Abstract
We investigated the neural processes, with a focus on subcortical circuits, which govern corrective submovements in visually targeted action. During event-related fMRI, subjects moved a cursor to capture targets presented at varying movement amplitudes. Movements were performed in a rehearsed null and a novel viscous (25% random trials) torque field. Movement error feedback was provided after each trial. The viscous field invoked a significantly larger error at the end of the primary movement. Subjects compensated by producing more corrections than they had in the null condition. Corrective submovements were appropriately scaled such that terminal error was similar between the two conditions. Parametric analysis identified two regions where the BOLD signal correlated with the number of submovements per trial: a cerebellar region similar to the one noted in the task contrast and the contralateral dorsal putamen. A separate parametric analysis identified brain regions where activity correlated with movement amplitude. This identified the same cerebellar region as above, bilateral parietal cortex, and left motor and premotor cortex. Our data indicate that the basal ganglia and cerebellum play complementary roles in regulating ongoing actions when precise updating is required. The basal ganglia have a key role in contextually-based motor decision-making, i.e. for deciding if and when to correct a given movement by initiating corrective submovements, and the cerebellum is more generally involved in amplifying and refining the command signals for movements of different amplitudes.
Keywords: Online adaptation, Motor control, fMRI
Introduction
A motor plan can be prone to imperfections due to poor calibration, neural noise, or subsequent changes in goal. Therefore, the use of online movement corrections to guide and update actions becomes critical. Indeed, the existence of corrective submovements has been well established for over a century (Crossman and Goodeve, 1983; Flash and Henis, 1990; Guigon et al., 2007; Meyer et al., 1988; Schmidt et al., 1979; Woodworth, 1899). Recent models of corrective submovements suggest that they are implemented discretely as the need to reconcile significant errors arises, particularly for high-precision tasks (Barringer et al., 2008; Fishbach et al., 2007; Milner, 1992). While investigations of neural mechanisms involved in online error-detection and recalibration of visuomotor tracking, reaching and grasping have identified a distributed frontal, parietal, basal ganglia, and cerebellar network (Desmurget et al., 2001; Ghilardi et al., 2000; Imamizu et al., 2000; Inoue et al., 1997, 2000; Martin et al., 1996; Diedrichsen et al., 2005; Floyer-Lea and Matthews, 2004; Graydon et al., 2005; Krakauer et al., 2004; Miall and Jenkinson, 2005; Smith and Shadmehr, 2005; Tunik et al., 2007b), the neural circuits governing discrete submovement corrections remain poorly understood. Understanding the neural circuitry underlying corrective submovements is critical not only for elucidating neural mechanisms of motor control but for understanding neural disorders of movement as well.
The current project is the first in a series of investigations by our group aimed at understanding the neural correlates of corrective submovements in human and non-human primates. Here we investigate this using an event-related fMRI design in which people were asked to capture targets by turning a rotary dial that controlled a cursor on a display. The dial was attached to a torque motor which was programmed to deliver either a null or a positive viscous torque perturbation (25% of randomly interleaved trials). Neural circuits mediating movement in both conditions had access to the efference copy of the motor plan (formed from past experience) as well as online proprioceptive feedback. Since the null condition was highly rehearsed, the majority of the variance was ascribed to the motor plan (i.e. formed on the basis of experience), and therefore constituted a feedforward mode of control. Movements in the viscous condition had to also deal with the unexpected variance arising from the perturbation, requiring control to rely more on online information than on past experience alone, and therefore constituted feedback-based control. These conditions, therefore, allowed us to contrast different mechanisms by which discrete corrective submovements could be formed.
We hypothesized that regions where the BOLD signal significantly correlated with the number of submovements may reflect processes related to their generation. Moreover, regions showing significantly stronger correlation to submovements in the viscous than the null condition should be associated with feedback-based corrections more so than processes related to planning or guiding rehearsed actions. Finally, to rule out non-specific effects related to movement amplitude, we varied the overall target distance from trial to trial and correlated the movement extent with the BOLD signal. We predicted that the regions selectively associated with the initiation of corrective submovements should be insensitive to effects of movement amplitude.
We were particularly interested in understanding the role that the basal ganglia may play in implementing discrete corrective submovements. Dysfunction of the basal ganglia leads to systematic impairments on tasks requiring online movement corrections for motor errors (Smith et al., 2000; Tunik et al., 2004a,b) and goal errors (Desmurget et al., 2004a). A parsimonious explanation suggested by Houk et al. (2007) is that the basal ganglia may be critical in making decisions about if and when movement corrections are necessary and that dysfunction of the basal ganglia may impair this very process. This theory fits well with empirical findings that a large portion of pallidal neurons change discharge rate only after a movement has been initiated (Mink and Thach, 1991b) and in many cases the change in discharge is time-locked to the onset of corrective submovements (Roy et al., 2003, 2008). This study, therefore, tested the prediction that the basal ganglia play a decisive role in corrective submovements in humans. Part of this study is published in abstract form (Tunik et al., 2008).
Materials and methods
Subjects
Eighteen right-handed (Oldfield, 1971) individuals (21.8± 2.6 years old; 9 males) with no history of neurological impairment participated after signing informed institutional consent.
Setup and procedure
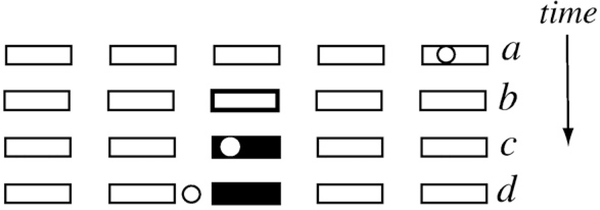
Participants viewed a display showing five rectangular targets equidistant from each other and horizontally aligned (Fig. 1). Subjects rotated a dial with their right hand to control the horizontal motion of a cursor (1° knob rotation=0.5 cm cursor translation). The dial was connected to a torque motor and optical encoder (Model# SM233BEN16N, Parker Automation). To start a trial, subjects aligned the cursor within a rectangle whose border was highlighted (start position). After 1 s, two simultaneous events served as the go cue: 1) another rectangle’s border was highlighted (target), and 2) the cursor vanished. Subjects were required to rapidly and accurately move the invisible cursor from the start rectangle and place it in the target rectangle within 1.5 s of the go cue and to maintain it at what the subject thought should be the correct position for at least 0.4 s. Knowledge of results was provided by making the cursor visible again when its position remained unchanged for more than 0.4 s. Targets were presented in an unpredictable order, counterbalanced across four possible movement amplitudes and two directions.
Fig. 1.
Subjects viewed a display with 5 rectangular targets (schematically drawn in each row of the figure) and controlled a round cursor (open circle). On each trial, subjects first aligned the cursor to a starting target (epoch a) and were then given a ‘go’ cue indicated by the disappearance of the cursor and simultaneous illumination of a target (epoch b). Knowledge of results was provided after each trial for accurate (epoch c) and inaccurate (epoch d) movements.
Each subject was brought in on the day prior to the imaging session and familiarized on a null perturbation (motor disabled) for 60 trials per block×6 blocks (360 trials). For the training session, subjects lay supine on a plinth with the monitor placed between their legs to simulate the same body position and joint movements as would be assumed the following day in the scanner. The following day, immediately prior to the first functional run in the scanner, subjects repeated 60 trials with the null torque field to refamiliarize with the task.
For the MRI portion, the motor apparatus was secured against a wall, 10 ft from the subject’s hand. The dial was connected to the torque motor via a 3.05 m (10 ft) delrin rod. The moment of inertia of the rod was 1.6×10−3 kg m2 (weight: 2 kg; diameter: 4 cm) and that of the motor rotor was 9.3×10−5 kg m2. Also, the rod was supported at each distal end by an Accrolon 9000 series non-metallic self-lubricating sleeve bearing (Accro-Seal, Inc.) and at its middle by a custom-designed plastic ball-bearing. Thus any resistance and friction was negligible.
During scanning, subjects performed 60 trials per block×4 blocks (functional runs) of randomly interleaved null (75% of trials) and velocity-dependent (viscous, 25% of trials) torque field trials. The perturbation was a positive viscous torque proportional to the subjects’ velocity (−0.5 oz-in/°s−1 [0.004 Nm/°s−1]). The intertrial interval was varied randomly between 2–7 s within each functional run in order to jitter the slice acquisition for each event, though the total elapsed inter-trial time remained constant across functional runs. Because the viscous perturbation occurred on a minority of randomly selected trials, it could not be anticipated and was therefore exclusively reliant upon online updating mechanisms.
Magnetic resonance imaging (MRI) — acquisition and analysis
Imaging was performed with a 3 T Phillips MRI scanner using an 8-channel phased array head coil. For each functional run, an echo planar gradient echo imaging sequence sensitive to blood oxygenation-level-dependent contrast was used to acquire 30 slices per TR (4 mm thickness, 0.5 mm gap), with a TR of 1976 ms, TE of 35 ms, flip angle of 90°, field of view (FOV) of 240 mm, and 80×80 matrix. Two hundred whole brain images were collected in each functional run. Afterwards, a high-resolution T1 weighted image of the whole brain was acquired using a spoiled gradient recalled 3D sequence (TR=9.9 ms; TE=4.6 ms; flip angle=8°, FOV=240 mm; slice thickness=1 mm, matrix=256×256). The functional imaging data was preprocessed and analyzed with Statistical Parametric Mapping (SPM2; Wellcome Department of Cognitive Neurology, London, UK). The first two brain images of each functional run were discarded and the following images collected and stored. Raw data for each participant was realigned, unwarped and normalized to the MNI template (Talairach and Tournoux, 1988) with a resolution of 2×2×2 mm, and smoothed using a 6 mm Gaussian kernel.
All trials were included in the analyses. Condition-specific differences in the BOLD signal were analyzed with a general linear model approach for event-related fMRI. A design matrix was created for each subject with vectors for the null and viscous conditions to identify the main effects of the two tasks. Additionally, we included separate vectors for the number of submovements per trial for the viscous and null conditions (center mean normalized). This made it possible to correlate the mean number of submovements (sm) with the corresponding magnitude of the BOLD response on a trial-by-trial basis independent of movement related activity. Finally, run-to-run regressors were included in the design matrix to account for any nonspecific run-to-run effects. The onset and duration of each event, obtained from time stamps recorded during the experiment, were entered into the model and convolved with the canonical hemodynamic response function. First, we created a task (viscous+null) versus rest contrast. Regions significantly activated in this contrast were used as an inclusive mask for all subsequent analyses of the fMRI data. Since activation in this contrast was not exclusively based on activation in a given condition or correlation with a given parameter, this mask served as an unbiased region of interest. Contrasts were estimated for: 1) null and viscous>rest to identify task-related sensorimotor circuits; 2) movement in the viscous>null to identify condition-related differences attributable to online error-detection and correction specific to a viscous field; 3) since our main argument was based on an a priori hypothesis of a relation between submovements and activation in the basal ganglia we modeled the number of corrective submovements (sm) in a given trial as a parametric modulator for BOLD signal in the corresponding trials of the viscous and null conditions. The contrast viscous sm>null sm identified neural circuits that scaled more strongly when generating corrective submovements (subtracting out activation related to visual, motor, and other non-specific stimuli); 4) as an alternative account for our findings, we performed a secondary analysis in which we modeled another kinematic variable, movement amplitude, as a parametric modulator. This too was a parameter of interest and helped us to understand whether regions correlating with submovements were also correlated with other kinematics, such as movement amplitude. However, since we found that the number of submovements was modestly correlated with movement amplitude (see Results), the statistical parametric map for amplitude and submovement were estimated using separate general linear models. Modeling the kinematic parameters using this two-stage estimation procedure avoided inaccurate beta estimations that would otherwise occur if we had included collinear kinematic parameters into a single design. Contrast images for each subject were passed on for random effects analysis at the group level. Our analyses were carried out within an a priori defined and unbiased ROI which dramatically reduced the number of multiple comparisons. Rather than imposing additional corrections to the threshold, which would considerably increase the risk of type II error, statistical significance in the imaging data was based on a conservative but uncorrected alpha of p<0.001 and an extent of 10 voxels.
Device shielding
Radiofrequency and electromagnetic interference between the electric motor and the scanner was minimized by several means (Chinzei et al., 1999). 1) The motor was housed in specially constructed copper and Mu metal nesting boxes (Magnetic Shield Corp., IL). 2) The nesting box housing the torque motor was placed as far as possible away from isocenter, within the 1–3 G range (zone 4 according to Chinzei et al., 1999). 3) The computer (NI-PXI 8176), digital servoamplifier (Accelus ASP-180–18, Copley Controls Corp.), and power supply (PST-070–08-DP-E, Copley Controls Corp.) were placed outside the scanner suite (in the technician room). 4) All wires connecting the controllers to the motor were twisted pair cables and triply shielded using the wires’ own shielding as well as copper mesh and Mu metal hoses. 5) All shielding materials were earth-grounded. We have previously verified the electromagnetic shielding of this device and the absence of noise in functional images when it is in operation (Tunik et al., 2007b).
Movement kinematics — acquisition and analysis
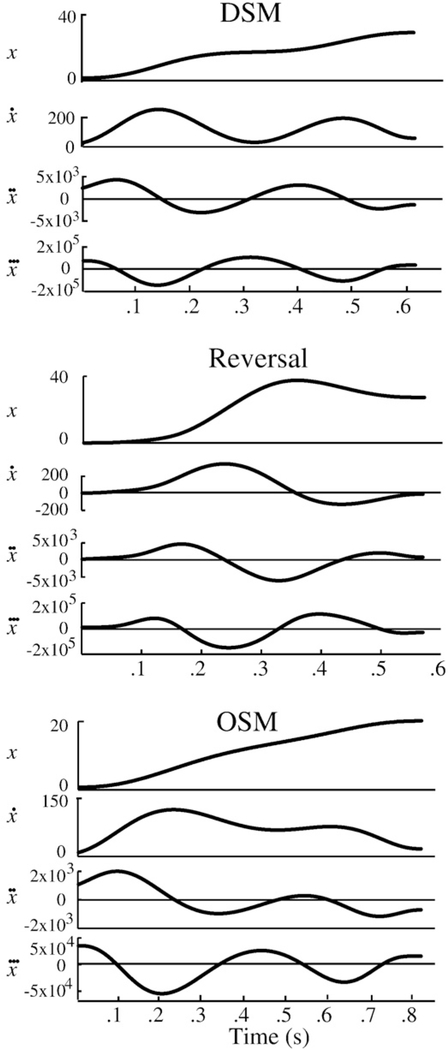
The dial (cursor) position was sampled at 1500 Hz and offline Butterworth lowpass filtered at 10 Hz. The position data was differentiated to yield a velocity trace and movement onsets and offsets were defined. Movement onset was defined as the time at which the angular velocity exceeded and remained above 5% of the peak angular velocity for >100 ms. Movement offset was defined as the time at which the knob angle did not change by more than 5° for >0.4 s. Movement time was defined as the interval between movement onset and offset. Movement amplitude was defined as the displacement (in angular terms) between the angle at the start and the maximal angular displacement in the corresponding trial. Movement error was computed in two ways: the final movement error was defined as the angular displacement between the dial’s position at movement offset and the center of the target; and primary movement error was defined as the displacement between the dial’s position at the end of the primary movement (see below) and the center of the target. Each movement was decomposed into a primary movement, and if present, any submovements (Novak et al., 2000, 2002, 2003). We used a method similar to that employed by Novak et al. to identify submovements, that is, we computed the third derivative of the position (jerk) and identified all zero crossings occurring between the movement onset and offset. The number of zero crossings was divided by two to determine the total number of movements (velocity peaks) in a given trajectory. Subtracting the single primary movement from this number determined the number of submovements. As an example, Fig. 3A shows a trial’s position trajectory and its first three derivatives. Note that the jerk profile for this trial is characterized by 4 zero crossings, equating to a primary movement and one submovement.
Fig. 3.
The position and its first three derivatives of representative trials having a delayed submovement (DSM, top panel), a reversal submovement (middle panel), and an overlapping submovement (OSM, bottom panel).
All trials were included in the analyses. For group-level analysis, the mean of each variable for each subject was submitted to a one-way analysis of variance (levels: null, viscous) using Statview statistical software. The significance level was set at p<0.05. To understand the source of variance in the data, we also performed a factor analysis on the same variables, separately for the null and viscous conditions.
Results
Behavioral data
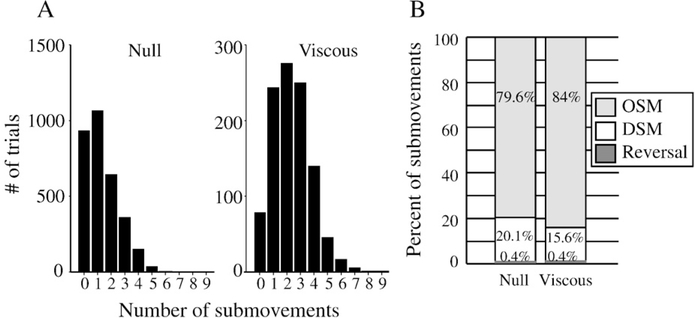
In the null and viscous fields, the terminal movement error was small and did not differ significantly between conditions (group mean±SD: null: 7°±1.8° and viscous: 7.5°±2°; p=0.2). To understand whether this level of accuracy was achieved through similar control mechanisms across conditions, we decomposed each movement into a primary movement component and, if present, subsequent submovement components. The mean number of submovements in the null condition was 1.3±0.7 (range: 0–8; Fig. 2A). Submovements occurred mostly in cases in which the primary movement significantly undershot the target (group mean error of primary movement: 20.8°±10°). In the viscous condition, the perturbation generally caused a significantly larger undershoot in the primary movement (group mean: 33.7°±5°) than that of the null condition (t(17)=8.4, p<0.0001) and led subjects to make a significantly greater number of corrective submovements (mean: 2.4±0.6; range: 0–10; t(17)=12.3, p<0.0001). Between-condition differences were also noted in the movement amplitude (null: 54.4°±2.2°; viscous: 57.1°±2.3°; t(17)= −5.8, p<.0001) and movement time (null: 432 ms±9.5 ms; viscous 523 ms±8.9 ms; t(17)=−11, p<.0001).
Fig. 2.
(A) Histogram showing the distribution of submovements in the null and viscous conditions. (B) Stacked bar plot showing the relative proportion of the three types of submovements expressed as a percentage of the total number of submovements in each perturbation condition.
To understand whether the types of submovements used in the null and viscous conditions were qualitatively different, we classified each submovement into one of three types (Wisleder and Dounskaia, 2007) (see Fig. 3): 1) those characterized by a change in sign in the velocity profile after the primary movement (i.e. the submovement occurred in the opposite direction to the primary movement). We refer to these as ‘reverse submovements’. 2) Those whose onset was separated in time from the primary movement by at least 50 ms of minimal motion (i.e. minimal motion was defined as a velocity less than 5% of peak velocity). We refer to these as ‘delayed submovements’. 3) Finally, those that overlapped in time with the primary movement. We refer to these as ‘overlapping submovements’. The incidence rate of each type of submovement is shown in Fig. 2B as a percentage of the total number of submovements. While the percent of ‘reverse submovements’ did not change significantly between the null and viscous conditions (null: 0.35%±1.5%; viscous: 0.39%±1.5%; p>0.3), the incidence rate of the other types of submovements was significantly affected by the perturbation. Specifically, subjects reduced the amount of delayed submovements used in the viscous trials by 4.5% (t(17)=−4.4, p=0.0004) but increased the number of overlapping submovements by 4.4% (t(17)=4.4, p=0.0003).
In accordance with previous reports (Fishbach et al., 2003, 2007), we noted a moderate correlation between submovements and movement amplitude (r=0.49; p<0.0001) or movement time (r=0.86, p<0.0001). To guide our selection of kinematics to correlate with the BOLD signal in the general linear model (GLM) we performed a factor analysis on the six kinematic measures. Factor analysis revealed that submovements and movement amplitude were associated with different sources of performance variance (Table 1). Specifically, for null and viscous conditions, the first factor accounted for 50% of the variance, with factor loadings driven by submovements, primary movement error, and movement time. The second factor accounted for 25% of the variance, with factor loadings driven by amplitude and final error. These findings are reasonable since submovement production should be associated with increased movement time and the primary movement error (which draws the need for making a submovement). Thus, we modeled the number of submovements and movement amplitude in separate fMRI analyses (in separate GLMs) to avoid non-orthogonality issues. Correlating these two kinematic measures with the fMRI data is in line with the main purpose of this study and is substantiated by the factor analysis which revealed strong loadings of submovements and movement amplitude, suggesting that these two variables may represent dissociable sources of performance variance — and may thus reflect different neural processes.
Table 1.
A summary of the behavioral data used in the factor analysis, the loadings of each variable on the first two factors, and percent variance explained by each variable and factor.
| Null |
Viscous |
|||||||
|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | |||||
| Submovments | 0.83 | 11.5% | − 0.09 | 0.1% | 0.95 | 15.0% | 0.05 | 0.0% |
| Primary error | 0.93 | 14.4% | 0.15 | 0.4% | 0.87 | 12.6% | − 0.08 | 0.1% |
| Final error | −0.01 | 0.0% | 0.86 | 12.3% | − 0.31 | 1.6% | 0.50 | 4.2% |
| Movement time | 0.82 | 11.2% | 0.00 | 0.0% | 0.94 | 14.7% | 0.14 | 0.3% |
| Movement amplitude | −0.15 | 0.4% | −0.90 | 13.5% | 0.46 | 3.5% | 0.88 | 12.9% |
| Primary movement amplitude | −0.24 | 1.0% | 0.19 | 0.6% | 0.03 | 0.0% | − 0.67 | 7.5% |
| % of total variance | 38.4% | 26.9% | 47.5% | 25.0% | ||||
The factor loadings are derived using the oblique solution.
Brain activation related to the overall task
Movements in the viscous and null conditions were associated with robust activation in a distributed sensorimotor network typically recruited for point-to-point movements requiring speed and precision (see Supplementary Fig. 1). Significantly activated regions included contralateral precentral gyrus, contralateral supplementary motor area, bilateral postcentral gyri, contralateral posterior parietal cortex, bilateral dorsal putamen, bilateral cerebellar cortex (anterior and posterior), and bilateral dentate nuclei. This contrast was used as a mask for regions of interest analyses in subsequent contrasts.
Brain activation scaling with the generation of submovements
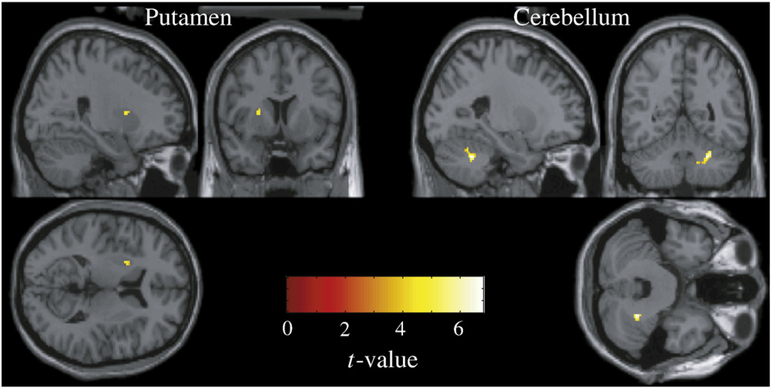
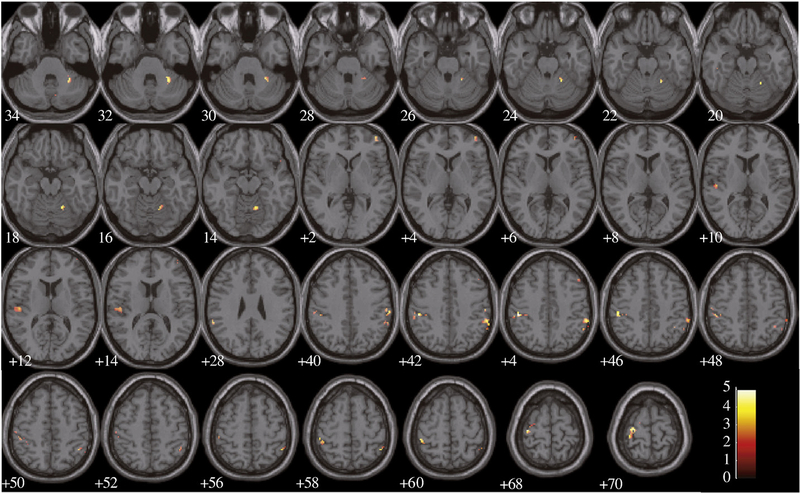
Subjects generated significantly more submovements in the viscous than the null condition. This compensatory response to the perturbation reduced final error such that viscous and null conditions had comparable final endpoint error. Regions where the BOLD response correlated with corrective submovements in the null condition included the right anterior-middle cingulate cortex and a caudal portion of the left middle frontal gyrus (see Table 2). At a slightly less conservative threshold of p=0.005, the left motor cortex was also recruited (cluster center: −40, −22, 60; Ke=64; t=4.07; z=3.29). Regions that significantly correlated with submovements in the viscous condition included the left posterior-middle putamen and the right posterior-intermediate cerebellum (see Table 2). A contrast to identify regions where the correlations with submovements were significantly stronger in the viscous than the null conditions revealed a set of loci in the putamen and cerebellum (see Fig. 4 and Table 2). The reverse contrast did not reveal any significant activation.
Table 2.
Regions that were significantly activated by the task and that significantly correlated with the number of submovements produced in the corresponding trial.
| Region | k | t | z score | p value | x | y | z |
|---|---|---|---|---|---|---|---|
| Activation related to the task | |||||||
| L caudate body | 338 | 8.97 | 5.29 | <.001 | −16 | −8 | 18 |
| L caudate head | 21 | 4.35 | 3.48 | <.001 | −10 | 14 | 10 |
| L precentral/postcentral gyri | 266 | 6.65 | 4.54 | <.001 | −22 | −36 | 76 |
| L superior temporal gyrus | 71 | 6.44 | 4.46 | <.001 | −56 | −20 | 12 |
| L posterior-intermediate cerebellum | 114 | 6.24 | 4.38 | <.001 | −48 | −58 | −34 |
| L posterior-inferior cerebellum | 34 | 5.44 | 4.04 | <.001 | −32 | −52 | −44 |
| R inferior parietal lobe | 185 | 7.14 | 4.72 | <.001 | 60 | −34 | 44 |
| 45 | 5.47 | 4.05 | <.001 | 48 | −56 | 50 | |
| R posterior parietal lobe | 80 | 5.1 | 3.87 | <.001 | 0 | −84 | 32 |
| R intraparietal sulcus | 21 | 5.09 | 3.87 | <.001 | 38 | −68 | 48 |
| R STG | 24 | 4.32 | 3.47 | <.001 | 58 | −20 | 12 |
| R lateral occipital lobe | 117 | 5.98 | 4.27 | <.001 | 32 | −98 | 0 |
| R posterior-intermediate cerebellum | 988 | 7.98 | 5 | <.001 | 44 | −64 | −32 |
| R dentate nucleus | 97 | 5.29 | 3.96 | <.001 | 10 | −42 | −36 |
| R ventral-anterior thalamus | 162 | 6 | 4.28 | <.001 | 16 | −6 | 14 |
| Regions that correlated with submovements in the null condition | |||||||
| L middle frontal gyrus, caudal | 47 | 5.78 | 4.13 | 0.001 | −32 | −6 | 62 |
| R anterior-middle cingulate cortex | 23 | 6.63 | 4.47 | 0.009 | 6 | −8 | 62 |
| Regions that correlated with submovements in the viscous condition | |||||||
| L posterior-middle putamen | 17 | 4.19 | 3.39 | 0.03 | −28 | 20 | 2 |
| L postcentral gyrus | 15 | 5.69 | 4.15 | 0.041 | −52 | −30 | 38 |
| L caudal superior frontal gyrus | 66 | 5.5 | 4.06 | <.0001 | −26 | −4 | 60 |
| L precentral gyrus | 49 | 4.71 | 3.68 | 0.001 | −36 | −22 | 62 |
| L ventroposteriolateral nucleus of thalamus | 10 | 4.7 | 3.67 | 0.088 | −18 | −22 | 4 |
| L caudal inferior frontal gyrus | 12 | 4.34 | 3.47 | 0.064 | −56 | 10 | 30 |
| R anterior-superior cerebellum | 49 | 5.07 | 3.86 | 0.001 | 12 | −54 | −18 |
| R caudal middle frontal gyrus | 32 | 6.71 | 4.56 | 0.005 | 38 | 0 | 42 |
| R rostral middle frontal gyrus | 21 | 4.37 | 3.49 | 0.019 | 40 | 40 | 28 |
| Regions that correlated with submovements more strongly in the viscous than the null condition | |||||||
| L posterior-middle putamen | 10 | 4.71 | 3.68 | 0.069 | −26 | 8 | 10 |
| R posterior-intermediate cerebellum | 50 | 6.76 | 4.58 | <.001 | 24 | −52 | −32 |
Statistical parametric maps were created with a p<0.001 threshold and a 10 voxel extent. Above p values are uncorrected at the voxel level. Coordinates are in MNI space.
Fig. 4.
Regions for which the BOLD signal was significantly more correlated with corrective submovements in the viscous than in the null condition. Significance threshold set at p<0.001 and extent of 10 voxels.
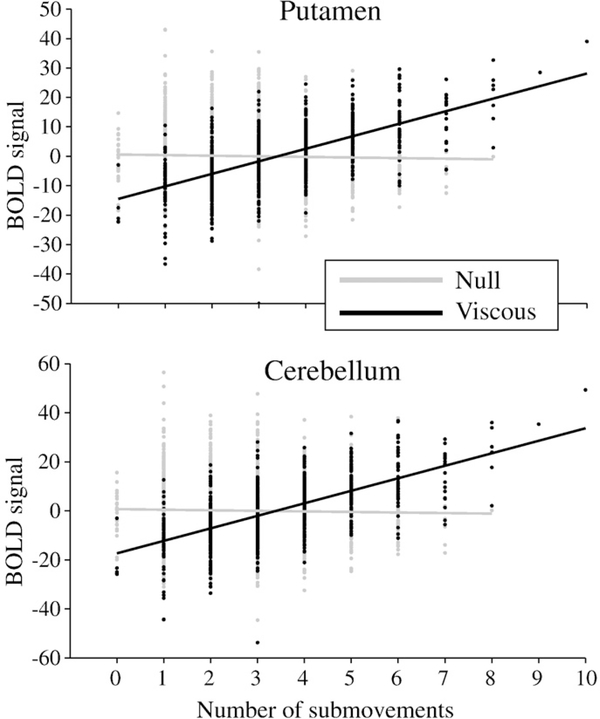
To confirm the parametric scaling of BOLD in the putamen and cerebellum with the number of submovements, we performed an additional analysis. For this, we extracted the amplitude of the smoothed and convolved event-related BOLD signal for the time course of each functional run and correlated that value with the number of submovements generated on the corresponding trial. The locations for these two sites were defined independently by the main effect of task versus baseline. Fig. 5 shows a scatter plot of the relationship between submovements and BOLD response for each condition (null, viscous) and brain loci (putamen, cerebellum). The solid line represents the best fit linear regression line. Correlation between submovements generated in the viscous condition and the BOLD response was highly significant in the putamen (r=0.628, p<0.0001) and cerebellum (r=0.629, p<0.0001). No such relationship existed in the null condition for either brain site (putamen: r=0.03; cerebellum: r=0.029; all p>0.1). As a precaution against outlier effects, we recalculated the correlation after removing trials with 8–10 submovements in the viscous condition (which did not occur in the null condition). This did not affect the results (putamen: r=0.603; cerebellum: r=0.602; all p<0.0001), suggesting that the effect in the viscous condition was robust within the same range of submovements that occurred in the null condition.
Fig. 5.
A scatter plot showing the relationship between the number of submovements (x-axis) in the null (gray) and viscous (black) conditions and the BOLD signal (y-axis) in the putamen (top) and cerebellar (bottom) regions.
Brain activation scaling with movement amplitude
It is possible that the correlation between brain activity and submovements could be confounded by movement amplitude, which also correlated with submovements. To address this, we performed a separate general linear model (to avoid non-orthogonality between amplitude and submovements) to identify brain regions that correlated with movement amplitude on our task. Fig. 6 shows that correlating movement extent with BOLD activity localized to the left motor cortex (x,y,z coordinates in MNI space: −34, −30, 70), left premotor cortex (−28, −18, 70), left and right anterior intraparietal sulcus (left: −50, −28, 42; right: 56, −38, 42), and the right cerebellar cortex (26, −46, −32). Notably, the basal ganglia did not show a significant correlation with movement amplitude even at a reduced threshold of p<0.05, suggesting that the basal ganglia’s involvement with corrective submovements was unlikely to be confounded by movement amplitude (which has been shown to be related to corrective submovements). Instead our data suggest, and we discuss below, that the basal ganglia may be involved in online control of movement by implementing corrective submovements in a context (i.e. perturbation)-specific manner.
Fig. 6.
Regions where BOLD signal significantly correlated with the movement amplitude. Significance threshold set at p<0.001 and extent of 10 voxels.
Brain activation specific to the viscous condition
To further characterize task specific recruitment of brain activity in the viscous condition, we estimated a contrast for viscousNnull within the mask created from the movement versus rest contrast. Supplementary Fig. 2 shows that the viscous condition was associated with significantly more activation only in an ipsilateral posterior-intermediate cerebellar region (MNI, 24, −76, −32). Given the difference we observed in the regression slope between the viscous and null conditions, we were surprised there was no significant main effect in the putamen at the a priori threshold of p<0.001. However, a post hoc examination of the putamen shown in Fig. 4 revealed a significant difference between viscous and null conditions (cluster center: −26, 8, 10; Ke=10, t=2.02, z=1.88, p=0.03).
Discussion
Brain activity related to the production of corrective submovements
Neural circuits mediating movement in both null and viscous conditions had access to the efference copy of the motor plan (formed from past experience) and online proprioceptive feedback. Since the null condition was highly rehearsed, the majority of the sensorimotor variance in each trial was ascribed to the motor plan (i.e. formed on the basis of experience). On the other hand, the viscous perturbation was unfamiliar and thus introduced an additional degree of unexpected sensorimotor variance to the neural circuits. To compensate for this, subjects used significantly more overlapping corrective submovements (i.e. an online control strategy). The above, and the finding that activity in the BG and cerebellum correlated with corrective submovements in the viscous condition (see Fig. 5), implicate these structures in a role for making online decisions about the need for and the type of corrective submovement to invoke under conditions of uncertainty in the sensorimotor plant.
Brain activity related to the viscous perturbation
In addition to relating functional anatomy and submovement formation at the individual trial level of analysis, we also directly compared the viscous and null conditions at a group level. Despite potential confounds introduced by this contrast (different levels of perceived error, different motor output, etc.) there were relatively few differences between the conditions. The viscous condition was associated with significantly stronger activation in the right anterior cerebellum and, at a lower threshold, in the left putamen. This highlights important distinctions between neural processing at the individual trial level versus general task effects and suggests that there may be weak differences of general task effects within the anatomical network for performing movements in viscous or null loads that are better captured through trial-by-trial modeling of performance. The lack of differences in cortical areas is not surprising given recent evidence that largely overlapping anatomical substrates govern online performance under kinematic and dynamic perturbations during 2D movements (Diedrichsen et al., 2005) or under positional and viscous perturbations during 1D movements (Tunik et al., 2007b). However, the unique activation in the cerebellar and putamen sites may be specifically related to the adaptive responses. It has been demonstrated that complex spikes in Purkinje cells may encode movement errors (Kitazawa et al., 1998; Pasalar et al., 2006). These signals could be critical for implementing movement corrections across trials, and the absence of these signals in cerebellar pathology, may underlie the basic deficits in adaptive responses commonly found in cerebellar-lesioned patients (Martin et al., 1996; Smith and Shadmehr, 2005; Tseng et al., 2007). Interestingly, our cerebellar region was very similar to one identified by Diedrichsen et al. (2005), who noted it to be recruited for error-detection during a motor task. It is possible therefore that this cerebellar region was more strongly recruited in our viscous condition as a result of more robust climbing fiber input to the Purkinje cells, arising from larger primary movement errors in this condition. The putamen site on the other hand, may have been involved in using this error information to initiate corrective submovements. We discuss this in more detail below.
Context-dependent action selection by the basal ganglia
The findings that the BG had trial-by-trial interactions with submovement formation whereas the cerebellum had a more general correlation with increasing task difficulty and error support a model in which the basal ganglia and cerebellum play complementary roles in specifying contextually-based motor decisions (Houk, 2005; Houk et al., 2007; Mink,1996; Mink and Thach,1993). However, because the putamen was selectively correlated only with submovements, unlike the cerebellum which also correlated with movement extent, the basal ganglia may play a particularly unique role in governing the initiation of online movement corrections.
Discrete online corrections are a means of controlling precise movement, particularly for unfamiliar skills (Milner, 1992). Our data support a model in which the basal ganglia may be important for this process, for example by making decisions regarding whether a discrete correction is necessary and if so, the timing of the correction given a particular context. Single unit recordings in monkeys support the role of the basal ganglia in making “whether to correct?” and “when to correct?” decisions. For example, pre-movement firing rate of pallidal neurons is strongly modulated by contextual demands, as a function of high-precision (targeted outward movements) and low-precision (uncued return movements) contexts (Gdowski et al., 2001, 2007; Turner and Anderson, 2005). Moreover, pre-movement changes in firing rate can be enhanced in the peri-movement epoch, particularly for self-timed movements (Turner and Anderson, 2005) and a large portion of pallidal neurons begin to burst only after a movement has been initiated (Mink and Thach, 1991a,b,c). Detailed analysis of perimovement discharge reveals that pauses and bursts in a large portion of pallidal neurons is time-locked to the onset of corrective submovements (Roy et al., 2003, 2008). These empirical data in monkeys are in close agreement with our event-related fMRI data in humans, and the congruency between our behavioral paradigm and that of Houk et al. (2007) make the results all the more compelling.
The critical role of the basal ganglia in timing and contextappropriate movement selection is further underscored by lesion studies. Neurotoxic lesions of basal ganglia nuclei clearly show that an animal’s ability to select and appropriately sequence motor elements according to contextual demands is severely impoverished, such as for example in monkeys required to select contextspecific reach-to-grasp movements (Pessiglione et al., 2003), in birds required to string together socially-appropriate songs (Kao and Brainard, 2006), and in rodents required to string together stereotypical grooming elements (Aldridge and Berridge, 1998) — without impairing the elements themselves (Cromwell and Berridge, 1996). In humans, pathology of the basal ganglia, such as due to Parkinson’s disease and Huntington’s disease, likewise leads to pronounced deficits in adapting to sudden changes in context, whether in the motor (Desmurget et al., 2004a; Smith et al., 2000; Tunik et al., 2004a) or cognitive domains (Cools et al., 2006; Ell et al., 2006). Interestingly, dopaminergic replacement for Parkinson’s disease does not remediate this impairment (Schettino et al., 2006; Tunik et al., 2007a) suggesting that non-selective disinhibition of BG-cortical pathways is not sufficient to restore the basal ganglia’s capacity to mediate timed and context-based motor behaviors.
A third line of evidence that substantiates our data is neuroimaging work showing activation in the basal ganglia for feedback-based adaptation of limb movement to novel dynamic environments (Krebs et al.,1998; Shadmehr and Holcomb,1997, 1999). In these studies, PET revealed increased regional cerebral blood flow to the cortico-striatal (putamen) circuits during the earlier stages of learning to adapt arm movements to a novel force-field perturbation. However, the limits of PET precluded the characterization of these circuits trial-by-trial. Our data are in line with and extend this work by showing that the putamen is activated on a trial-by-trial basis for adaptation to dynamics in novel but not familiar contexts and that the specific role may involve the generation of corrective submovements. Related neuroimaging work using fMRI shows that the globus pallidus and putamen are also activated when learning to adapt to a novel kinematic perturbation (a rotational mismatch between cursor and joystick position) (Seidler et al., 2006). When interpreted with our current data, this suggests that putamen involvement in generating corrective submovements may occur irrespective of the type of perturbation. Our ongoing studies use a similar paradigm to the current design to get at these issues.
One interesting finding arising from some of the neuroimaging work referenced above is that earlier stages of learning are dominated by basal ganglia involvement and later stages by cerebellar involvement. Since our paradigm was not designed to lead to across-trial learning (due to random perturbations occurring on a minority of trials) we are limited in drawing parallels with this data. Instead, we note a difference in the role played by the putamen and cerebellum, with the putamen’s involvement being more limited to corrective submovements and the cerebellum’s involvement being more general to scaling movement extent. These data suggest that the corticostriatal and cortico-cerebellar systems may be acting in parallel for unique aspects of online motor control. This scheme is supported by our previous data showing that while overall activity in cortical, basal ganglia, and cerebellar regions may remain similar when learning two different dynamic fields, the functional interactions among these circuits on a trial-by-trial basis (measured with BOLD coherence) can be quite different (Tunik et al., 2007a,b).
Role of cortical and subcortical circuits in online error correction
Neural control of online updating is not exclusive to the basal ganglia, but extends to frontoparietal circuits as well. Our own work using transcranial magnetic stimulation to induce virtual lesions implicates a region in the anterior intraparietal sulcus (aIPS) in online updating. TMS to aIPS causes significant delays in online adjustment of grasp (Rice et al., 2006, 2007; Tunik et al., 2005) and forearm movements in a target capture task much like the one used in the present experiment (unpublished data), indicating that aIPS’s role may have less to do with representing effectors and more to do with representing action goals (Tunik et al., 2007b). Basal ganglia and cerebellar neurons project onto aIPS (Clower et al., 2005) and may have complementary roles in maintaining a target goal during movement. Another area of interest is the primary motor cortex. In an early report, Georgopoulos et al. (1983b) noted that perturbing target location during a reaching movement led to an interruption in the firing rate of neurons in the primary motor cortex (M1) and that resumption of neural activity in M1 reflected the new, rather than the initial, movement. More recently, the activity of M1 neurons was analyzed with respect to the occurrence of corrective submovements and found to be time-locked to just prior to the initiation of the movement corrections (Fishbach et al., 2003; Roy et al., 2003), much like the activity of GPi neurons (Roy et al., 2008). The submovementtime-locked changes in GPi and M1 support a model in which the basal ganglia release wanted motor commands and inhibit unwanted motor commands at the motor cortical level, but in a contextually-defined manner (Mink, 1996; Mink and Thach, 1993).
Neural circuits governing movement extent
Subjects accomplished the task by accurately scaling movement amplitude on a trial-by-trial basis to home in on the target. One possibility is that the dorsal putamen and the anterior-intermediate cerebellum finding was confounded by changes in movement extent (i.e. more submovements, therefore more movement) thereby reflecting control of basic movement parameters, such as the scaling of movement extent. This possibility gains support from imaging and neurophysiological recording studies showing regional cerebral blood flow to the posterior putamen and cerebellum (Krakauer et al., 2004; Turner et al., 2003) as well as other cortical and subcortical regions (DeLong et al., 1984a,b; Desmurget et al., 2003, 2004b; Fu et al., 1997; Georgopoulos et al., 1983a; Sergio et al., 2005; Sergio and Kalaska, 2003; Turner et al., 2003; Turner et al., 1998; Winstein et al., 1997) that are modulated by kinematic variables such as movement extent and velocity. However, other studies report an equal and at times even a stronger relationship of, for example, basal ganglia activity to movement direction and context when compared against their relationship to amplitude and muscle activation (Crutcher and DeLong, 1984; Gdowski et al., 2007; Georgopoulos et al.,1983a;Mitchell et al.,1987). One difference between previous imaging studies showing basal ganglia amplitude modulation and our data may be explained by their use of a less constrained task (joystick movement), use of a continuous tracking movement rather than a discrete point-to-point movement, and a blocked experimental design (which yields a much stronger hemodynamic response). Also, their analyses did not account for a potential relationship between submovements and movement amplitude. In our study, the factor analysis confirmed that submovements and amplitude were primary contributors to different components of the overall performance variance. Further, our data indicated that BOLD signal in the motor/premotor cortex, anterior intraparietal sulcus, and cerebellum correlated with movement extent, while the basal ganglia nuclei did not, suggesting that the putamen was unlikely mediating basic movement parameters, such as scaling movement amplitude. A similar argument has been made based on direct neuronal recording from the globus pallidus (Gdowski et al., 2007). It may be that our event-related design and a more constrained task captured a subtle function of the basal ganglia that has previously been overlooked in human neuroimaging studies. It has recently been proposed that the BG are more involved in setting movement “vigor” based on a given task context, rather than the direct scaling of amplitude (Desmurget and Turner, 2008; Mazzoni et al., 2007). The relationship between submovement formation and control of motor vigor remains to be determined.
Conclusion
The idea that the basal ganglia may be critical for signaling online motor decision-making processes, particularly during feedback- rather than feedforward-based motor control, fits well with a long-held view of the their role in filtering competing motor programs — by inhibiting unwanted motor programs and disinhibiting wanted programs (Houk, 2005; Houk et al., 2007; Mink, 1996; Mink and Thach, 1993). In the case of precision-guided movements, the decisions may pertain to whether or not discrete submovement corrections are necessary, perhaps based on a threshold mechanism that takes into account the goal, current movement, and predicted consequences of the ongoing movement (Fishbach et al., 2007). Current work in our lab is addressing this possibility. Our current data indicate that the basal ganglia and cerebellum play complementary roles in regulating ongoing actions when precise updating is required. The basal ganglia may play a key role in contextually-based motor decision-making, i.e. for deciding if and when to correct a given movement by initiating corrective submovements, and the cerebellum is more generally involved in amplifying and refining the command signals to specify movements with different amplitudes, velocities and directions.
Supplementary Material
Acknowledgments
Thanks to Dr. Antonia Hamilton for help with batch fMRI 674 preprocessing scripts and Stephane Roy for development of methods to analyze submovements.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2009.04.077.
References
- Aldridge JW, Berridge KC, 1998. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J. Neurosci 18, 2777–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barringer CW, Barto AG, Fishbach A, Houk JC, 2008. Simulated Reaching Supports Discrete Control Hypothesis for Error-correction in Voluntary Limb Movements.. Society for Neuroscience, Washington D.C. [Google Scholar]
- Chinzei K, Kikinis R, Jolesz F, 1999. MR compatibility of mechatronic devices: design criteria. Proc. MICCAI ‘99 Lect. Notes Comput. Sci 1679, 1020–1031. [Google Scholar]
- Clower DM, Dum RP, Strick PL, 2005. Basal ganglia and cerebellar inputs to ‘AIP’. Cereb. Cortex 15, 913–920. [DOI] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D’Esposito M, 2006. The human striatum is necessary for responding to changes in stimulus relevance. J. Cogn. Neurosci 18, 1973–1983. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC,1996. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J. Neurosci 16, 3444–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman ER, Goodeve PJ, 1983. Feedback control of hand-movement and Fitts’ Law. Q. J. Exp. Psychol., A 35, 251–278. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR, 1984. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp. Brain Res 53, 244–258. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Alexander GE, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, 1984a. Role of basal ganglia in limb movements. Hum. Neurobiol 2, 235–244. [PubMed] [Google Scholar]
- Delong MR, Georgopoulos AP, Crutcher MD, Mitchell SJ, Richardson RT, Alexander GE, 1984b. Functional organization of the basal ganglia: contributions of single-cell recording studies. Ciba Found. Symp 107, 64–82. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Turner RS, 2008. Testing basal ganglia motor functions through reversible inactivations in the posterior internal globus pallidus. J. Neurophysiol 99, 1057–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST, 2001. Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J. Neurosci 21, 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS, 2003. Basal ganglia network mediates the control of movement amplitude. Exp. Brain Res 153, 197–209. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S, 2004a. Online motor control in patients with Parkinson’s disease. Brain 127, 1755–1773. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton ST, Vindras P, Grea H, Turner RS, 2004b. The basal ganglia network mediates the planning of movement amplitude. Eur. J. Neurosci 19, 2871–2880. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R, 2005. Neural correlates of reach errors. J. Neurosci 25, 9919–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ell SW, Marchant NL, Ivry RB, 2006. Focal putamen lesions impair learning in rule-based, but not information-integration categorization tasks. Neuropsychologia 44, 1737–1751. [DOI] [PubMed] [Google Scholar]
- Fishbach A, Roy SA, Bastianen C, Miller LE, Houk JC, 2003. Neural correlates of online error correction in M1 of behaving macaque monkeys., Society for the Neural Control of Movement Abstracts. [Google Scholar]
- Fishbach A, Roy SA, Bastianen C, Miller LE, Houk JC, 2007. Deciding when and how to correct a movement: discrete submovements as a decision making process. Exp. Brain Res 177, 45–63. [DOI] [PubMed] [Google Scholar]
- Flash T, Henis E, 1990. Arm trajectory modifications during reaching towards visual targets. J. Cogn. Neurosci 3, 220–230. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM, 2004. Changing brain networks for visuomotor control with increased movement automaticity. J. Neurophysiol 92, 2405–2412. [DOI] [PubMed] [Google Scholar]
- Fu QG, Mason CR, Flament D, Coltz JD, Ebner TJ, 1997. Movement kinematics encoded in complex spike discharge of primate cerebellar Purkinje cells. Neuroreport 8, 523–529. [DOI] [PubMed] [Google Scholar]
- Gdowski MJ, Miller LE, Parrish T, Nenonene EK, Houk JC, 2001. Context dependency in the globus pallidus internal segment during targeted arm movements. J. Neurophysiol 85, 998–1004. [DOI] [PubMed] [Google Scholar]
- Gdowski MJ, Miller LE, Bastianen CA, Nenonene EK, Houk JC, 2007. Signaling patterns of globus pallidus internal segment neurons during forearm rotation. Brain Res. 1155, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, DeLong MR, Crutcher MD, 1983a. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J. Neurosci 3, 1586–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT, 1983b. Interruption of motor cortical discharge subserving aimed arm movements. Exp. Brain Res 49, 327–340. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D, 2000. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 871, 127–145. [DOI] [PubMed] [Google Scholar]
- Graydon FX, Friston KJ, Thomas CG, Brooks B, Menon RS, 2005. Learning-related fMRI activation associated with a rotational visuo-motor transformation. Brain Res. Cogn. Brain Res 22, 373–383. [DOI] [PubMed] [Google Scholar]
- Guigon E, Baraduc P, Desmurget M, 2007. Computational motor control: redundancy and invariance. J. Neurophysiol 97, 331–347. [DOI] [PubMed] [Google Scholar]
- Houk JC, 2005. Agents of the mind. Biol. Cybern 92, 427–437. [DOI] [PubMed] [Google Scholar]
- Houk JC, Bastianen C, Fansler D, Fishbach A, Fraser D, Reber PJ, Roy SA, Simo LS, 2007. Action selection and refinement in subcortical loops through basal ganglia and cerebellum. Philos. Trans. R. Soc. Lond., B Biol. Sci 362, 1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M, 2000. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403, 192–195. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Goto R, Sugiura M, Ito M, Fukuda H, 1997. Activity in the parietal area during visuomotor learning with optical rotation. Neuroreport 8, 3979–3983. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawashima R, Satoh K, Kinomura S, Sugiura M, Goto R, Ito M, Fukuda H, 2000. A PET study of visuomotor learning under optical rotation. Neuroimage 11, 505–516. [DOI] [PubMed] [Google Scholar]
- Kao MH, Brainard MS, 2006. Lesions of an avian basal ganglia circuit prevent contextdependent changes to song variability. J. Neurophysiol 96, 1441–1455. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kimura T, Yin PB, 1998. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature 392, 494–497. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Mentis M, Barnes A, Veytsman M, Eidelberg D, Ghez C, 2004. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J. Neurophysiol 91, 924–933. [DOI] [PubMed] [Google Scholar]
- Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM, 1998. Robot-aided functional imaging: application to a motor learning study. Hum. Brain Mapp 6, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT, 1996. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119 (Pt 4), 1183–1198. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW, 2007. Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J. Neurosci 27, 7105–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JE, 1988. Optimality in human motor performance: ideal control of rapid aimed movements. Psychol. Rev 95, 340–370. [DOI] [PubMed] [Google Scholar]
- Miall RC, Jenkinson EW, 2005. Functional imaging of changes in cerebellar activity related to learning during a novel eye-hand tracking task. Exp. Brain Res 166,170–183. [DOI] [PubMed] [Google Scholar]
- Milner TE, 1992. A model for the generation of movements requiring endpoint precision. Neuroscience 49, 487–496. [DOI] [PubMed] [Google Scholar]
- Mink JW,1996. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol 50, 381–425. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT, 1991a. Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J. Neurophysiol 65, 273–300. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT, 1991b. Basal ganglia motor control. II. Late pallidal timing relative to movement onset and inconsistent pallidal coding of movement parameters. J. Neurophysiol 65, 301–329. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT, 1991c. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J. Neurophysiol 65, 330–351. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT, 1993. Basal ganglia intrinsic circuits and their role in behavior. Curr. Opin. Neurobiol 3, 950–957. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Richardson R, Baker F, MR D,1987. The primate globus pallidus: neuronal activity related to direction of movement. Exp. Brain Res 68, 491–505. [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LE, Houk JC, 2000. Kinematic properties of rapid hand movements in a knob turning task. Exp. Brain Res 132, 419–433. [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LE, Houk JC, 2002. The use of overlapping submovements in the control of rapid hand movements. Exp. Brain Res 144, 351–364. [DOI] [PubMed] [Google Scholar]
- Novak KE, Miller LE, Houk JC, 2003. Features of motor performance that drive adaptation in rapid hand movements. Exp. Brain Res 148, 388–400. [DOI] [PubMed] [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Pasalar S, Roitman AV, Durfee WK, Ebner TJ, 2006. Force field effects on cerebellar Purkinje cell discharge with implications for internal models. Nat. Neurosci 9, 1404–1411. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Agid Y, Hirsch EC, Feger J, Tremblay L, 2003. Impairment of context-adapted movement selection in a primate model of presymptomatic Parkinson’s disease. Brain 126, 1392–1408. [DOI] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Grafton ST, 2006. The anterior intraparietal sulcus mediates grasp execution, independent of requirement to update: new insights from transcranial magnetic stimulation. J. Neurosci 26, 8176–8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice NJ, Tunik E, Cross ES, Grafton ST, 2007. On-line grasp control is mediated by the contralateral hemisphere. Brain Res. 1175, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SA, Bastianen C, Nenonene E, Fishbach A, Miller LE, Houk JC, 2003. Neural correlates of corrective submovement formation in the basal ganglia and motor cortex ., Society for the Neural Control of Movement Abstracts.
- Roy SA, Tunik E, Bastianen C, Fishbach A, Grafton ST, Houk JC, 2008. Firing Patterns of GPi Neurons Associated with Primary Movements and Corrective Submovements. Society for Neuroscience, Washington D.C. [Google Scholar]
- Schettino LF, Adamovich SV, Hening W, Tunik E, Sage J, Poizner H, 2006. Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state. Exp. Brain Res 168, 186–202. [DOI] [PubMed] [Google Scholar]
- Schmidt RA, Zelaznik H, Hawkins B, Frank JS, Quinn JT Jr, 1979. Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol. Rev 47, 415–451. [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Chintalapati P, 2006. Bilateral basal ganglia activation associated with sensorimotor adaptation. Exp. Brain Res 175, 544–555. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Kalaska JF, 2003. Systematic changes in motor cortex cell activity with arm posture during directional isometric force generation. J. Neurophysiol 89, 212–228. [DOI] [PubMed] [Google Scholar]
- Sergio LE, Hamel-Paquet C, Kalaska JF, 2005. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J. Neurophysiol 94, 2353–2378. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH, 1997. Neural correlates of motor memory consolidation. Science 277, 821–825. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH, 1999. Inhibitory control of competing motor memories. Exp. Brain Res 126, 235–251. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R, 2005. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J. Neurophysiol 93, 2809–2821. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brandt J, Shadmehr R, 2000. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature 403, 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P,1988. Co-planar Stereotaxic Atlas of the Human Brain. Thieme, New York. [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ, 2007. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol [DOI] [PubMed] [Google Scholar]
- Tunik E, Adamovich SV, Poizner H, Feldman AG, 2004a. Deficits in rapid adjustments of movements according to task constraints in Parkinson’s disease. Mov. Disord 19, 897–906. [DOI] [PubMed] [Google Scholar]
- Tunik E, Poizner H, Adamovich SV, Levin MF, Feldman AG, 2004b. Deficits in adaptive upper limb control in response to trunk perturbations in Parkinson’s disease. Exp. Brain Res 159, 23–32. [DOI] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST, 2005. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat. Neurosci 8, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Feldman AG, Poizner H, 2007a. Dopamine replacement therapy does not restore the ability of Parkinsonian patients to make rapid adjustments in motor strategies according to changing sensorimotor contexts. Parkinsonism Relat. Disord [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Schmitt PJ, Grafton ST, 2007b. BOLD coherence reveals segregated functional neural interactions when adapting to distinct torque perturbations. J. Neurophysiol 97, 2107–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Roy S, Hou JC, Grafton ST, 2008. Basal Ganglia Contribution to the Initiation of Corrective Submovements. Society for Neuroscience, Washington D.C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Anderson ME, 2005. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J. Neurosci 25, 2965–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RS, Grafton ST, Votaw JR, Delong MR, Hoffman JM,1998. Motor subcircuits mediating the control of movement velocity: a PET study. J. Neurophysiol 80, 2162–2176. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST, 2003. Motor subcircuits mediating the control of movement extent and speed. J. Neurophysiol 90, 3958–3966. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS, 1997. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J. Neurophysiol 77, 1581–1594. [DOI] [PubMed] [Google Scholar]
- Wisleder D, Dounskaia N, 1997. The role of different submovement types during pointing to a target. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale 176, 132–149. [DOI] [PubMed] [Google Scholar]
- Woodworth RS, 1899. The accuracy of voluntary movement. Psychol. Rev 3, 1–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.