Abstract
Lung ultrasound surface wave elastography (LUSWE) is a novel noninvasive technique for measuring superficial lung tissue stiffness. The purpose of this study was to develop LUSWE for assessing disease progression for patients with interstitial lung disease (ILD). In this study, LUSWE was used to measure the changes of lung surface wave speeds at 100 Hz, 150 Hz and 200 Hz through six intercostal lung spaces for 52 patients with ILD. Patients’ mean age was 63.1 ± 12.0 years (range 20-85, 23 male and 29 female). The follow up interval was 9.2 ± 3.5 months depending on each patient’s return appointment and availability. The disease progression of each patient was evaluated clinically between the follow-up and the baseline tests using a 7-point Likert scale that includes 3 grades of improvement (mild, moderate, marked), unchanged, and 3 grades of worsening (mild, moderate, marked). Clinical assessments were based on changes of pulmonary function tests (PFT) together with high-resolution computed tomography (HRCT), echocardiograms, and clinical evaluations. This study demonstrates correlations between the changes of lung surface wave speed and clinical assessments. The changes of lung surface wave speed at lower lateral and posterior lung portions showed good correlation with clinical assessments. LUSWE provides quantitative global and regional changes of lung surface wave speed that may be useful for quantitative assessment of disease progression of ILD.
Keywords: lung ultrasound surface wave elastography (LUSWE), lung, interstitial lung disease (ILD), lung surface wave speed, disease progression tracking
INTRODUCTION
Ultrasonography is not widely used for clinically assessing lung disease. Most of the energy of the ultrasound wave is reflected from the lung surface because of large differences in acoustic impedance between lung parenchyma and air inside the lung. Ultrasonography evaluation of the thorax is limited to evaluating structures outside of the lung such as pleural fluid, thoracic superficial masses, or adenopathy (Volpicelli 2013). Lung ultrasonography is the standard for diagnosing pleural diseases and is very useful in the emergency and critical care settings (Hakimisefat 2010, Mathis 2008). Lung ultrasound imaging typically presents artifacts such as A-lines and B-lines and features such as lung sliding and lung point (Hakimisefat 2010). These artifacts and features may be used to assess various lung disorders including lung fibrosis (Sperandeo, et al. 2009), pneumothorax (Noble 2012), and lung consolidations (Barillari 2011). However, analysis of these artifacts is qualitative and relies on visual interpretation, which is subject to inter-operator variability (Corradi, et al. 2016).
We have developed a lung ultrasound surface wave elastography (LUSWE) technique to measure superficial lung tissue stiffness safely and quickly (Clay, et al. 2018, Zhang, et al. 2017, Zhang, et al. 2017, Zhang, et al. 2017). In LUSWE, a 0.1 second harmonic vibration at a given low frequency between 100 Hz and 200 Hz is generated on the chest wall of a subject using a handheld vibrator. The ultrasound probe is positioned about 5 mm away from the indenter of the vibrator in the same intercostal space to measure the generated surface wave propagation on the lung in that intercostal space. The measurement of surface wave speed on the lung is determined from the change in wave phase with distance and independent of the location of wave excitation on the chest wall.
We are evaluating LUSWE for assessing patients with interstitial lung disease (ILD) in a prospective clinical research study. Patients with ILD have fibrotic and stiff lungs leading to symptoms, especially dyspnea, and may eventually lead to respiratory failure (Coultas, et al. 1994). Many ILDs typically are distributed in the peripheral, subpleural regions of the lung (Desai, et al. 2004, Wells, et al. 1993). The superficial distribution of lung fibrosis is especially suited for LUSWE. Diagnosis of lung fibrosis can be difficult, especially early in the disease course, because the symptoms are nonspecific (most commonly shortness of breath and a dry cough) (Loscalzo 2010, Mathis 2008, Sperandeo, et al. 2009). High-resolution computed tomography (HRCT) is the clinical standard for diagnosing lung fibrosis (Mathieson, et al. 1989, Verschakelen 2010), but it substantially increases radiation exposure for patients. Various HRCT scanning techniques were proposed to reduce the dose (Mayo 2009). Lung fibrosis results in stiffened lung tissue. However, HRCT does not directly measure lung stiffness.
Our previous papers were to demonstrate the feasibility of LUSWE for assessing ILD by comparing the measurements between the patients and healthy control subjects. After baseline testing, these patients are followed up with LUSWE testing when they return for routine clinical appointments. The purpose of this study is to evaluate the change of surface wave speed in the follow-up of LUSWE and correlate it with clinical scores based on pulmonary function test (PFT) and high resolution computed tomography (HRCT) so as to validate the use of LUSWE for tracking the disease progression.
MATERIALS AND METHODS
Lung ultrasound surface wave elastography (LUSWE)
In LUSWE, a 0.1s harmonic vibration at a frequency is generated on the skin of the chest wall in an intercostal space (Figure 1a). The resulting wave propagation at that frequency travels through the intercostal muscle and propagates on the surface of the lung. The wave motions on the selected locations on the lung surface are noninvasively measured using our ultrasound-based method (Zhang, et al. 2017). The phase change with distance of the harmonic wave propagation on the lung surface is analyzed, and from which the surface wave speed is measured,
| (1) |
where Δr is the distance of two measuring locations, Δϕ is the wave phase change over distance, and f is the frequency.
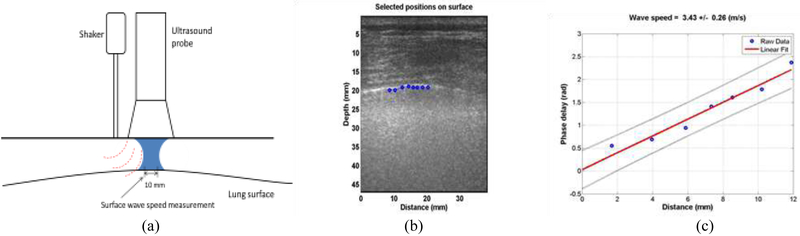
Figure 1.
(a) In LUSWE, a 0.1s harmonic vibration at a frequency is generated on the skin of the chest wall in an intercostal space. The resulting wave propagation at that frequency travels through the intercostal muscle and propagates on the surface of the lung. The wave motions on the selected locations on the lung surface are noninvasively measured using our ultrasound-based method. (b) LUSWE analysis for a patient subject. The wave motions are measured at eight locations on the lung surface. (c) The wave phase delay of the remaining locations, relative to the first location, is used to measure the surface wave speed.
The measurement of wave speed can be improved by using multiple phase change measurements over distances. The regression of the phase change Δϕ with distance Δr can be obtained by “best fitting” a linear relationship between them, and the equation is
| (2) |
where Δϕ denotes the value of Δϕ on the regression for a given distance of Δr, and α is the regression parameter.
The surface wave speed can be estimated by
| (3) |
where csr is the estimation of wave speed from the regression analysis.
Measurement of lung surface wave speed is noninvasive. Representative LUSWE analysis for a patient is shown in Figure 1(b). The wave motions are measured at eight locations on the lung surface. The normal component of the lung surface motion can be analyzed by cross-correlation analysis of the ultrasound tracking beams (Hasegawa and Kanai 2006, Zhang, et al. 2018). In this study, eight locations over a length of approximately 10-12 mm on the lung surface were used to measure the normal component of the lung surface motion. The tissue motion is measured at these locations in response to the harmonic wave excitation on the chest wall. A high pulse repetition rate of 2000 pulse/s is used to detect tissue motion in response to the wave excitation at 100, 150, or 200 Hz. A Verasonics ultrasound system (Verasonics, Inc; Kirkland, WA) is used that collects up to a few thousand imaging frames per second by using a plane-wave pulse transmission method.
The surface wave speed on the lung is estimated by determining the change in wave phase with distance along the lung surface. The lung motion at the first location is measured and used as a reference. The wave phase delay of the lung motions at the remaining locations, relative to the reference at the first location, is used to measure lung surface wave speed. The surface wave speed is estimated by the phase changes simultaneously at the 8 locations. Figure 1(c) shows a representative wave speed at 100 Hz for the patient in the left second intercostal space. The surface wave speed was 3.43 ± 0.26 m/s (mean ± standard error from the regression analysis) at 100 Hz for the patient. The wave speed on the lung surface is determined by analyzing ultrasound data directly from the lung. Therefore, the wave speed measurement is local and independent of the location and amplitude of excitation.
Human study protocol
Human studies were approved by the Mayo Clinic Institutional Review Board (IRB). The subject is tested in a sitting position. The lung testing takes about 20-30 minutes. Both lungs of the subject are tested through six intercostal spaces. The upper anterior lungs are tested at the second intercostal space in the mid-clavicular line. The lower lateral lungs are tested at one intercostal space above the level of the diaphragm in the mid-axillary line. The lower posterior lungs are tested at one intercostal space above the level of the diaphragm in the mid-scapular line. Ultrasound imaging is used to identify the lungs and select appropriate intercostal spaces to measure the upper and lower lungs. A 0.1-second harmonic vibration is generated by the indenter of the handheld shaker (Model: FG-142, Labworks Inc., Costa Mesa, CA 92626, USA) on the chest wall. The excitation force from the indenter is much less than 1 Newton and the subject only feels a small vibration on his/her skin. The indenter of the handheld shaker is placed on the chest wall in an intercostal space. The ultrasound probe is positioned about 5 mm away from the indenter in the same intercostal space to measure the resulting surface wave propagation on the lung. An ultrasound probe L11-4 with a central frequency of 6.4 MHz is positioned about 5 mm away from the indenter in the same intercostal space to measure the resulting surface wave propagation on the lung. A Verasonics ultrasound system (Verasonics V1, Verasonics, Inc., Kirkland, WA 98034, USA) is used in this research. Images of the lung and skin are acquired by compounding 11 successive angles at a pulse repetition frequency (PRF) of 2 kHz. The lung is tested at total lung capacity when the subject takes a deep breath and holds for a few seconds. The surface wave speeds are measured at three excitation frequencies of 100 Hz, 150 Hz, or 200 Hz. Three measurements are performed at each location and at each frequency. A small tissue motion in tens of μm is enough for sensitive ultrasound detection of the generated tissue motion. The 100 Hz wave motion is stronger than those of higher frequency waves. The higher frequency waves have smaller wave length but decay more rapidly over distance than the lower frequency waves. The frequency ranges chosen in this study consider the wave motion amplitude, spatial resolution, and wave attenuation.
Patient’s baseline and follow up tests
Each participant completed an informed consent form. Patients were enrolled in this research based on their clinical diagnoses. 91 patients with ILD were enrolled from Mayo Clinic Departments of Rheumatology and Pulmonary and Critical Care Medicine. Patients’ mean age was 62.4 ± 13.0 years (range 20-85, 39 male and 52 female). These patients were confirmed ILD patients with clinical assessments together with pulmonary function tests (PFT) and high resolution CT scans. These ILD patients also had various diseases including systemic sclerosis (SSc) or scleroderma, rheumatoid arthritis, connective tissue disease, idiopathic pulmonary fibrosis, anti-synthetase, Sjögren’s syndrome, polymyositis, and systemic lupus erythematosus. We have performed follow-up tests on 52 patients. In this paper, we are analyzing the baseline and follow up tests for these 52 patients. Patients’ mean age was 63.1 ± 12.0 years (range 20-85, 23 male and 29 female). The follow up interval was 9.2 ± 3.5 months depending on each patient’s return appointment and availability.
Clinical assessment of disease progression
The disease progression of each patient was evaluated between the follow-up and the baseline test using a 7-point Likert scale that includes 3 grades of improvement (mild, moderate, marked), unchanged, and 3 grades of worsening (mild, moderate, marked) (Clements, et al. 2000). A numerical scale of (−3 −2 −1 0 1 2 3) is used to score the changes of disease for each patient. Therefore, a score of −2 means moderate improvement. Clinical scores were based on changes of pulmonary function tests. The TLC, FEV1 and DLCO percent predicted changes were used over 1 – 3 years depending on the data available. Other clinical findings such as HRCT, echocardiograms, and clinical assessments were also used to score the change of patient’s status.
Quantitative assessment of disease progression using LUSWE
The change of lung surface wave speed is analyzed,
| (4) |
where Cc is the change of surface wave speed, C1 is the surface wave speed measured at the baseline, and C2 is the surface wave speed measured at the follow up.
RESULTS
We have performed follow-up tests on 52 patients. In this paper, we analyze the changes of lung surface wave speed between the follow-up and the baseline tests. Patients’ mean age was 63.1 ± 12.0 years and the follow up interval was 9.2 ± 3.5 months. One patient was removed due to death and another patient was removed because there was only one set of data and we could not compare two time points. Most patients had relatively mild changes (score 1) or moderate changes (score 2). Four patients had marked changes (score 3) but the number of patients with score 3 is small and the four patients are removed from analysis.
Table 1(a) shows the surface wave speed measurements for a patient at the baseline for 100 Hz, 150 Hz and 200 Hz and through six intercostal spaces. The patient’s lung was tested on both sides. The right and left lungs are designated by letters R and L, respectively. The three intercostal spaces are designated by a number from 1 to 3. The upper anterior lung is designated by 1. The lower lungs at the lateral and posterior positions are designated by 2 and 3, respectively. Therefore, L1 represents the left anterior lung in the second intercostal space. Table 1(b) shows the surface wave speed measurements for the patient at the follow up test. The changes of surface wave speeds between the follow up and the baseline tests are shown in Table 1(c).
Table 1.
(a) Surface wave speeds at the baseline test for a patient. (b) Surface wave speeds at follow-up testing for same patient. (c) The changes of surface wave speeds between the second and first tests for the patient.
| 1st | 2nd | change [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Surface wave speed (m/s) | Surface wave speed (m/s) | Surface wave speed | ||||||||
| 100 Hz | R1 | 4.43 | 100 Hz | R1 | 3.51 | 100 Hz | R1 | −20.79 | ||
| R2 | 1.92 | R2 | 3.19 | R2 | 66.27 | |||||
| R3 | 3.89 | R3 | 3.62 | R3 | −6.99 | |||||
| L1 | 3.92 | L1 | 3.36 | L1 | −14.33 | |||||
| L2 | 2.53 | L2 | 3.03 | L2 | 19.94 | |||||
| L3 | 2.75 | L3 | 2.97 | L3 | 8.12 | |||||
| 150 Hz | R1 | 6.74 | 150 Hz | R1 | 4.57 | 150 Hz | R1 | −32.16 | ||
| R2 | 3.42 | R2 | 3.88 | R2 | 13.57 | |||||
| R3 | 3.50 | R3 | 5.29 | R3 | 51.41 | |||||
| L1 | 6.71 | L1 | 5.43 | L1 | −19.16 | |||||
| L2 | 3.66 | L2 | 3.95 | L2 | 7.69 | |||||
| L3 | 4.06 | L3 | 4.94 | L3 | 21.82 | |||||
| 200 Hz | R1 | 8.41 | 200 Hz | R1 | 5.99 | 200 Hz | R1 | −28.79 | ||
| R2 | 5.19 | R2 | 4.85 | R2 | −6.47 | |||||
| R3 | 6.43 | R3 | 6.68 | R3 | 3.82 | |||||
| L1 | 6.31 | L1 | 7.51 | L1 | 19.01 | |||||
| L2 | 5.35 | L2 | 5.32 | L2 | −0.60 | |||||
| L3 | 4.37 | L3 | 5.62 | L3 | 28.63 | |||||
| (a) | (b) | (c) | ||||||||
Table 2 is similar to Table 1(c) but with color coded. The red color means increase of wave speed but the blue color shows the decrease of wave speed. This table presents the change of wave speed for each frequency and each location. The average global changes of the surface wave speed for all six intercostal spaces are respectively, 8.70 %, 7.20%, and 2.60% at 100 Hz, 150 Hz, and 200 Hz. The average global change of the surface wave speed is 6.17% for six intercostal spaces and three frequencies.
Table 2.
The regional changes of surface wave speeds in percentage (%) for each intercostal space with color coded. The average global changes of the surface wave speed for all six intercostal spaces are respectively, 8.70 %, 7.20%, and 2.60% at 100 Hz, 150 Hz, and 200 Hz. The average global change of the surface wave speed is 6.17% for six intercostal spaces and three frequencies.
| R1 | R2 | R3 | L1 | L2 | L3 | |
|---|---|---|---|---|---|---|
| 100 Hz | −20.79 | 66.27 | −6.99 | −14.33 | 19.94 | 8.12 |
| 150 Hz | −32.16 | 13.57 | 51.41 | −19.16 | 7.69 | 21.82 |
| 200 Hz | −28.79 | −6.47 | 3.82 | 19.01 | −0.60 | 28.63 |
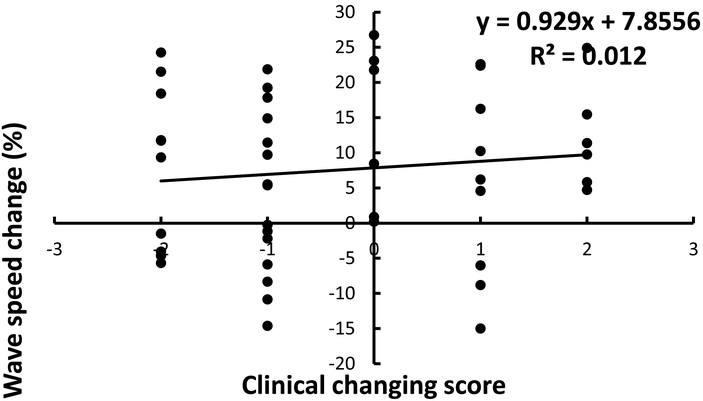
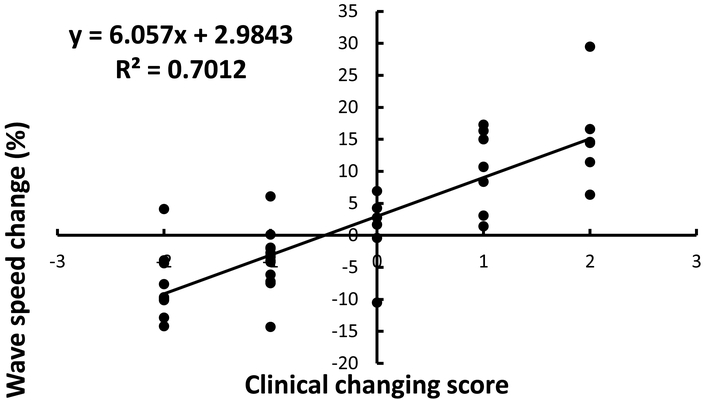
Figure 2 shows the correlation of the average global changes of wave speed for the six locations and three frequencies with clinical scores. Figure 3 shows the correlation of the average global changes of wave speed for the four locations of lateral and posterior lung zones (R2, R3, L2, L3) and three frequencies with clinical scores. It can be seen that the correlations between the average global changes of wave speed and clinical scores for the lower lateral and posterior lung zones (R2 = 0.701) was better than that of six lung zones (R2 = 0.012). The slopes of the curve between wave speed change and clinical assessment were 6.057 for the lower lung zones and 0.929 for all lung zones.
Figure 2.
Correlation of the average global changes of wave speed for the six locations and three frequencies with clinical scores.
Figure 3.
Correlation of the average global changes of wave speed for the four locations of lateral and posterior lung zones (R2, R3, L2, L3) and three frequencies with clinical scores.
DISCUSSION
LUSWE is a safe and noninvasive technique for measuring the surface wave speed of lung. In LUSWE, the surface wave propagation on the superficial lung tissue is safely generated by using a gentle mechanical harmonic vibration on the skin of chest wall. Diagnostic ultrasound is only used for detection of wave propagation along the lung. Therefore, LUSWE is safe for both generation and detection of lung surface wave propagation (Zhang, et al. 2017, Zhang, et al. 2018). This technique can be also safely used for noninvasive measurement of elastic properties of eye (Sit, et al. 2018, Zhou, et al. 2017). Currently, most shear wave elastography (SWE) techniques use ultrasound radiation force (URF) to generate tissue motion. However, URF may not be applied to some tissues such as the lung because the relatively high-intensity ultrasound energy may cause alveolar hemorrhage or lung injury (Zachary, et al. 2006).
Our results showed that the correlations of the average global changes of wave speed for four (lateral and posterior) lung portions with clinical scores was better than that for six (anterior, lateral and posterior) lung portions. This is important because the lung fibrosis affects more on the lower lobes of lung. This is in agreement with our previous findings that LUSWE was less sensitive for discriminating normal from abnormal lung at the apices (Clay, et al. 2018). There was actually more “normal” lung as quantitatively assessed in subjects with ILD at the upper lung zones when compared with healthy controls. This may reflect compensatory hyperinflation of the upper lung as a reaction to basilar-predominant fibrosis. This makes the argument that the lower lateral and lower posterior lung zones may be best to assess for the presence of ILD.
Diagnosis of lung fibrosis can be difficult, especially early in the disease course, because the symptoms are nonspecific (most commonly shortness of breath and a dry cough) (Loscalzo 2010, Mathis 2008, Sperandeo, et al. 2009). Current diagnostic tools include medical history and physical examination, chest radiography, high-resolution computed tomography (HRCT), and pulmonary function tests (PFTs) (Ruppel 2009). The findings of physical examinations are usually nonspecific. ILD may be first suspected after an abnormal chest radiograph, but in most cases, the radiographs also show nonspecific (nondiagnostic) findings. HRCT is the clinical standard for diagnosing lung fibrosis (Mathieson, et al. 1989, Verschakelen 2010), but it substantially increases radiation exposure for patients, even when using various techniques to reduce the dose (Mayo 2009). In addition, the potential for frequent HRCT use is limited by its expense. Most ILDs are typically distributed in the peripheral, subpleural regions of the lung (Desai, et al. 2004, Wells, et al. 1993). LUSWE provides a noninvasive and safe method for measuring superficial lung tissues for assessing lung fibrosis.
Systemic progression of tissue fibrosis most often occurs early in the disease course, and during that time, close monitoring for signs predicting severe organ damage is necessary (Steen and Medsger 2000). Providing effective treatment for patients with tissue fibrosis currently is challenged by the lack of clinical methods that accurately and reproducibly assess fibrosis progression (Lott and Girardi 2011). In this research, we propose a method to measure the changes of lung surface wave speed for assessing the progression of lung fibrosis. We demonstrated that the global changes of lung surface wave speed correlated with clinical assessment of disease progression. Compared with clinical assessment, LUSWE provides quantitative measurements of changes of lung surface wave speed. In addition, LUSWE can provide these changes for different intercostal spaces. Local measurements at each intercostal space may provide sensitive information about disease progression. Precise measures of lung improvement or deterioration are important, particularly in the first few years, to determine whether patients are 1) receiving the best available treatment to suppress the fibrotic process and 2) avoiding toxicities and adverse effects of medications, which can include immunosuppression. We will continue to improve this method for assessing disease progression and also assessing patient’s response to treatment.
We have previously proposed a concept of start frequency for generating the surface waves in a finite phantom structure (Zhang 2016). It was shown that different standing wave modes were generated below the start frequency because of wave reflection. However, the pure symmetric surface waves were generated from the excitation above the start frequency. Our experiences on multiple tissues suggested us to measure the wave speed starting 100 Hz. Sometimes, the 50 Hz may generate standing wave due to wave reflection. Also, the wavelength of the 50 Hz is relative large which needs longer distance to accurately measure the wave speed. In various situations, we measured the wave speed up to 400 Hz. However, the wave decays very quickly over a few millimeters for high frequencies. Therefore, the current frequency range is standardized for this larger patient study.
The LUSWE was performed through intercostal spaces. LSWE generates a vibration on the chest between 2 ribs and detects the surface wave propagation in parallel with the ribs. Therefore, the induced surface wave propagation on the lung should not be affected by the ribs. However, a directional filter technique was used to remove reflected waves and analyze wave propagation along the lung surface. Physiologic motions such as heart beat and respiration may affect the measurements; however, these motions are typically of low frequency and were removed during data processing using bandpass filter.
In this research, we provide surface wave speeds at 100 Hz, 150 Hz, and 200 Hz. The elasticity and viscosity of the lung can be estimated from the wave speed dispersion with frequency. However, lung mass density is unknown for fibrotic lungs. Also, lung density is dependent on the pulmonary pressure. In this research, a subject is tested at total lung capacity (TLC) when taking a deep breath and holding. In a paper using an x-ray technique (Garnett, et al. 1977), lung density was averaged to be 0.32 g/cm3 for healthy lungs. The measurements were made with the patient sitting either on a chair or in bed and breathing quietly. Lung density was 0.33-0.93 g/cm3 for patients with pulmonary congestion and edema. In an ex vivo study on sheep lungs (Jahed, et al. 1989), lung density of 0.19-0.26 g/cm3 was used. We expect that the lung density of ILD would be higher than that of healthy lungs. However, there are no data of lung density for ILD patients. Lung mass density is directly associated with lung pathology. Computed tomography (CT) is the major clinical imaging modality for assessing various lung diseases. The mechanism of CT is based on the changes of tissue mass density, but the CT system uses the Hounsfield unit (HU) and not lung density to image the lung. We are developing a deep neural network (DNN) model to predict lung mass density on lung phantoms based on the LUSWE measurements (Zhou and Zhang 2018). We will study how to develop and apply the DNN models for the patient’s data.
LUSWE may be useful for assessing other lung diseases. Pulmonary edema is a fundamental feature of congestive heart failure and inflammatory conditions such as acute respiratory distress syndrome (Picano and Pellikka 2016). The presence of extravascular lung water (EVLW) predicts a worse prognosis in critically ill patients (Sakka, et al. 2002) and increased risk of death or heart failure readmission (Coiro, et al. 2015). The features of “B-lines” are used to evaluate pulmonary edema (Picano, et al. 2006) and congestive heart failure (Cardinale, et al. 2014). However, analysis of B-line artifacts is qualitative and relies on visual interpretation which is subject to inter-operator variability (Corradi, et al. 2016). We have started to evaluate LUSWE for assessing EVLW on patients with heart failure.
CONCLUSION
LUSWE was used to measure the changes of lung surface wave speeds at 100 Hz, 150 Hz and 200 Hz through six intercostal lung spaces for 52 patients with ILD. The disease progression of each patient was evaluated clinically between the follow-up and the baseline tests using a 7-point Likert scale that includes 3 grades of improvement (mild, moderate, marked), unchanged, and 3 grades of worsening (mild, moderate, marked). This study demonstrates correlations between the changes of lung surface wave speed and clinical assessments. The changes of lung surface wave speed at lower lateral and posterior lung portions showed good correlation with clinical assessments. LUSWE provides quantitative global and reginal changes of lung surface wave speed that may be useful for quantitative assessment of disease progression of ILD.
Acknowledgment
This study is supported by NIH R01HL125234 from the National Heart, Lung, and Blood Institute. We thank Mrs. Jennifer Poston for editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barillari A, Franco FD, Colonna F Chest ultrasound helps to diagnose pulmonary consolidations in pediatric patients. J Medical Ultrasound 2011; 19:27–31. [Google Scholar]
- Cardinale L, Priola AM, Moretti F, Volpicelli G. Effectiveness of chest radiography, lung ultrasound and thoracic computed tomography in the diagnosis of congestive heart failure. World J Radiol 2014; 6:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay R, Bartholmai BJ, Zhou B, Karwoski R, Peikert T, Osborn T, Rajagopalan S, Kalra S, Zhang X. Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography: A Novel Technique With Clinicoradiologic Correlates. J Thorac Imaging 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, Wigley F, Weisman M, Barr W, Moreland L, Medsger TA Jr., Steen VD, Martin RW, Collier D, Weinstein A, Lally E, Varga J, Weiner SR, Andrews B, Abeles M, Furst DE. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum 2000; 43:2445–54. [DOI] [PubMed] [Google Scholar]
- Coiro S, Rossignol P, Ambrosio G, Carluccio E, Alunni G, Murrone A, Tritto I, Zannad F, Girerd N. Prognostic value of residual pulmonary congestion at discharge assessed by lung ultrasound imaging in heart failure. Eur J Heart Fail 2015; 17:1172–81. [DOI] [PubMed] [Google Scholar]
- Corradi F, Brusasco C, Vezzani A, Santori G, Manca T, Ball L, Nicolini F, Gherli T, Brusasco V. Computer-Aided Quantitative Ultrasonography for Detection of Pulmonary Edema in Mechanically Ventilated Cardiac Surgery Patients. Chest 2016; 150:640–51. [DOI] [PubMed] [Google Scholar]
- Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994; 150:967–72. [DOI] [PubMed] [Google Scholar]
- Desai SR, Veeraraghavan S, Hansell DM, Nikolakopolou A, Goh NS, Nicholson AG, Colby TV, Denton CP, Black CM, du Bois RM, Wells AU. CT features of lung disease in patients with systemic sclerosis: comparison with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Radiology 2004; 232:560–7. [DOI] [PubMed] [Google Scholar]
- Garnett ES, Webber CE, Coates G, Cockshott WP, Nahmias C, Lassen N. Lung density: clinical method for quantitation of pulmonary congestion and edema. Can Med Assoc J 1977; 116:153–4. [PMC free article] [PubMed] [Google Scholar]
- Hakimisefat B, Mayo PH Lung ultrasonography. The Open Critical Care Medicine Journal 2010; 3:21–25. [Google Scholar]
- Hasegawa H, Kanai H. Improving accuracy in estimation of artery-wall displacement by referring to center frequency of RF echo. IEEE Trans Ultrason Ferroelectr Freq Control 2006; 53:52–63. [DOI] [PubMed] [Google Scholar]
- Jahed M, Lai-Fook SJ, Bhagat PK, Kraman SS. Propagation of stress waves in inflated sheep lungs. J Appl Physiol 1989; 66:2675–80. [DOI] [PubMed] [Google Scholar]
- Loscalzo J 2010. Harrison's Pulmonary and Critical Care Medicine. 17 edn. New York: Mc Graw Hill Medical. [Google Scholar]
- Lott JP, Girardi M. Practice gaps. The hard task of measuring cutaneous fibrosis. Arch Dermatol 2011; 147:1115–6. [DOI] [PubMed] [Google Scholar]
- Mathieson JR, Mayo JR, Staples CA, Muller NL. Chronic diffuse infiltrative lung disease: comparison of diagnostic accuracy of CT and chest radiography. Radiology 1989; 171:111–6. [DOI] [PubMed] [Google Scholar]
- Mathis G, Lessnau KD Atlas of Chest Sonography. Berlin: Springer-Verlag, 2008. [Google Scholar]
- Mayo JR. CT evaluation of diffuse infiltrative lung disease: dose considerations and optimal technique. J Thorac Imaging 2009; 24:252–9. [DOI] [PubMed] [Google Scholar]
- Noble VE. Think ultrasound when evaluating for pneumothorax. J Ultrasound Med 2012; 31:501–04. [DOI] [PubMed] [Google Scholar]
- Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr 2006; 19:356–63. [DOI] [PubMed] [Google Scholar]
- Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J 2016; 37:2097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppel GL. Manual of Pulmonary Function Testing. St. Louis: Mosby Elsevier, 2009. [Google Scholar]
- Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest 2002; 122:2080–6. [DOI] [PubMed] [Google Scholar]
- Sit AJ, Kazemi A, Zhou B, Zhang X. Comparison of Ocular Biomechanical Properties in Normal and Glaucomatous Eyes Using Ultrasound Surface Wave Elastography. Investigative Ophthalmology & Visual Science 2018; 59:1218–18. [Google Scholar]
- Sperandeo M, Varriale A, Sperandeo G, Filabozzi P, Piattelli ML, Carnevale V, Decuzzi M, Vendemiale G. Transthoracic ultrasound in the evaluation of pulmonary fibrosis: our experience. Ultrasound Med Biol 2009; 35:723–9. [DOI] [PubMed] [Google Scholar]
- Steen VD, Medsger TA Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 2000; 43:2437–44. [DOI] [PubMed] [Google Scholar]
- Verschakelen JA. The role of high-resolution computed tomography in the work-up of interstitial lung disease. Curr Opin Pulm Med 2010; 16:503–10. [DOI] [PubMed] [Google Scholar]
- Volpicelli G Lung sonography. J Ultrasound Med 2013; 32:165–71. [DOI] [PubMed] [Google Scholar]
- Wells AU, Hansell DM, Rubens MB, Cullinan P, Black CM, du Bois RM. The predictive value of appearances on thin-section computed tomography in fibrosing alveolitis. Am Rev Respir Dis 1993; 148:1076–82. [DOI] [PubMed] [Google Scholar]
- Zachary JF, Blue JP Jr., Miller RJ, Ricconi BJ, Eden JG, O'Brien WD Jr. Lesions of ultrasound-induced lung hemorrhage are not consistent with thermal injury. Ultrasound Med Biol 2006; 32:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X Identification of the Rayleigh surface waves for estimation of viscoelasticity using the surface wave elastography technique. The Journal of the Acoustical Society of America 2016; 140:3619–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Osborn T, Zhou B, Bartholmai B, Greenleaf JF, Kalra S. An ultrasound surface wave elastography technique for noninvasive measurement of surface lung tissue. The Journal of the Acoustical Society of America 2017; 141:3721–21. [Google Scholar]
- Zhang X, Osborn T, Zhou B, Meixner D, Kinnick RR, Bartholmai B, Greenleaf JF, Kalra S. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans Ultrason Ferroelectr Freq Control 2017; 64:1298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou B, Clay R, Zhou J, Osborn T, Bartholmai B, Greenleaf JF, Kalra S. Lung ultrasound surface wave elastography for assessing interstitial lung disease. The Journal of the Acoustical Society of America 2018; 143:1776–76. [Google Scholar]
- Zhang X, Zhou B, Kalra S, Bartholmai B, Greenleaf J, Osborn T. An Ultrasound Surface Wave Technique for Assessing Skin and Lung Diseases. Ultrasound Med Biol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou B, Miranda AF, Trost LW. A Novel Noninvasive Ultrasound Vibro-elastography Technique for Assessing Patients With Erectile Dysfunction and Peyronie Disease. Urology 2018; 116:99–105. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou B, Osborn T, Bartholmai B, Greenleaf J, Sanjay K. 2017. Assessment of interstitial lung disease using lung ultrasound surface wave elastography. Ultrasonics Symposium (IUS), 2017 IEEE International, 1–4. [Google Scholar]
- Zhou B, Sit AJ, Zhang X. Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures. Ultrasonics 2017; 81:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhang X. Lung mass density analysis using deep neural network and lung ultrasound surface wave elastography. Ultrasonics 2018; 89:173–77. [DOI] [PMC free article] [PubMed] [Google Scholar]