Abstract
OBJECTIVE:
We hypothesized that red blood cell (RBC) transfusions influence intestinal inflammation in very low birth weight (VLBW) infants. We also suspected that hematocrit (Hct) at transfusions and RBC storage time correlate with intestinal inflammation.
STUDY DESIGN:
VLBW infants, without major congenital defects, intestinal perforation or necrotizing enterocolitis, were enrolled prospectively. Fecal calprotectin (FC) levels were measured from stool samples collected before and after RBC transfusions. Data on Hct and RBC storage time were collected.
RESULT:
Data from 42 RBC transfusions given to 26 infants revealed that FC levels increased faster than baseline after RBC transfusions (P = 0.018) and were higher in multiple-transfused infants (0 to 48 and >48 h post transfusion, P = 0.007 and P = 0.005, respectively). Lower Hct and RBC storage >21 days correlated with higher FC levels (P = 0.044 and P = 0.013, respectively).
CONCLUSION:
RBC transfusions, anemia and prolonged RBC storage were associated with an increase in intestinal inflammation.
INTRODUCTION
Necrotizing enterocolitis (NEC) continues to be a devastating gastrointestinal complication of very low birth weight (VLBW) infants born before 33 weeks of gestation.1 The disease affects 4 to 11% of these infants, with mortality rates as high as 15 to 30%.2–4 The etiology of NEC is likely multifactorial, and it is affected by factors including but not limited to genetic predisposition,5–7 excessive inflammatory response, immature intestinal barrier and abnormal microbial flora.1,8 In recent years, several retrospective studies have reported that 25 to 40% of all cases of NEC may occur within 48 h of receiving a red blood cell (RBC) transfusion.9–23 The underlying mechanism(s) are unclear; plausible factors may include immaturity of mesenteric vascular autoregulation, microvascular sludging by stored RBCs, inflammatory factors accumulated in the transfused RBC units and immaturity of the host immune responses.24
Fecal calprotectin (FC) is derived mainly from activated neutrophils present in the intestinal mucosa and lumen, and it has been investigated as a biomarker of mucosal inflammation in diverse conditions such as NEC, an inflammatory bowel disease and intestinal ischemia–reperfusion.25–30 FC is a useful biomarker in preterm neonates for several reasons: stool samples can be easily collected even in the smallest neonates, calprotectin is stable at room temperature for up to 1 week and calprotectin can be measured easily using a robust, commercially available immunoassay.31 We hypothesized that RBC transfusions cause subclinical intestinal inflammation in VLBW infants. To investigate this hypothesis, we measured calprotectin levels in serially collected stool samples from premature infants receiving RBC transfusions, postulating that FC levels would increase after RBC transfusions. We also measured the relationship between FC levels and prenatal or postnatal clinical factors, storage time of transfused RBCs and the hematocrit (Hct) at the time of transfusion.
METHODS
This prospective observational study was conducted from April 2012 to November 2013 at the Level III Neonatal Intensive Care Unit at Tampa General Hospital/University of South Florida after approval by the Institutional Review Board. Infants with a birth weight of <1500 g or a gestational age of <33 weeks were eligible for the study. Exclusion criteria included lethal genetic disorders, major congenital anomalies, abdominal wall or gastrointestinal defects and intestinal perforation or NEC before enrollment. A written informed consent in the parents’ preferred language was obtained before initiation of sample collection. The decisions to transfuse were made by the medical teams following a standardized transfusion guideline. During the study period, we did not routinely withhold enteral feeds during transfusions.
Sample size
On the basis of existing literature on FC levels in VLBW infants, we used a s. d. of 189 μg g− 1 stool and expected a change in FC of 85 μg g − 1 stool to be biologically significant. To investigate the hypothesis that FC levels increase significantly after RBC transfusion, we estimated our sample size to be 39 RBC transfusions to achieve statistical power of 0.8 and α = 0.05.
Data collection
Maternal and infant characteristics were collected from electronic charts. Chorioamnionitis was identified based on placental histopathology reports. Gestational age was recorded as completed weeks of gestation based on the first trimester ultrasound or maternal last menstrual period. The classification of patent ductus arteriosus (PDA) was based on echocardiographic evaluation, and the largest ductal size was documented. Clinically significant PDAs were defined by the presence of persistent metabolic acidosis, O2 requirement >40% and need for assisted ventilation. Intraventricular hemorrhage was graded using Papile’s classification.32 The highest stage of retinopathy of prematurity during hospital stay was recorded. Corrected gestational age was calculated by adding the postnatal age in weeks to the gestational age at birth and recorded as completed weeks of gestation. Our unit provided donor breast milk for qualified infants (birth weight <1500 g), which was fortified with powdered cow’s milk-based human milk fortifier, as needed. Feeding intolerance was defined as abdominal distension, residuals of >50% of feeding volume, lack of radiographic findings suggestive of NEC and withholding of feeds for 48 to 72 h. We recorded NEC stage IIb or greater based on the modified Bell’s staging criteria.33 For each transfusion, the duration of RBC storage (days) was provided by the blood bank. All transfused RBCs were cytomegalovirus negative and irradiated.
Stool sample collection
We collected serial stool samples before and after RBC transfusions so that FC levels could be compared in each infant before and after RBC transfusion. After an infant was enrolled, the nursing staff saved one stool diaper during each 12-h shift in a closed box at the patient’s bedside. During the weeks when the infant did not receive an RBC transfusion, one stool sample was saved per week and stored for analysis. When an RBC transfusion order was placed, stool diapers were collected more frequently, including the last stool before transfusion, the first stool after transfusion and one stool every 24 h for up to 3 days after the transfusion. Stool samples were collected in 2-ml plastic cryovials and kept at room temperature for no more than 5 days. Samples were then frozen at − 80 °C until analysis. Stool collection was stopped at 37 weeks corrected gestational age or at the time of discharge, whichever came first.
Fecal calprotectin
FC levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA; PhiCal/Genova Diagnostics, Ashville, NC, USA). This ELISA kit is FDA-approved for clinical laboratory usage and has a linear range of measurements from 25 to 2500 μg per g stool. At the time of analysis, samples were thawed to room temperature and homogenized in the extraction buffer. FC levels were measured in duplicate according to the manufacturer’s protocol.
Statistical analysis
Demographic and clinical characteristics are expressed as percentages and as means ± s.d. (SPSS version 22; SPSS IBM, New York, NY, USA). To analyze longitudinal changes in FC, panel plots with Loess curves were produced. A generalized linear mixed model with radial smoothing was used to fit the longitudinal data by using SAS PROC GLIMMIX version 13.1 (SAS Institute, Cary, NC, USA). An indicator variable was created to represent the before and after transfusion period for each infant. An interaction between this indicator and time was tested for the rate of change of FC values. To analyze temporal changes in FC levels from 0 to >48 h and 448 h before and after RBC transfusions, natural log transformation was applied to approximate the normal distribution, and a fixed-effects model was used to control for measured and unmeasured confounders. To estimate the effects of RBC storage time and Hct on the trajectory of posttransfusion FC levels, we fitted a linear mixed-effects model. The log-transformed FC levels were regressed on RBC storage time (⩽21 days vs >21 days) and Hct at the time of transfusion. Clustering of observations within patients over time was modeled with a random intercept for each patient. Regressions were performed using R 3.0.1 software with nlme package (R Foundation for Statistical Computing, Vienna, Austria). P-values <0.05 were considered statistically significant.
RESULTS
Patient population
Of the 167 infants enrolled, 47 (28%) infants were transfused. We analyzed stool samples associated with the first 42 RBC transfusions as we determined a priori from our sample size calculation. These transfusions were from a total of 26 infants. Of these 42 transfusions, 19 represented first transfusions, whereas 23 were repeat transfusions given to previously transfused infants. The postnatal age at the time of enrollment ranged from 0 to 21 days of life; 21 of 26 infants (81%) were enrolled within the first week of life and all infants were enrolled before the first transfusion.
Infants and maternal characteristics
The clinical and demographic characteristics of the studied infants are given in Table 1. The gestational age of infants was 27.6 ± 2.6 (mean ± s.d.) weeks, and the birth weight was 1170 ± 407 g. For 3 of the 26 infants, the placental pathology to diagnose chorioamnionitis was not performed. Thirty-eight percent had a moderate or large PDA and one infant was treated with indomethacin; none underwent PDA ligation; 12% had evidence of intraventricular hemorrhage on cranial ultrasounds and one received a ventriculoperitoneal shunt; and 23% had retinopathy of prematurity but did not require intervention. The incidences of PDA, intraventricular hemorrhage and retinopathy of prematurity were similar to those typically seen in this unit.
Table 1.
Maternal and infant characteristics
| Characteristic | (n = 26) |
|---|---|
| Maternal age (years) | 25.6 ± 0.7 |
| Maternal race | |
| White | 12 (46%) |
| Black | 8 (31%) |
| Others | 6 (23%) |
| Maternal hypertension | 6 (23%) |
| Maternal diabetes | 3 (12%) |
| Histologic chorioamnionitis | 13 (50%) |
| Antenatal antibiotics | 18 (69%) |
| Antenatal steroids | 23 (88%) |
| Smoking during pregnancy | 4 (15%) |
| Gestational age (weeks) | 27.6 ± 2.6 |
| Birth weight (g) | 1170 ± 407 |
| Male | 13 (50%) |
| Vaginal delivery | 12 (46%) |
| Patent ductus arteriosusa | |
| None | 5 (19%) |
| Small | 2 (8%) |
| Moderate/large with clinical significance | 10 (38%) |
| Intraventricular hemorrhage | |
| None | 22 (85%) |
| Grades 1 and 2 | 3 (11%) |
| Grades 3 and 4 | 1 (4%) |
| Retinopathy of prematurity | |
| None | 20 (77%) |
| Stages 1 and 2 | 6 (23%) |
| Stage >2 | 0 |
Values were not total 100% because not all infants were evaluated for the condition. Data reported as n(%) or mean ± s.d.
Clinical characteristics at the time of RBC transfusions
The mean postnatal age and corrected gestational age at the time of RBC transfusions were 27 ± 16 days and 31 ± 3 weeks, respectively (Table 2). Most transfusions (83%) were administered while infants were being fed human milk or fortified human milk. For 25 (60%) of the transfusions, infants were given antibiotics for late-onset sepsis evaluation within 48 h of the transfusions, and 8 (19%) of those had a positive blood culture. Two infants (4.8%) had NEC (⩾Bell stage IIb). Four (9.5%) transfusions were administered in infants whose enteral feeds were withheld because of feeding intolerance or indomethacin.
Table 2.
Infant’s clinical characteristics within 48 h of PRBC transfusions
| Characteristic | Number of transfusions (n = 42) |
|---|---|
| Diet | |
| Human milk/human milk+fortifier | 35 (83%) |
| Formula only | 6 (14%) |
| Human milk and formula | 1 (2%) |
| Antibiotics within 48 h | 25 (60%) |
| Bacteremia within 48 h | 8 (19%) |
| Hematocrit before tranfusion (%) | 28 ± 4 |
| Feeding intolerance | 4 (9.5%) |
| Necrotizing enterocolitis stage IIb or greater | 2 (4.8%) |
| Age (days) | 27 ± 16 |
| Corrected gestational age (weeks) | 31 ± 3 |
Abbreviation: PRBC, packed red blood cells. Data are reported in n(%) or as mean ± s.d.
Changes in FC levels in relation to RBC transfusions
The linear mixed model with radial smoothing showed a significant increase in FC over time (B = 3.6062, P = 0.0021), and the rate of change was higher after RBC transfusions (slope before transfusion = 74.78 vs slope after transfusion = 77.69, P = 0.0176). Using fixed-effects regression, we compared FC levels (natural log-transformed to approximate the normal distribution) before and after transfusion. Comparing 0 to 48 h before vs 0 to 48 h after transfusion, we observed a significant association between FC levels and the number of prior transfusions (Coefficient = 1.67, P = 0.007). A similar association between FC and the number of prior transfusions was observed in >48 h before vs >48 h after transfusion (Coefficient = 1.27, P = 0.005).
Association between FC, Hct at transfusion, RBC storage and clinical characteristics
For 3 of the 42 transfusions administered, the storage duration of the transfused RBCs could not be confirmed by the blood bank. For the remaining 39 transfusions, RBC storage duration >21 days Was associated with higher logFC (B = 0.428, s.e. = 0.170, t(95) = 2.52, P = 0.013; Figure 1). A lower Hct at the time of transfusion was associated with higher logFC levels (B = −0.0496, s.e.=0.024, t(95)=2.04, P=0.044) even when controlled for the effect of RBC storage time >21 days using a dichotomous covariate method (B=0.53, s.e.=0.17, t(95)=2.52, P=.013; Figure 2). We did not find a significant difference in FC levels in relation to storage time of 1 week or 2 weeks, or a significant (linear) association when treating storage age as a continuous variable. Only two infants developed NEC. Infant 1 was a 32-week 2.25-kg boy with prenatal diagnosis of obstructive uropathy, who was mechanically ventilated for 10 days plus 30 more days of nasal continuous positive airway pressure. Formula feedings were initiated on day 1 with transition to breast milk fortified with bovine-based fortifier starting on day 3 and reached full feed by day 10. He was diagnosed with NEC presenting with feeding intolerance, abdominal distension and focal pneumatosis on abdominal film on day 17 with full recovery after 10 days of Piperacilin/tasobactam and bowel rest. The transfusion was given at the time of NEC diagnosis. His peak pretransfusion and posttransfusion FC levels were 797 and 362 μg per g stool, respectively. Infant 2 was a 26-week 0.95-kg male twin with mild surfactant deficiency treated with continuous positive airway pressure, who was started on formula feeding on day 2, transitioned to breast milk and bovine-based fortifier starting on day 4 and reached full feeds on day 13. He was treated with vancomycin for 7 days for coagulase-negative staphylococcal bacteremia on days 8 to 15 and received the first RBC transfusion on day 8 for apnea and tachycardia. On day 18, he developed abdominal distension with diffuse pneumatosis on abdominal film. He underwent laparotomy and bowel resection on day 20 after presenting with radiographic evidence of free air. He received second RBC transfusion on day 18, 6 h before the onset of abdominal symptoms. His immediate pretransfusion and posttransfusion FC levels were 68 and 166 μg per g stool, respectively. We did not find a correlation between FC levels and persistent ductal patency, antibiotic use, intrauterine growth restriction, histologic chorioamnionitis, type of feeds, nil per os status or bacteremia.
Figure 1.
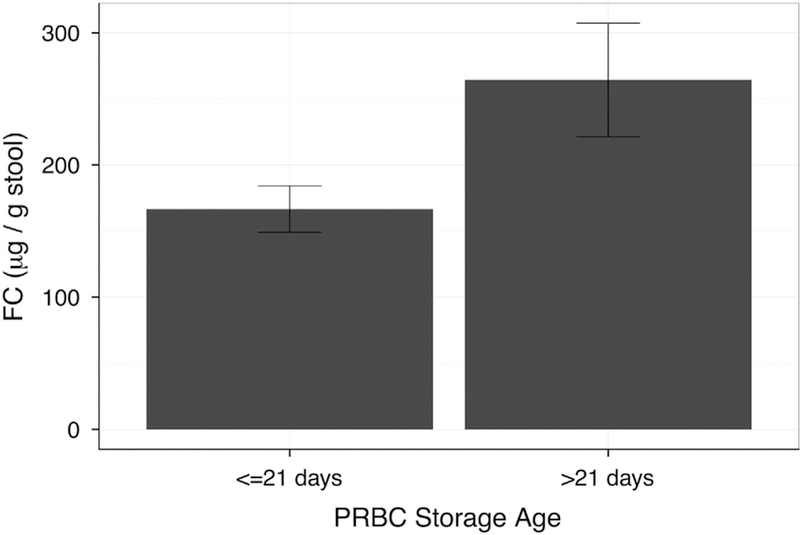
Difference in posttransfusion fecal calprotectin (FC) levels (mean ± 95% confidence interval) based on packed red blood cell (PRBC) storage age. Infants were transfused with RBCs ⩽21 days of age (n = 20) or >21 days of age (n = 19). A linear mixed-effect model of FC levels regressed on RBC storage age and controlled for hematocrit at transfusion, confirmed a significant difference in the means (B = 0.428, s.e. = 0.170, t(95) = 2.52, P = 0.013).
Figure 2.
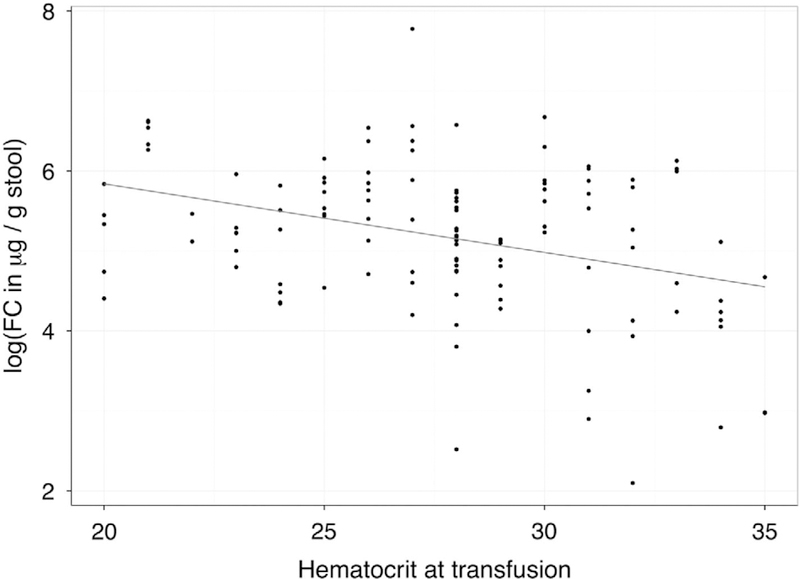
The correlation between post-transfusion fecal calprotec-tin (FC) levels and hematocrit at the time of transfusion. The line represents the fit of a linear model. A linear mixed-effect model of log-transformed FC levels regressed on hematocrit at the time of transfusion controlled for red blood cell storage time >21 days or not confirmed a significant negative relationship (B = 0.53, s. e. = 0.17, t(95) = 2.52, P = 0.013).
DISCUSSION
We found a significant association between RBC transfusions and FC levels in VLBW infants. FC levels were higher in infants with lower pretransfusion Hct, in infants who received transfusions with RBCs stored for >21 days and in infants who received more than one RBC transfusion. The rate of increase in FC levels was also higher after transfusions, indicating that RBC transfusions caused a subclinical inflammatory response in the intestinal tract. These findings are of importance as a plausible, potential mechanistic basis for the association between RBC transfusions and NEC.9–23,34 In this pathophysiological model, RBC transfusions induce a mucosal inflammatory response that remains subclinical in most recipients, but in susceptible infants an exaggerated mucosal reaction could become clinically evident as NEC.
FC is a promising biomarker of gut mucosal inflammation.25–30 In the inflamed intestine, circulating neutrophils are actively recruited to the mucosa, and some of these migrate into the intestinal lumen. Although several neutrophil-derived biomarkers have been examined in feces, calprotectin is the most widely studied.35 Calprotectin is released mainly by activated or damaged neutrophils, although small amounts are also expressed by activated macrophages and monocytes.35 Following RBC transfusions, neutrophil activation is a well-recognized phenomenon and has been noted in a variety of settings, including in transfusionassociated acute lung injury, surgical patients, cancer and trauma.36 Transfused RBC units are known to contain several neutrophil priming agents and mechanisms, including inflammatory cytokines, vesicular microparticles, membrane fragments, bioactive lipids such as lysophosphatidylcholines and antineutrophil antigen antibodies.36
In our study, RBC transfusions triggered the greatest rise in FC levels in infants with the lowest pretransfusion Hct. These findings are consistent with the findings of Singh et al.,17 who previously showed that lower Hct at the time of transfusions was associated with increased odds of NEC (odds ratio 1.10, P = 0.01) after controlling for other factors. They also showed that RBC transfusions had a temporal relationship with onset of NEC, in which a transfusion within the preceding 24 h (odds ratio 7.60, P = 0.001) and 48 h (odds ratio 5.55, P = 0.001) was associated with increased odds of developing NEC. Studies in animal models demonstrate that anemia can impair mesenteric perfusion and increase oxygen extraction, leading to anaerobic metabolism and accumulation of its by-products such as lactic acid.37 Anemia can also alter the normal change in intestinal vascular resistance during the transition from fetal to neonatal life, predisposing the developing intestine to hypoxemic–ischemic gut mucosal injury, and possibly NEC.38
We also detected an association between FC levels and the storage age of RBCs. These finding are significant because blood banks often dedicate single RBC units for sequential transfusions in a single infant. Although this practice may reduce alloimmunization and infections, it may also result in transfusions with RBCs that have been stored for longer periods. Stored RBCs are less deformable, are ‘sticky’ and lose nitric oxide, which can result in decreased O2 delivery, vasoconstriction and ischemia.39 Stored RBCs also contain microparticles, heme and cytokines (IL-1β, IL-6 and IL-8), which can activate neutrophils and cause inflammation.40 All of these mechanisms are plausible explanations for the influx of neutrophils into the intestine in response to RBC transfusions. Although RBC storage has not been associated with NEC in retrospective clinical studies published to date,24 there is renewed interest in this question because several studies now show that the storage age of transfused RBCs correlates with adverse outcomes in critically ill adults.
In agreement with previous studies, we found that FC levels correlated positively with postnatal age.41,42 This finding suggests that the microbial colonization of the gut influences FC levels in premature infants. Future studies may correlate intestinal inflammatory responses with differences in microbial colonization.
Our study is limited to the fact that a small number of infants had multiple RBC transfusions. This may act as a confounding factor, as our analysis demonstrates that RBC transfusions may alter the infant’s baseline FC levels. In the study unit, RBC transfusions were not administered routinely, but most commonly in the setting of symptomatic anemia. Hence, 60% of the transfusions were given to infants with nonspecific systemic symptoms that led to work-up for late-onset infection including antibiotic administration. In all, 19% of those work-ups resulted in a positive blood culture, and there were two cases of NEC stage IIb or greater. Interestingly, Josefsson et al.42 showed no correlation between C-reactive protein, a systemic inflammatory marker and FC levels in infants without severe abdominal symptoms; their study also showed lower FC values in infants treated with antibiotics. It remained unclear how other inflammatory processes and antibiotic administration influenced the changes in FC levels in our study.
This study was also limited by the small number of infants with NEC, which limits our ability to draw conclusions regarding a causal relationship between RBC transfusions and NEC. Our observational study was not designed to investigate the clinical significance of increased FC levels in association with RBC transfusions, which would require a larger cohort. We cannot yet caution against restrictive transfusion practices or recommend the use of RBCs <21 days old to prevent intestinal inflammation and/ or NEC in VLBW infants. Further study is needed to validate these data in a larger cohort with a larger proportion of infants with NEC. However, this study is the first to provide a surrogate measure of the mucosal inflammatory response immediately after RBC transfusions in preterm infants. The availability of serial stool samples is also a major strength, which allowed us to compare pretransfusion and post-transfusion FC values for each individual infant and thus minimize confounding factors.
In summary, we report significant changes in FC levels after RBC transfusions: FC levels were higher in infants who received more than one transfusion, and the rate of increase in FC levels was higher after transfusions. We also found significant associations between levels of FC and lower pretransfusion Hct, and between FC levels and RBC storage time >21 days. The combination of FC levels and other biomarkers of gut well-being such as peripheral monocyte counts, plasma and urinary biomarkers of inflammation, or measurements of intestinal blood flow and perfusion may help further elucidate the relationship between packed RBC transfusion and intestinal injury.43
ACKNOWLEDGEMENTS
We thank the research nurses, Judy Zaritt and Shari Roberts, for their valuable contribution in the identification of eligible infants, collection of clinical information and collection of stool samples; Tampa General Hospital NICU nurses for saving the stool samples; laboratory technicians, Bradley Kane and Shaunte Williams, for their assistance with processing of samples and with ELISA; Dr Emmanuel for her encouragement and enduring support; and Dr Jane Carver for patiently editing the grant proposal and manuscript. The study was funded by the Gerber Foundation Novice Research Award (to TTBH), Fremont, MI, USA, and by the National Institution of Health (NIH) award R01HD059142 (to AM).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011; 364: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003; 23: 278–285. [DOI] [PubMed] [Google Scholar]
- 3.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol 2006; 20: 498–506. [DOI] [PubMed] [Google Scholar]
- 4.Llanos AR, Moss ME, Pinzon MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol 2002; 16: 342–349. [DOI] [PubMed] [Google Scholar]
- 5.Radulescu A, Yu X, Orvets ND, Chen Y, Zhang HY, Besner GE. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases sus-ceptibility to necrotizing enterocolitis. J Pediatr Surg 2010; 45: 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekman EF, Boelen CC, Prinsen BH, Ijlst L, Duran M, de Koning TJ et al. Necro-tizing enterocolitis and respiratory distress syndrome as first clinical presentation of mitochondrial trifunctional protein deficiency. JIMD Rep 2013; 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz S, Wong RJ, Jang KY, Kalish F, Chisholm KM, Zhao H et al. Heme oxygenase-1 deficiency promotes the development of necrotizing enterocolitis-like intestinal injury in a newborn mouse model. Am J Physiol Gastrointest Liver Physiol 2013; 304: G991–G1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maheshwari A, Corbin LL, Schelonka RL. Neonatal necrotizing enterocolitis. Res Rep Neonatol 2011; 1: 39–53. [Google Scholar]
- 9.Mally P, Golombek SG, Mishra R, Nigam S, Mohandas K, Depalhma H et al. Asso-ciation of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol 2006; 23: 451–458. [DOI] [PubMed] [Google Scholar]
- 10.Perciaccante JV, Young TE. Necrotizing enterocolitis associated with packed red blood cell transfusions in premature neonates. E-PAS 2008. 5839.8.
- 11.Holder GL, Dohert DA, Patole SK. Elective red cell transfusion for anemia of pre-maturity and development of necrotizing enterocolitis in previously well preterm neonates: incidence and difficulties in proving a cause-effect association. J Neo-natal Perinatal Med 2009; 2: 181–186. [Google Scholar]
- 12.Christensen RD, Lambert DK, Henry E, Wiedmeier SE, Snow GL, Baer VL et al. Is ‘transfusion-associated necrotizing enterocolitis’ an authentic pathogenic entity? Transfusion 2010; 50: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 13.Josephson CD, Wesolowski A, Bao G, Sola-Visner MC, Dudell G, Castillejo MI et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? J Pediatr 2010; 157: 972–978 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter BM, Holditch-Davis D, Tanaka D, Schwartz TA. Relationship of neonatal treatments with the development of necrotizing enterocolitis in preterm infants. Nurs Res 2012; 61: 96–102. [DOI] [PubMed] [Google Scholar]
- 15.Couselo M, Aguar M, Ibanez V, Mangas L, Garcia-Sala C. [Relation between packed red blood cell transfusion and severity of necrotizing enterocolitis in premature infants]. Cir Pediatr 2011; 24: 137–141. [PubMed] [Google Scholar]
- 16.Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics 2011; 127: 635–641. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Visintainer PF, Frantz ID 3rd, Shah BL, Meyer KM, Favila SA et al. Asso-ciation of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol 2011; 31: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol 2011; 31: 183–187. [DOI] [PubMed] [Google Scholar]
- 19.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr 2011; 158: 403–409. [DOI] [PubMed] [Google Scholar]
- 20.Ghirardello S, Lonati CA, Dusi E, Pugni L, Mosca F. Necrotizing enterocolitis and red blood cell transfusion. J Pediatr 2011; 159: 354–355 author reply 355–356. [DOI] [PubMed] [Google Scholar]
- 21.Stritzke AI, Smyth J, Synnes A, Lee SK, Shah PS. Transfusion-associated necrotising enterocolitis in neonates. Arch Dis Child Fetal Neonatal Ed 2013; 98: F10–F14. [DOI] [PubMed] [Google Scholar]
- 22.Wan-Huen P, Bateman D, Shapiro DM, Parravicini E. Packed red blood cell transfusion is an independent risk factor for necrotizing enterocolitis in premature infants. J Perinatol 2013; 33: 786–790. [DOI] [PubMed] [Google Scholar]
- 23.Elabiad MT, Harsono M, Talati AJ, Dhanireddy R. Effect of birth weight on the association between necrotising enterocolitis and red blood cell transfusions in ≤ 1500 g infants. BMJ Open 2013; 3: e003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amin SC, Remon JI, Subbarao GC, Maheshwari A. Association between red cell transfusions and necrotizing enterocolitis. J Matern Fetal Neonatal Med 2012; 25: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Inca R, Dal Pont E, Di Leo V, Ferronato A, Fries W, Vettorato MG et al. Calpro-tectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis 2007; 22: 429–437. [DOI] [PubMed] [Google Scholar]
- 26.Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnorm-ality. Lancet 2000; 356: 1783–1784. [DOI] [PubMed] [Google Scholar]
- 27.Aydemir O, Aydemir C, Sarikabadayi YU, Emre Canpolat F, Erdeve O, Biyikli Z et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med 2012; 25: 2237–2241. [DOI] [PubMed] [Google Scholar]
- 28.Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet 2003; 361: 310–311. [DOI] [PubMed] [Google Scholar]
- 29.Thuijls G, Derikx JPM, van Wijck K, Zimmermann LJI, Degraeuwe PL, Mulder TL et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg 2010; 251: 1174. [DOI] [PubMed] [Google Scholar]
- 30.Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology 2008; 94: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapel N, Campeotto F, Kalach N, Baldassare M, Butel MJ, Dupont C. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr 2010; 51: 542. [DOI] [PubMed] [Google Scholar]
- 32.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978; 92: 529–534. [DOI] [PubMed] [Google Scholar]
- 33.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987; 17: 213–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics 2012; 129: 529–540. [DOI] [PubMed] [Google Scholar]
- 35.Leach ST, Yang Z, Messina I, Song C, Geczy CL, Cunningham AM et al. Serum and mucosal S100 proteins, calprotectin (S100A8/S100A9) and S100A12, are elevated at diagnosis in children with inflammatory bowel disease. Scand J Gastroenterol 2007; 42: 1321–1331. [DOI] [PubMed] [Google Scholar]
- 36.Neal MD, Raval JS, Triulzi DJ, Simmons RL. Innate immune activation after transfusion of stored red blood cells. Transfus Med Rev 2013; 27: 113–118. [DOI] [PubMed] [Google Scholar]
- 37.Szabo JS, Mayfield SR, Oh W, Stonestreet BS. Postprandial gastrointestinal blood flow and oxygen consumption: effects of hypoxemia in neonatal piglets. Pediatr Res 1987; 21: 93–98. [DOI] [PubMed] [Google Scholar]
- 38.Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol 2002; 29: 23–39. [DOI] [PubMed] [Google Scholar]
- 39.Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfus Sci 1997; 18: 447–458. [DOI] [PubMed] [Google Scholar]
- 40.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiol Scand 1996; 40: 496–501. [DOI] [PubMed] [Google Scholar]
- 41.Baldassarre ME, Altomare MA, Fanelli M, Carbone D, Di Bitonto G, Mautone A et al. Does calprotectin represent a regulatory factor in host defense or a drug target in inflammatory disease? Endocr Metab Immune Disord Drug Targets 2007; 7: 1–5. [DOI] [PubMed] [Google Scholar]
- 42.Josefsson S, Bunn SK, Domellof M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr 2007; 44: 407–413. [DOI] [PubMed] [Google Scholar]
- 43.Remon J, Kampanatkosol R, Kaul RR, Muraskas JK, Christensen RD, Maheshwari A. Acute drop in blood monocyte count differentiates NEC from other causes of feeding intolerance. J Perinatol 2014; 34: 549–554 [DOI] [PMC free article] [PubMed] [Google Scholar]


