1.33) | 0.55 | 122 (26.2) | 125 (31.0) | 0.79 (0.59 to 1.06) | 0.12 | 0.17 |
| Score on modified Rankin scaleb, median (IQR) | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 0.78 | 1.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.45 | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 0.39 | 0.35 | |||
| Participants, n (%) | |||||||||||||
| 0 (no symptoms) | 123 (17.5) | 138 (18.3) | 1.03 (0.85 to 1.24)c | 0.78 | 167 (20.4) | 141 (17.0) | 0.94 (0.79 to 1.11)c | 0.45 | 87 (18.7) | 62 (15.4) | 0.90 (0.71 to 1.14)c | 0.39 | 0.35 |
| 1 (no significant disability despite symptoms) | 264 (37.6) | 279 (37.0) | 257 (31.3) | 273 (32.9) | 145 (31.1) | 138 (34.2) | |||||||
| 2 (slight disability) | 160 (22.8) | 175 (23.2) | 173 (21.1) | 201 (24.2) | 112 (24.0) | 78 (19.4) | |||||||
| 3 (moderate disability) | 85 (12.1) | 87 (11.5) | 115 (14.0) | 109 (13.1) | 53 (11.4) | 69 (17.1) | |||||||
| 4 (moderately severe disability) | 39 (5.6) | 45 (6.0) | 60 (7.3) | 58 (7.0) | 37 (7.9) | 27 (6.7) | |||||||
| 5 (severe disability) | 11 (1.6) | 14 (1.9) | 18 (2.2) | 27 (3.3) | 14 (3.0) | 12 (3.0) | |||||||
| 6 (dead) | 20 (2.9) | 16 (2.1) | 30 (3.7) | 21 (2.5) | 18 (3.9) | 17 (4.2) | |||||||
| Death, n (%) | 20 (2.9) | 16 (2.1) | 1.35 (0.70 to 2.63) | 0.37 | 30 (3.7) | 21 (2.5) | 1.46 (0.83 to 2.58) | 0.19 | 18 (3.9) | 17 (4.2) | 0.91 (0.46 to 1.80) | 0.79 | 0.39 |
| Recurrent stroke, n (%) | 6 (0.9) | 13 (1.7) | 0.49 (0.19 to 1.31) | 0.16 | 14 (1.7) | 16 (2.0) | 0.88 (0.43 to 1.82) | 0.73 | 8 (1.7) | 14 (3.5) | 0.48 (0.20 to 1.16) | 0.10 | 0.84 |
| Vascular events, n (%)d | 11 (1.6) | 19 (2.5) | 0.62 (0.29 to 1.31) | 0.21 | 26 (3.2) | 22 (2.7) | 1.20 (0.67 to 2.14) | 0.54 | 11 (2.4) | 18 (4.5) | 0.51 (0.24 to 1.10) | 0.09 | 0.63 |
| Death or vascular events, n (%) | 26 (3.7) | 27 (3.6) | 1.04 (0.60 to 1.79) | 0.90 | 43 (5.2) | 37 (4.5) | 1.19 (0.76 to 1.86) | 0.46 | 23 (4.9) | 30 (7.4) | 0.65 (0.37 to 1.13) | 0.13 | 0.21 |
Difference in mean blood pressure between the antihypertensive treatment and control groups was tested using a Student’s t test, the percentages of composite death or major disability, all-cause mortality, recurrent stroke, vascular events, and use of antihypertensive medication using a χ2 test, the medians of Rankin score using the Wilcoxon rank-sum test, and odds ratios of ordinal Rankin scores using ordinal logistic regression. BP, blood pressure; CI, confidence interval; OR, odds ratio.
Modified Rankin Score of 3 or greater.
Scores on the modified Rankin Scale, for which a score of 0 indicates no symptoms, a score of 5 indicates severe disability, and a score of 6 indicates death.
Odds of a 1-unit higher modified Rankin score.
Includes vascular deaths, nonfatal stroke, nonfatal myocardial infarction, hospitalized and treated angina, hospitalized and treated congestive heart failure, and hospitalized and treated peripheral arterial disease.
Sensitivity Analysis
After adjustment for important prognostic variables, the effects of treatment on clinical outcomes were consistent with the results from unadjusted analyses (Table 4). Primary outcome (death or major disability) and ordinal modified Rankin score were not significantly different between the treatment and control groups by baseline systolic BP at both 14 days or hospital discharge and 3 months. However, all-cause mortality was significantly reduced in patients with baseline systolic BP ≥180 mmHg and non-significantly increased in patients with baseline systolic BP <180 mmHg (p=0.01 for interaction). Recurrent stroke, vascular events, and the composite outcome of death and vascular events at 3 months were not significantly different between the treatment and control groups by baseline systolic BP.
Table 4.
Adjusted odds ratio (95% CI) at 14 days/hospital discharge or at 3-month post-treatment follow-up visit according to baseline systolic blood pressure levelsa
| <160 mm Hg | 160-179 mm Hg | ≥180 mm Hg | P value for homogeneity | ||||
|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | ||
| 14 days or hospital discharge | |||||||
| Composite death or major disability | 1.12 (0.82 to 1.52) | 0.47 | 1.10 (0.84 to 1.44) | 0.48 | 1.01 (0.69 to 1.46) | 0.97 | 0.63 |
| Ordinal modified Rankin score | 1.09 (0.88 to 1.34) | 0.42 | 1.03 (0.86 to 1.25) | 0.73 | 0.90 (0.70 to 1.16) | 0.40 | 0.36 |
| All-cause mortality | 2.41 (0.56 to 10.30) | 0.24 | 1.57 (0.57 to 4.36) | 0.39 | 0.08 (0.01 to 0.71) | 0.02 | 0.01 |
| 3-month post-treatment follow-up visit | |||||||
| Composite death or major disability | 1.03 (0.75 to 1.42) | 0.87 | 1.30 (0.98 to 1.73) | 0.07 | 0.84 (0.58 to 1.21) | 0.34 | 0.38 |
| Ordinal modified Rankin score | 1.04 (0.84 to 1.28) | 0.74 | 1.07 (0.89 to 1.30) | 0.45 | 0.99 (0.77 to 1.28) | 0.96 | 0.86 |
| All-cause mortality | 1.23 (0.55 to 2.71) | 0.62 | 2.27 (1.14 to 4.52) | 0.02 | 0.45 (0.18 to 1.15) | 0.10 | 0.57 |
| Recurrent stroke | 0.69 (0.25 to 1.94) | 0.48 | 0.92 (0.43 to 2.00) | 0.84 | 0.49 (0.20 to 1.17) | 0.11 | 0.49 |
| Vascular events | 0.72 (0.32 to 1.61) | 0.43 | 1.31 (0.70 to 2.43) | 0.40 | 0.51 (0.23 to 1.14) | 0.10 | 0.46 |
| Death or vascular events | 1.06 (0.56 to 2.01) | 0.85 | 1.47 (0.88 to 2.45) | 0.14 | 0.64 (0.34 to 1.20) | 0.16 | 0.20 |
Abbreviations: CI = confidence interval; NIHSS = NIH stroke scale
Adjusting for age, sex, baseline systolic blood pressure, body-mass index, diabetes, cigarette smoking, stroke subtype, and NIH Stroke Scale score at baseline as well as time from onset to randomization.
Discussion
The current subgroup analysis of the CATIS trial found no significant difference in stroke adverse outcomes between early antihypertensive treatment and discontinued antihypertensive medications during hospitalization among acute ischemic stroke patients with various systolic BP levels at baseline. However, this analysis also observed an interaction between antihypertensive treatment and baseline systolic BP on death and suggested a greater reduction in case-fatality rate during hospitalization among patients with a systolic BP ≥180 mmHg at baseline.
These study findings may have important clinical significance. Although current clinical guidelines recommend that antihypertensive medications should be withheld unless systolic BP is >220 mmHg or diastolic BP is >120 mmHg, it is common to lower BP when systolic BP is >180 mmHg in clinical practice.16,17 The findings from this analysis indicate that there was no statistically significant difference in the composite primary outcome of death or major disability between the treatment and control groups among patients with a systolic BP between 180-220 mmHg. Therefore, antihypertensive treatment should be up to the discretion of health care providers based on individual clinical judgment as long as the patients have systolic BP <220 mmHg.
While the most prospective cohort analyses reported an U-shaped associations between BP during acute phase and adverse clinical outcomes in patients with ischemic stroke, extremely elevated BP was consistently associated with worse clinical outcomes.13–15, 26–29 In 17,398 patients with confirmed ischemic stroke from the International Stroke Trial, early death increased by 17.9% for every 10 mmHg fall in systolic BP among patients with a systolic BP <150 mmHg (P<0.0001) and increased by 3.8% for every 10 mmHg increase in systolic BP among patients with a systolic BP >150 mm Hg (P=0.016).13 Among 1,874 patients with first-ever acute ischemic stroke from the Fukuoka Stroke Registry, systolic BP at admission ≥166 mmHg was associated with neurological deterioration (odds ratio 1.92; 95% CI 1.15-3.27) and poor functional outcome (odds ratio 2.51, 95% CI 1.69-3.74).28 The current subgroup analyses of the CATIS trial did not show a reduced mortality or disability related to antihypertensive treatment among acute ischemic stroke patients with a systolic BP 160-180 mmHg, or even ≥180 mmHg. Observational studies might be biased by confounding factors, such as severity of stroke, and underlying mechanisms of BP increase during acute phase of ischemic stroke.30
Our study suggested a greater reduction of in-hospital mortality associated with early antihypertensive treatment among acute ischemic stroke patients with a baseline systolic BP 180-220 mmHg. In the Efficacy of Nitric Oxide in Stroke (ENOS) trial, a suggestive beneficial effect of glyceryl trinitrate on functional outcome at 90 days was observed only among acute ischemic stroke patients with a systolic BP ≥200 mmHg (odds ratio 0.74, 95% CI 0.48-1.15).11 These findings warrant future randomized clinical trials to test the effect of antihypertensive treatment among acute ischemic stroke patients with a systolic BP 180-220 mmHg at admission.
The mean time from onset to randomization was 15.3 hours (SD=12.9) in the treatment group and 14.9 hours (SD=13.0) in the control group in the CATIS trial.10 Time from stroke onset to initiation of antihypertensive treatment has been associated with clinical outcomes in patients with acute ischemic stroke.24,31
As a subgroup analysis of a clinical trial, this study had some limitations, such as multiple comparisons, loss of statistical power, and difficulty in interpretation.32 For example, interactions between antihypertensive treatment and baseline BP have been tested on multiple primary and secondary study outcomes. Therefore, chance or confounding factors could explain the subgroup study findings. However, the study findings did not change after adjusting for important observed prognostic variables. Rather than testing predefined hypotheses, this subgroup analysis has generated study hypotheses for future clinical trials. Additionally, our study participants were exclusively Chinese stroke patients and findings from our study might not be generalizable to other populations in whom the management of acute ischemic stroke might be different. Furthermore, our trial did not compare the appropriate BP treatment targets, times of starting treatment, and classes of antihypertensive drug for early BP lowering in patients with acute ischemic stroke. Finally, cerebral blood flow data were not collected in the CATIS trial. Future trials should collect these data to guide BP management in acute ischemic stroke.
In conclusion, this subgroup analysis of the CATIS trial indicates that early antihypertensive treatment had a neutral effect on clinical outcomes among acute ischemic stroke patients with various levels of systolic BP at baseline. Future trials are warranted to test early BP-lowering effects in acute ischemic stroke patients by baseline systolic BP levels.
Acknowledgements
We thank the clinical staff at all participating hospitals for their support and contribution to this project and patients and their families for their collaboration.
Sources of Funding
This study was partially supported by the National Institute of General Medical Sciences of the National Institutes of Health (P20GM109036), Bethesda, MD; Soochow University, a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the National Natural Science Foundation of China (grant No. 81320108026), all in China. Dr. Chongke Zhong was supported by a research training grant (D43TW009107) from the NIH Fogarty International Center, Bethesda, MD. We acknowledge that the Changzhou Pharmaceutical Factory provided the study drug (Enalapril) for this trial.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT01840072
Conflicts of interest
None.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Gu D, Chen J, Wu X, Kelly TN, Huang JF, et al. Premature deaths attributable to blood pressure in China: a prospective cohort study. Lancet. 2009;374(9703):1765–72. [DOI] [PubMed] [Google Scholar]
- 3.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383(9932):1899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–67. [DOI] [PubMed] [Google Scholar]
- 5.Bundy JD, Li C, Stuchlik P, Bu X, Kelly TN, Mills KT, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. January 2007;25(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation. 2008;118(2):176–187. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL. Blood pressure management in early ischemic stroke. JAMA. 2014;311(5):469–470. [DOI] [PubMed] [Google Scholar]
- 9.Sandset EC, Bath PM, Boysen G, Jatuzis D, Kõrv J, Lüders S, et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. Lancet. 2011;377(9767):741–750. [DOI] [PubMed] [Google Scholar]
- 10.He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen CS, et al. Effects of immediate blood pressure reduction on death and major disability in patients with acute ischemic stroke: the CATIS randomized clinical trial. JAMA. 2014;311(5):479–489. [DOI] [PubMed] [Google Scholar]
- 11.Bath PM, Woodhouse L, Scutt P, Krishnan K, Wardlaw JM, Bereczki D, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015;385(9968):617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M, Ovbiagele B, Hong KS, Wu YL, Lee JE, Rao NM, et al. Effect of blood pressure lowering in early ischemic stroke: meta-analysis. Stroke. 2015;46(7):1883–9. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA; IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33(5):1315–20. [DOI] [PubMed] [Google Scholar]
- 14.Ntaios G, Lambrou D, Michel P. Blood pressure change and outcome in acute ischemic stroke: the impact of baseline values, previous hypertensive disease and previous antihypertensive treatment. J Hypertens. 2011;29(8):1583–9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Reilly KH, Tong W, Xu T, Chen J, Bazzano LA, et al. Blood pressure and clinical outcome among patients with acute stroke in Inner Mongolia, China. J Hypertens. 2008;26(7):1446–52. [DOI] [PubMed] [Google Scholar]
- 16.Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. [DOI] [PubMed] [Google Scholar]
- 17.Grise EM, Adeoye O, Lindsell C, Alwell K, Moomaw C, Kissela B, et al. Emergency department adherence to American Heart Association guidelines for blood pressure management in acute ischemic stroke. Stroke. 2012;43(2):557–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyden P, Raman R, Liu L, Grotta J, Broderick J, Olson S, et al. NIHSS training and certification using a new digital video disk is reliable. Stroke. 2005;36(11):2446–2449. [DOI] [PubMed] [Google Scholar]
- 19.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. [DOI] [PubMed] [Google Scholar]
- 20.Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988;19(12):1497–1500. [DOI] [PubMed] [Google Scholar]
- 21.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Manual of Operations. Available at: https://ccct.sph.uth.tmc.edu/ALLHAToutreach/Documents/ALLHATmoo.pdf. Accessed June 2017.
- 22.Wilcoxin F Probability tables for individual comparisons by ranking methods. Biometrics. September 1947;3(3):119–122. [PubMed] [Google Scholar]
- 23.Armstrong BG, Sloan M. Ordinal regression models for epidemiologic data. Am J Epidemiol. 1989;129(1):191–204. [DOI] [PubMed] [Google Scholar]
- 24.Xu T, Zhang Y, Bu X, et al. Blood pressure reduction in acute ischemic stroke according to time to treatment: a subgroup analysis of the China Antihypertensive Trial in Acute Ischemic Stroke trial. J Hypertens. 2017;35(6):1244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bu X, Li C, Zhang Y, et al. Early Blood Pressure Reduction in Acute Ischemic Stroke with Various Severities: A Subgroup Analysis of the CATIS Trial. Cerebrovasc Dis. 2016;42(3-4):186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berge E, Cohen G, Lindley RI, Sandercock P, Wardlaw JM, Sandset EC, et al. Effects of blood pressure and blood pressure-lowering treatment during the first 24 hours among patients in the Third International Stroke Trial of Thrombolytic Treatment for Acute Ischemic Stroke. Stroke. 2015; 46:3362–9. [DOI] [PubMed] [Google Scholar]
- 27.Geeganage C, Tracy M, England T, Sare G, Moulin T, Woimant F, et al. Relationship between baseline blood pressure parameters (including mean pressure, pulse pressure, and variability) and early outcome after stroke: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST). Stroke. 2011;42(2):491–3. [DOI] [PubMed] [Google Scholar]
- 28.Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, et al. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014; 63:54–60. [DOI] [PubMed] [Google Scholar]
- 29.Okumura K, Ohya Y, Maehara A, et al. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens. 2005; 23:1217–23. [DOI] [PubMed] [Google Scholar]
- 30.McManus M, Liebeskind DS. Blood Pressure in Acute Ischemic Stroke. J Clin Neurol. 2016; 12(2):137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodhouse L, Scutt P, Krishnan K, et al. Effect of Hyperacute Administration (Within 6 Hours) of Transdermal Glyceryl Trinitrate, a Nitric Oxide Donor, on Outcome After Stroke: Subgroup Analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) Trial. Stroke. 2015;46(11):3194–201. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine-reporting of subgroup analyses in clinical trials. N Engl J Med 2007; 357(21):2189–94. [DOI] [PubMed] [Google Scholar]


