Abstract
Background:
Late Life Depression (LLD) has been associated with alterations in intrinsic functional networks, best characterized in the Default Mode Network (DMN), the Cognitive Control Network (CCN), and the Salience Network (SN). However these findings often derive from small samples and it is not well understood how network findings relate to clinical and cognitive symptomatology.
Methods:
We studied 100 older adults (n=79 with LLD, n=21 nondepressed) and collected resting state functional MRI, clinical measures of depression, and performance on cognitive tests. We selected canonical network regions for each intrinsic functional network (DMN, CCN, and SN) as seeds in seed-to-voxel analysis. We compared connectivity between depressed and non-depressed groups and correlated connectivity with depression severity among depressed subjects. We then investigated whether the observed connectivity findings were associated with greater severity of common neuropsychiatric symptoms or poorer cognitive performance.
Results:
LLD was characterized by decreased DMN connectivity to the frontal pole, a CCN region (Wald χ2=22.33, P<0.001). No significant group differences in connectivity were found for the CCN or SN. However, in the LLD group increased CCN connectivity was associated with increased depression severity (Wald χ2>20.14, p<0.001), greater anhedonia (Wald χ2=7.02, p=0.008) and fatigue (Wald χ2=6.31, p=0.012), and poorer performance on tests of episodic memory (Wald χ2>4.65, p<0.031), executive function (Wald χ2=7.18, p=0.007), and working memory (Wald χ2>4.29, p<0.038).
Conclusions:
LLD is characterized by differences in DMN connectivity, while CCN connectivity is associated with LLD symptomology, including poorer performance in several cognitive domains.
Keywords: Aging, Default Mode Network, Cognitive Control Network, Functional Connectivity, Cognition, Geriatric
INTRODUCTION
Late Life Depression (LLD), or Major Depressive Disorder (MDD) in adults 60 years or older, is a clinically heterogeneous syndrome characterized by both neuropsychiatric symptoms and multi-domain cognitive deficits (1). Converging evidence supports intrinsic brain network dysfunction as an underlying neural mechanism contributing to the pathogenesis of LLD (2). Specifically, adult MDD and LLD are associated with alterations in function and resting state connectivity in intrinsic brain networks, best characterized in the Default Mode Network (DMN), Cognitive Control Network (CCN), and Salience Network (SN) (3). However these findings often derive from small samples and it is not well understood how network differences relate to clinical and cognitive symptomatology (4).
The DMN is a set of regions exhibiting increased activity during rest and decreased activity during externally-driven attention-demanding tasks (5). The DMN is related to spontaneous or self-generated cognition, with its anterior hub contributing to self-referential processing and emotion regulation of present-states and its posterior hub being associated with episodic memory retrieval and scene construction (6, 7). Although DMN activity decreases during externally-directed attention, in MDD DMN activity is higher when assessing external stimuli (8) and during maladaptive ruminative self-focus (9). In both adult MDD and LLD, functional connectivity within the anterior and posterior hubs is increased, (10–12), but there is reduced connectivity between the anterior and posterior DMN (13). DMN connectivity to other networks including the CCN is increased in LLD (4).
The CCN is engaged during externally-directed cognitive tasks (14) and involved in attentional control, emotional regulation (15) and higher-order functions including decision making and conflict resolution (14). In MDD, reduced CCN activity is observed at rest, in response to negative stimuli (16) and during attempts to regulate emotional responses (17). Although not universally observed (18), most studies in MDD and LLD demonstrate reduced within-network connectivity (3, 10, 11). Additionally, CCN connectivity to the SN is increased in LLD and associated with greater depression severity (4).
The SN facilitates switching between the DMN and CCN as needed to shift attention from internal states to external stimuli. The SN is activated in response to various salient stimuli (19) and includes the insula, the dACC, and the amygdala. In MDD, SN regions are generally over-responsive to affective challenges, particularly negatively valenced stimuli (20). MDD is characterized by altered SN connectivity of the amygdala and insula with frontal and ACC regions, but also with regions of the CCN and DMN (4, 21, 22).
The purpose of this study was to examine differences in intrinsic functional network connectivity in LLD. Based on past work, in our primary analyses we hypothesized that LLD and greater depression severity would be associated with higher DMN connectivity and with lower CCN connectivity. Exploratory analyses examined the SN. In further exploratory analyses, we hypothesized that connectivity findings would be clinically meaningful and associated with greater severity of neuropsychiatric symptoms or poorer cognitive performance. Because cognitive impairment in MDD and LLD has been hypothesized to be related to abnormal connectivity of the CCN and DMN (8, 23), we further examined whether connectivity-cognition relationships differed between the depressed and nondepressed groups.
METHODS
Participants
Participants were recruited at Vanderbilt University Medical Center from clinical referrals and community advertisements as part of three research studies with common entry criteria. Core entry criteria focused on adults aged 60 years or older with a current DSM-IV-TR diagnosis of Major Depressive Disorder and a Montgomery-Asberg Depression Rating Scale (MADRS) (24) score of ≥15. Participants were also cognitively intact without a clinical diagnosis of mild cognitive impairment or dementia, plus a Montreal Cognitive Assessment (MoCA) (25) score of ≥ 24 or Mini Mental State Exam (MMSE) score ≥ 24.
Common exclusion criteria included: 1) Current or past diagnoses of other psychiatric disorders, except for anxiety symptoms occurring during a depressive episode; 2) History of alcohol or drug abuse over last three years; 3) Acute grief; 4) Acute suicidality; 5) Current or past psychosis; 6) Primary neurological disorders including dementia; 7) Current psychotherapy; 8) ECT in the last 2 months; 9) Contraindications to MRI.
For two of the three studies, entry criteria specified no antidepressant use in the last two weeks. Antidepressant medications were allowed in one study, with 9 of 14 depressed participants taking antidepressant monotherapy at the time of MRI. For that study, participants taking antidepressant monotherapy needed to be on a stable dose for at least eight weeks.
Eligible nondepressed participants adhered to similar age requirements and exclusion criteria. They had no lifetime history of psychiatric disorders and no history of psychotropic medication use, psychotherapy, or brain stimulation treatment.
All studies were approved by the Vanderbilt University Institutional Review Board. All participants provided written informed consent.
Clinical Assessments
Diagnostic and Medical Assessments.
The Mini-International Neuropsychiatric Interview (MINI, version 5.0) (26) assessed current and lifetime depression and other psychiatric disorders. Diagnoses and duration of current episode were confirmed by clinical interview with a geriatric psychiatrist. Antidepressant treatment in the current episode was assessed using the Antidepressant Treatment History Form (ATHF) (27). Medical burden was quantified using the clinician-rated Cumulative Illness Rating Scale (CIRS) (28).
Mood and Neuropsychiatric Assessments.
MADRS was assessed by the study psychiatrist on the day of MRI. For two studies (N=56), additional neuropsychiatric symptoms were assessed in depressed participants through self-report questionnaires. Symptom domains and questionnaires included: Anhedonia, using the Snaith-Hamilton Pleasure Scale (SHAPS) (29); Anxiety using the Penn State Worry Questionnaire (PSWQ) (30); Apathy using the Apathy Evaluation Scale (AES) (31); Fatigue using the Fatigue Severity Scale (FSS) (32); and Rumination using the Ruminative Response Scale (RRS) that includes a total score and subscales for depressive rumination, reflective rumination, and brooding rumination (33).
Cognitive Assessments.
83 subjects (62 depressed, 21 nondepressed) completed paper-and-pencil neuropsychological test batteries. The specific tests probed specific cognitive domains affected by aging or impaired in LLD (34, 35). Tests in each domain included:
Episodic Memory: Word List Memory Recall (immediate and delayed), Paragraph Recall test, Constructional Praxis test, and Benton Visual Retention Test (BVRT).
Executive Function: Symbol-Digit Modality Test (SDMT), Trail-Making Test Part B, and the Stroop test color-word interference condition.
Working Memory: Digits Forwards and Digits Backwards.
Processing Speed: Stroop test color naming condition, Trail-Making Test Part A.
Language Processing: Stroop test word reading condition and the Shipley vocabulary test.
MRI Acquisition
Participants were scanned on a research-dedicated 3.0T Philips Achieva whole-body scanner (Philips Medical Systems, Best, The Netherlands) using body coil radiofrequency transmission and a 32-channel head coil for reception. Structural imaging included a whole-brain T1- weighted MPRAGE image with TR = 8.75ms, TE = 4.6ms, flip angle=9 degrees, and spatial resolution = 0.89 × 0.89 × 1.2 mm3 plus a FLAIR T2-weighted imaging conducted with TR = 10000ms, TE = 125ms, TI = 2700ms, flip angle = 90 degrees, and spatial resolution = 0.7 × 0.7 × 2.0mm3. Resting state functional MRI was conducted eyes-open, using parameters of TR = 2000ms, TE = 35ms, flip angle = 77 degrees, spatial resolution = 2.75 × 2.75 × 3.7mm3, and 35 axial slices.
Functional MRI Analyses
Resting fMRI images were preprocessed using the CONN toolbox (version 15.g) in SPM 12, including: realignment of the functional runs and correction for head motion, coregistration of functional and anatomical images for each participant, normalization of the anatomical and functional images to the standard MNI template, and spatial smoothing with a Gaussian filter (6 mm at full width half maximum). Motion artifacts were further detected by applying the Artifact Detection Toolbox (ART) as implemented in CONN. We used a displacement threshold of 0.9 mm and a global signal threshold of Z = 5. In order to effectively mitigate the effects of head motion, denoising in CONN was conducted for white matter (5 components extracted) and cerebral spinal fluid (5 components extracted) signal, and realignment parameters (36) with outlier volumes identified by ART. We retained all participants with greater than 5 minutes of scan time after excluding outlier volumes. The resulting BOLD time series were band-pass filtered (0.01–0.1 Hz) to further reduce noise and increase sensitivity.
We selected canonical network regions for each intrinsic functional network to use as key network seed ROIs: 1) DMN seed (posterior cingulate cortex, PCC (37); 2) CCN seed (left dorsolateral prefrontal cortex, left dLPFC (14); 3) SN seed (right anterior insula (14). First-level whole brain seed-to-voxel individual subject functional connectivity maps were created for each network ROI seed. We then conducted two second-level analyses. First, a second-level two-sided two-sample t-test examined differences in functional connectivity maps between diagnostic groups utilizing FDR=0.05 and peak significance threshold of uncorrected p<0.001. Second, a second-level linear regression examined the relationship of functional connectivity (both positive and negative connectivity) with depression severity (MADRS) among depressed subjects utilizing FDR=0.05 and peak significance threshold of uncorrected p<0.001. After identifying seed to cluster connectivity relationships using these methods, we extracted beta-values (a measure of functional connectivity) for those seed to cluster pairs for use in subsequent statistical analyses.
Structural MRI Analyses
White matter hyperintensity (WMH) volumes, findings on T2- weighted or FLAIR images related to cerebral ischemia, were measured using the Lesion Segmentation Toolbox (38). These analyses, identical to those previously described (39), were implemented through the VBM8 toolbox in SPM8 using the threshold of 0.3. This threshold was selected based on data from a sample dataset, where we compared segmented images with the native FLAIR image, examining a threshold range from 0 to 1 in 0.05 increments. In native space, each voxel on the T1 image is assigned as gray matter, white matter, or CSF. After bias-correction the FLAIR is co-registered to the T1 image. The toolbox initially creates a conservative binary WMH map based on outlier values across the T1 and FLAIR images. Next, a lesion-growth algorithm using Markov Random Fields modeling extends this conservative map to define the extent of the WMH. This lesion map is then used to calculate total cerebral WMH volume. We then applied FreeSurfer cortical parcellation of the T1 data to the WMH map, allowing us to calculate WMH volume for the frontal lobe.
Statistical Analysis
Statistical analyses examining demographic measures and extracted beta-value connectivity measures were conducted in SAS Studio 3.6 (Cary, NC, USA). Participant characteristics were summarized using mean (standard deviation) for continuous variables and N (%) for categorical variables and compared using two-tailed t-tests for continuous variables and chi-square test for categorical variables.
Seed-to-voxel relationships between functional connectivity, diagnosis of depression, and depression severity by MADRS were identified using CONN. After extracting the connectivity beta values, initial statistical models confirmed our second-level seed-to-voxel analyses testing for differences between diagnostic groups and relationships with depression severity. For group differences, we created a regression model with functional connectivity as the dependent variable and diagnostic group, age, sex, and medical morbidity by CIRS as the independent variables. For depression severity, using only the depressed group we created a regression model with MADRS as the dependent variable and functional connectivity, age, sex, and medical morbidity as the independent variables. We also examined whether the subsample of participants on antidepressant medications at time of MRI (N=9) influenced our findings. We re-ran the statistical models described above without those participants in order to determine whether they affected the results.
As WMH volume is associated with altered network functional connectivity (40, 41), we examined whether WMHs were associated with observed regional connectivity measures. We constructed regression models with functional connectivity as the dependent variable and whole brain or frontal lobe WMH volume as independent variables with additional covariates of age, sex, diagnostic group, and medical morbidity.
Final analyses examined the relationship between observed connectivity measures and clinical and cognitive measures. For the subset of depressed subjects with neuropsychiatric symptom data, we constructed models with each neuropsychiatric symptom as the dependent variable and functional connectivity, age, sex, medical morbidity, and MADRS score as independent variables. For the subset of subjects with neuropsychological test data, we examined how functional connectivity measures were related to cognitive performance. To test for group differences in the connectivity-cognitive performance relationships, we constructed models for each test with a group by connectivity (beta-value) interaction term, controlling for age, sex, medical morbidity, and education. For those cognitive tests without a significant interaction effect, we removed the interaction term and reran the model to test for primary effects of connectivity. If the interaction term was statistically significant, we conducted post-hoc analyses within each diagnostic group.
RESULTS
The study included 100 subjects, 79 depressed and 21 never-depressed elders. There were no significant differences between diagnostic groups in demographic measures or medical morbidity (Table 1). The sample was cognitively intact with a mean MMSE = 28.9 (SD = 1.31, range 26–30; N=86) and mean MOCA = 27.9 (SD = 1.46, range 25–30; N=14). There was no significant difference in MMSE score between diagnostic groups (t=0.82, df = 82, p=0.414). Per the ATHF, the sample could not overall be considered treatment-resistant, although there were exceptions as the score ranged from 0 to 18.
Table 1:
Demographics
| Depressed (n=79) | Nondepressed (n=21) | Test Statistic | P-value | |
|---|---|---|---|---|
| Age | 66.3 (5.9) | 68.3 (5.7) | t=1.41 | p=0.169 |
| Female | 27 (34) | 9 (43) | χ2=0.54 | p=0.461 |
| Education | 16.5 (2.3) | 16.4 (1.7) | t=0.24 | p=0.808 |
| MADRS | 27.3 (4.7) | 0.4 (0.7) | t=49.41 | p<0.001 |
| ATHF Score | 2.5 (3.0) | - | - | - |
| CIRS | 5.1 (3.1) | 4.4 (2.4) | t=1.16 | p=0.255 |
| WMH, total cerebral | 7.0 (13.2) | 3.9 (1.5) | t=1.67 | p=0.099 |
| WMH, frontal | 2.2 (4.9) | 1.0 (1.4) | t=1.94 | p=0.055 |
Data presented as mean (SD) for continuous variables and N (%) for categorical variables. Categorical variables compared using chi-square test with 1 degree of freedom. Analyses of continuous variables used pooled, two-tailed t-tests with 98 degrees of freedom, except for analyses of WMH that used Satterthwaite t-tests due to unequal variance. These analyses of total cerebral WMH had 84.9 degrees of freedom, while the frontal WMH comparison had 97.1 degrees of freedom. ATHF = antidepressant treatment history form, CIRS = Cumulative Illness Rating Scale, MADRS = Montgomery-Asberg Depression Rating Scale, WMH = white matter hyperintensity, volumes measured in milliliters
A subset of 83 subjects (N=62 depressed, N=21 nondepressed), completed neuropsychological testing. After controlling for covariates, the depressed group exhibited significantly poorer performance on measures of episodic memory, executive function, working memory, processing speed, and language processing (Table 2). However, in the depressed group, performance on cognitive tests was not associated with variability in depression severity except on the Digits Forward test (Table 2).
Table 2:
Cognitive Test Performance by Diagnostic Group and Relationship to Depression Severity
| Group Comparison | Relationship with Depression Severity | |||||
|---|---|---|---|---|---|---|
| Depressed (n=62) | Nondepressed (n=21) | Wald χ2 | p-value | Wald χ2 | p-value | |
| Episodic Memory | ||||||
| Word List Memory Recall | 7.5 (1.9) | 8.1 (1.7) | 5.32 | P=0.021 | 0.01 | P=0.926 |
| Paragraph Recall | 25.5 (7.8) | 32.5 (5.8) | 21.81 | P<0.001 | 0.14 | P=0.706 |
| Constructional Praxis | 8.8 (2.4) | 9.3 (2.1) | 3.37 | P=0.066 | 0.78 | P=0.378 |
| BVRT | 6.8 (1.7) | 7.2 (1.4) | 3.07 | P=0.080 | 0.07 | P=0.788 |
| Executive Function | ||||||
| SDMT | 42.0 (10.2) | 50.5 (8.4) | 30.79 | P<0.001 | 0.23 | P=0.632 |
| Trails B | 96.7 (54.2) | 68.9 (21.3) | 12.18 | P<0.001 | 0.00 | P=0.959 |
| Stroop-Color Word | 34.1 (8.5) | 43.3 (11.2) | 24.84 | P<0.001 | 2.19 | P=0.139 |
| Working Memory | ||||||
| Digits Forward | 8.5 (2.4) | 9.4 (1.9) | 2.48 | P=0.115 | 4.64 | P=0.031 |
| Digits Backward | 7.1 (2.2) | 8.2 (2.7) | 4.84 | P=0.028 | 0.23 | P=0.631 |
| Processing Speed | ||||||
| Stroop-Color | 63.3 (13.7) | 71.2 (10.2) | 14.71 | p<0.001 | 0.67 | P=0.413 |
| Trails A | 39.0 (32.6) | 32.1 (8.7) | 1.87 | P=0.171 | 0.02 | P=0.890 |
| Language Processing | ||||||
| Stroop-Word | 90.1 (15.4) | 98.0 (12.7) | 6.24 | P=0.013 | 0.07 | P=0.790 |
| Shipley | 32.6 (5.0) | 35.6 (2.4) | 8.75 | P=0.003 | 0.04 | P=0.839 |
“Group Comparison” analyses describe regression models with each cognitive test performance as the dependent variable and independent variables of age, sex, medical morbidity, education, and diagnostic group. This allowed us to test whether cognitive performance differed by group. “Relationship with Depression Severity” analyses examined regression models in the depressed group alone with cognitive test performance as the dependent variable and independent variables of age, sex, medical morbidity, education, and MADRS. This allowed us to test whether cognitive performance was a surrogate marker for depression severity. MADRS = Montgomery Asberg Depression Rating Scale, SDMT = Symbol Digit Modality Test, BVRT = Benton Visual Retention Test
Diagnostic Group Differences in Resting State Functional Connectivity and Relationship with Depression Severity
In whole-brain seed to voxel analyses, no CCN or SN regions exhibited statistically significant group differences in connectivity. Examining the DMN, depressed subjects exhibited lower positive resting functional connectivity between the PCC seed and a region in the left frontal pole (Figure 1A, Table 3). In models controlling for age, sex, and medical morbidity, depressed subjects continued to exhibit lower PCC-frontal pole connectivity (Wald χ2=22.33, p<0.001, Figure 1B), but PCC-frontal pole connectivity was not significantly associated with depression severity (Wald χ2=0.87, p=0.352, Figure 1C).
Figure 1. DMN Functional Connectivity Differences in Depressed Elders.
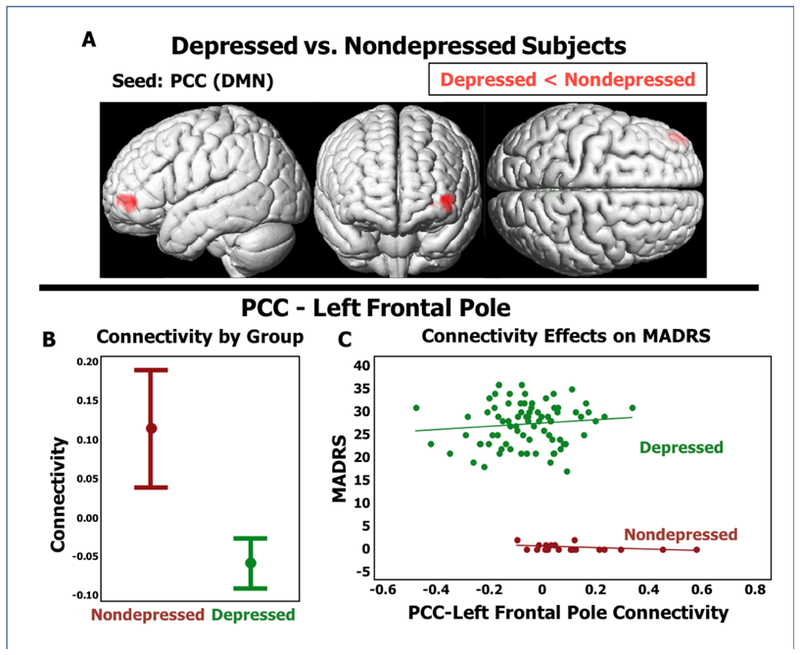
A) Resting functional connectivity pattern with lower connectivity in depressed subjects compared to nondepressed subjects using the posterior cingulate cortex (PCC) seed for the Default Mode Network. B) Comparison of 95% Confidence Intervals of beta-value between depressed and nondepressed diagnostic groups. C) X-axis is functional connectivity beta-value, Y-axis is MADRS. MADRS = Montgomery Asberg Depression Rating Scale.
Table 3:
Identified Functionally Connected Pairs
| Seed | MNI coordinates (x, y, z) | Cluster Size | Regions of Significant Clusters | p-value uncorrected |
|---|---|---|---|---|
| Depressed vs. Nondepressed | ||||
| PCC - Default Mode Network | −42, +48, −02 | 228 | Left Frontal Pole | P<0.001 |
| MADRS Regression in Depressed | ||||
| Left dlPFC - Cognitive Control Network | +10, +20, +28 | 338 | Bilateral Anterior Cingulate, Paracingulate Gyrus | P<0.001 |
| +12, +06, +60 | 296 | Bilateral Supplemental Motor Cortex, Right Superior Frontal Gyrus | P<0.001 | |
PCC = Posterior Cingulate Cortex, dlPFC = dorsolateral Prefrontal Cortex
In seed to voxel analyses in the depressed cohort (N=79), we did not observe any DMN or SN regions where functional connectivity was associated with MADRS score. In the CCN, MADRS score was positively associated with functional connectivity between the left dlPFC and two regions: the bilateral dorsal anterior cingulate cortex (dACC) and bilateral supplemental motor cortex (SMC) (Figure 2A, Table 3). In models controlling for age, sex, and medical morbidity, extracted functional connectivity measures between the left dlPFC and both regions continued to be significantly associated with depression severity (dACC: Wald χ2=20.14, p<0.001; SMC: Wald χ2=23.04, p<0.001, Figures 2C and 2E). When examining both depressed and nondepressed subjects, there was no longer a significant association between connectivity and depression severity. Hypothesizing that this may reflect a lack of diagnostic group differences between these regions, we found no statistically significant differences in pairwise connectivity measures between diagnostic groups (dACC: Wald χ2=3.35, p=0.067; SMC: Wald χ2=0.28, p=0.596, Figures 2B and 2D).
Figure 2. CCN Functional Connectivity Differences Associated with Depression Severity in Depressed Elders.
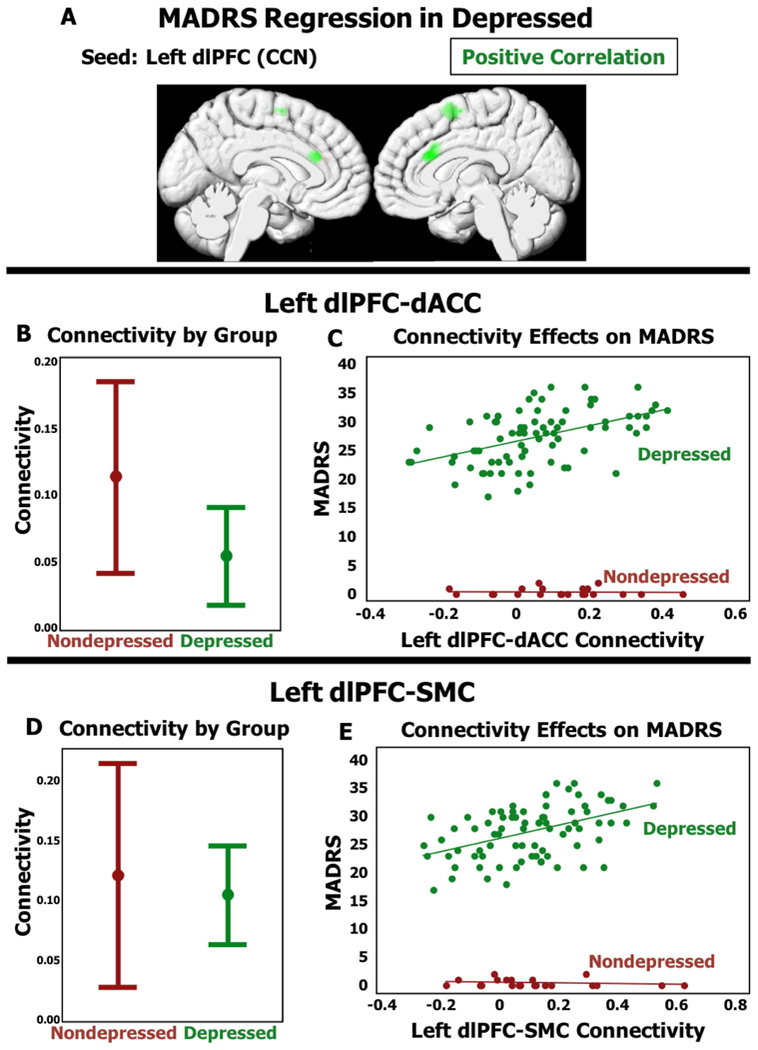
A) Resting functioal connectivity pattern associated with higher MADRS in depressed subjects using the Left dorsolateral prefrontal cortex (dlPFC) seed for the Cognitive Control Network. B and D) Comparison of 95% Confidence Intervals of beta-value between depressed and nondepressed diagnostic groups. C and E) X-axis is functional connectivity beta-value, Y-axis is MADRS. MADRS = Montgomery Asberg Depression Rating Scale.
Finally, we examined whether the subsample of participants on an antidepressant medication at time of scanning (N=9) influenced these findings. We reran the statistical models described above without those participants, and the results did not appreciably change. Subsequent analyses included all study participants.
Effects of White Matter Hyperintensities on Connectivity Measures
For the three identified functionally connected pairs (PCC-Left Frontal Pole, dlPFC-dACC, dlPFC-SMC) we examined whether total cerebral or frontal lobe white matter hyperintensity (WMH) volumes were related to extracted individual-level connectivity values (beta-value). One subject was an outlier with total brain WMH volume of 92.8 mL (7.2 SDs above the mean). When eliminated from our models (N=99), there was no significant effect of whole brain or frontal lobe WMH volume on pairwise connectivity.
Relationships between Connectivity Measures and Neuropsychiatric Symptoms
In the three identified functionally connected pairs (PCC-Left Frontal Pole, dlPFC-dACC, dlPFC-SMC), for the subset of 56 depressed subjects with available data, we constructed models controlling for age, sex, medical morbidity, and MADRS score while examining the relationship between connectivity and neuropsychiatric symptoms. The only statistically significant relationships were positive associations between dlPFC-SMC connectivity with anhedonia (SHAPS total score, Wald χ2=7.02, p=0.008, Figure 3A) and fatigue (FSS score, Wald χ2=6.31, p=0.012, Figure 3B). There were no significant connectivity-symptom associations for measures of worry, apathy, or rumination.
Figure 3. Functional Connectivity Relationship with Neuropsychiatric Symptoms.
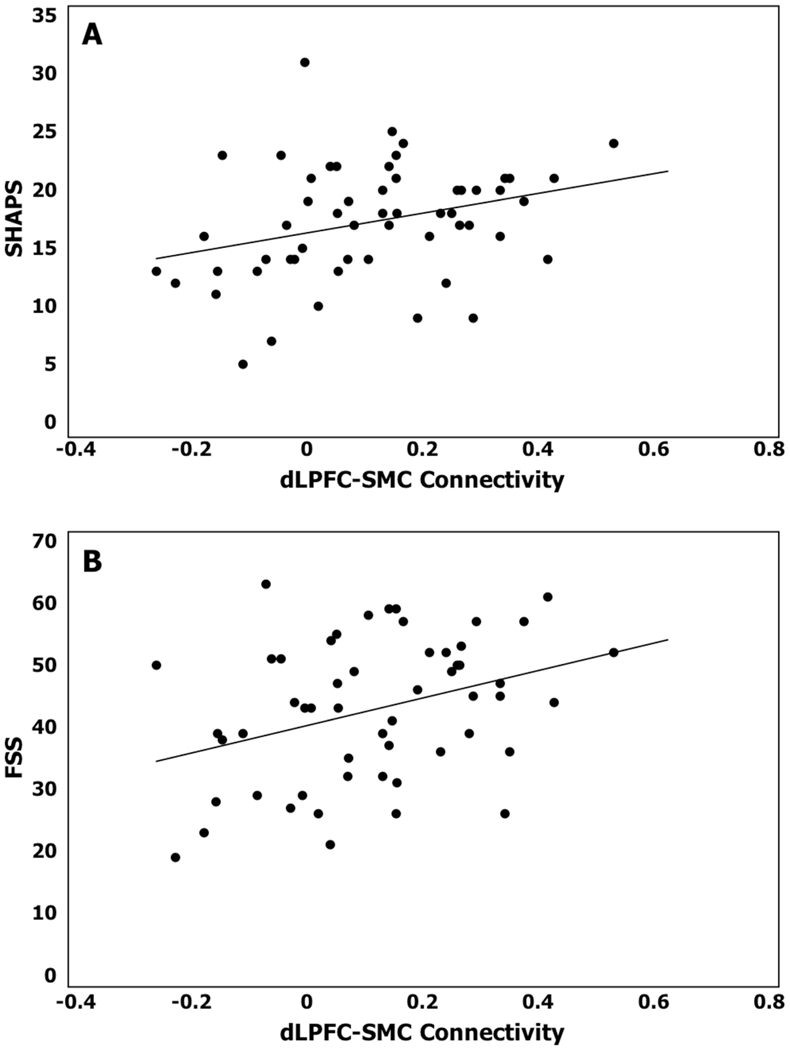
X-axis is functional connectivity beta-value for dlPFC-SMC, Y-axis is severity of specified neuropsychiatric symptom. Higher scores on both scales indicate greater symptom severity. SHAPS = Snaith-Hamilton Pleasure Scale, FSS = Fatigue Severity Scale.
Relationships between Connectivity Measures and Cognitive Performance
For subjects with neuropsychological data (62 depressed, 21 nondepressed), we examined relationships between pairwise connectivity and cognitive performance. To test for potential group differences in connectivity-performance relationships, we included a group–connectivity interaction term that was removed if not statistically significant.
PCC-left frontal pole connectivity exhibited a statistically significant interaction term only for Word List Memory Recall (Table 4, Figure 4A). However in post-hoc analysis, neither group on their own exhibited a significant functional connectivity-performance relationship. There were no statistically significant primary effects.
Table 4:
Group by Functional Connectivity Interactions Related to Cognitive Test Performance
| Functional Connectivity Pair | Cognitive Test | Group*FC Interaction | Post-Hoc Analysis: Correlation by Diagnostic Group | ||||
|---|---|---|---|---|---|---|---|
| Nondepressed | Depressed | ||||||
| Wald χ2 | p-value | Wald χ2 | p-value | Wald χ2 | p-value | ||
| PCC - Left Frontal Pole | Word List Memory Recall | 4.19 | 0.041 | 3.07 | 0.080 | 1.07 | 0.301 |
| Left dlPFC - dACC | SDMT | 7.18 | 0.007 | 18.35 | <0.001 | 0.45 | 0.501 |
| Word List Memory Recall | 5.89 | 0.015 | 0.20 | 0.656 | 14.26 | <0.001 | |
| Paragraph Recall | 10.82 | 0.001 | 9.14 | 0.003 | 6.20 | 0.013 | |
| Digits Forward | 5.35 | 0.021 | 5.62 | 0.018 | 0.19 | 0.6627 | |
| Left dlPFC - SMC | Paragraph Recall | 4.65 | 0.031 | 4.01 | 0.045 | 2.25 | 0.134 |
| Digits Forward | 4.29 | 0.038 | 1.29 | 0.256 | 3.14 | 0.076 | |
| Digits Backwards | 5.83 | 0.016 | 8.92 | 0.003 | 0.79 | 0.374 | |
Regression models with cognitive test performance as the dependent variable and independent variables of age, sex, medical morbidity, education, plus a diagnostic group by connectivity interaction term. This allowed us to test whether the relationship between connectivity and cognitive performance differed by group. Cognitive tests with significant group by functional connectivity interactions are reported above. If not listed, there was no significant group by functional connectivity interaction relationship. In post-hoc analyses, regression models were run separately for each diagnostic group, with cognitive performance as the dependent variable and independent variables of age, sex, medical morbidity, education, and connectivity. PCC = Posterior Cingulate Cortex, dlPFC = dorsolateral Prefrontal Cortex, SMC = Supplemental Motor Cortex, SDMT= Symbol Digit Modality Test
Figure 4. Functional Connectivity Relationship with Cognitive Test Performance.
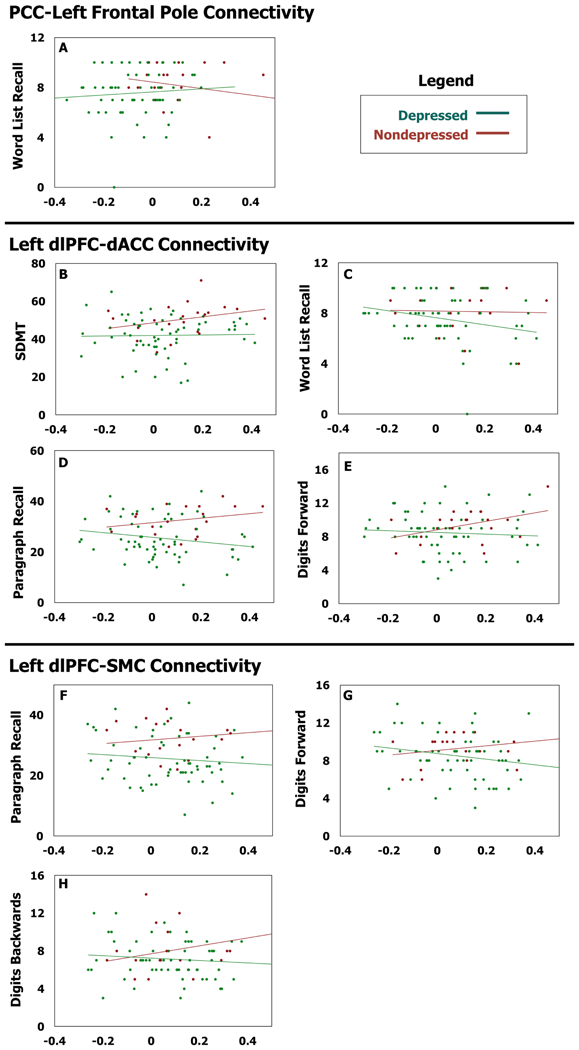
X-axis is functional connectivity beta-value for the specified functionally connected pair, Y-axis is performance on the specified cognitive test. SDMT= Symbol Digit Modality Test.
Left dlPFC-dACC connectivity exhibited multiple statistically significant group – connectivity interaction relationships with cognitive performance (Table 4, Figure 4B–E). Post-hoc analyses in the nondepressed group demonstrated that greater dlPFC-dACC connectivity was associated with significantly better performance on SDMT, Paragraph Recall, and Digits Forward. In the depressed group, greater dlPFC-dACC connectivity was associated with significantly poorer performance on Word List Memory Recall and Paragraph Recall. For all tests, with increasing dlPFC-dACC connectivity, the depressed group performed progressively worse than the nondepressed group (Table 4, Figure 4B–E). dlPFC-dACC connectivity exhibited a primary effect only for Stroop performance, with positive relationships observed for color naming (Wald χ2=4.29, p-value=0.038) and word naming (Wald χ2=7.79, p-value=0.005) conditions, with a trend for the interference condition (Wald χ2=3.50, p-value=0.0615).
Finally, dlPFC-SMC connectivity exhibited significant group differences for Paragraph Recall, Digits Forward, and Digits Backwards performance (Table 4, Figure 4F–H). Post-hoc analyses in the nondepressed group associated greater dlPFC-SMC connectivity with better Paragraph Recall and Digits Backwards performance, but did not observe significant associations in the depressed group. Thus with greater dlPFC-SMC connectivity, depressed participants performed relatively more poorly (Table 4, Figure 4F–H). There were no significant primary effects for other cognitive tests.
DISCUSSION
Our primary finding is that LLD is characterized by decreased connectivity between the PCC in the DMN and the frontal pole, a CCN region. Contrary to our initial hypothesis, no significant group differences in connectivity were found for the CCN or SN seeds. However, in the LLD group, increased CCN connectivity was associated with greater depression severity, greater anhedonia and fatigue, and poorer performance on tests of episodic memory, executive function, and working memory. Thus, despite no significant group differences and a comparable range of regional connectivity (Figure 2), connectivity between the left dlPFC, dACC, and SMC is associated with depressive symptomatology and poorer cognitive performance.
Decreased PCC-Frontal Pole Connectivity Differentiated LLD from Nondepressed Older Adults
LLD subjects exhibited lower positive functional connectivity (loss of positive correlation and reversal to anti-correlation) between the PCC and Left Frontal Pole, indicating lower connectivity between these regions (42). Past work similarly reports decreased PCC connectivity patterns in LLD (40, 41), although others report different patterns (11, 43). These inconsistent findings may be partly explained by methodological differences across studies or population heterogeneity. Additionally, these studies often had smaller sample sizes and different entry criteria that may contribute to discrepant findings. Importantly, although others have associated DMN connectivity with neuropsychiatric symptoms (11), the observed group difference in DMN connectivity was largely unrelated to our examined neuropsychiatric or neuropsychological measures. Thus, as hypothesized by others (44), decreased DMN connectivity may be a biomarker of depression vulnerability that does not drive symptoms during an episode.
Increased CCN Connectivity is Associated with Depression Severity, Anhedonia, Fatigue, and Poorer Cognitive Performance
Despite no significant group differences, CCN connectivity was associated with neuropsychiatric symptom severity. Additionally, the relationship between CCN connectivity and cognitive performance differed between groups (Table 4, Figure 4). Thus, although functional connectivity between the dlPFC, dACC, and SMC is comparable between depressed and nondepressed subjects (Figure 2), connectivity measures between these regions has clinical implications during depressive episodes. This supports that circuit influences on clinical or cognitive symptoms is not limited only to circuits exhibiting differences between diagnostic groups.
Past work implicates these regions in LLD. The dACC is involved in conflict monitoring, processing of cognitively demanding information, response selection and inhibition (45). Both structural and functional dACC abnormalities predict antidepressant response in LLD (11, 46). Our results are concordant with an ICA study in LLD that associated connectivity between the left CCN and dACC with depression severity (4). The SMC is related to implicit motor learning capacity and motor planning. However, the SMC exhibits reduced volume in melancholic depression (47). This structural association is concordant with our finding that increased left dlPFC-SMC connectivity was associated with increased anhedonia and fatigue, characteristics of melancholic depression.
The explanation is less clear for the different relationships between CCN connectivity and cognitive performance. The CCN broadly and the dACC specifically facilitates cognitive control by directing attentional resources to relevant stimuli (48). We propose that negativity bias in directing attention, a characteristic of depression, may subvert this process. In depression we hypothesize that increased CCN connectivity in context of negativity bias may result in greater attention being directed towards negatively valenced stimuli. This persistent negative focus could contribute to worsening depression severity with resultant worsening cognitive performance. Importantly, this hypothesis cannot be tested in our current study as we neither assessed negativity bias nor included emotional valenced tests assessing attention. It should also be noted that the negativity bias observed in depression may be related to circuit changes outside the CCN, although some CCN regions are implicated as contributing to negativity bias (49). Future studies could test this theory by incorporating measures of negativity bias as well as assessments of emotional and non-emotional attention performance.
White Matter Hyperintensity Burden is Not Associated with Network Differences
An important negative finding was that the observed connectivity findings were not associated with WMH burden. This is in contrast to previous studies reporting an association between WMH volume and connectivity in these networks (40, 41), however these studies studied older participants than in our analysis. It is possible that WMH severity affects network connectivity broadly even if not related to connectivity between the regions we examined.
Limitations
There are several important limitations to our analyses. First, we combined data across three studies, but not all studies gathered the same neuropsychiatric and cognitive data, resulting in some analyses examining a subsample. This may have reduced power to detect relationships between connectivity, neuropsychiatric symptoms, and cognitive performance. Moreover, these analyses of neuropsychiatric symptoms and cognitive performance involved multiple comparisons, so results should be viewed as exploratory and require confirmation. Second, the sample sizes for the diagnostic groups were unequal, which limits the power of our group comparisons and may have reduced our ability to detect group differences. Third, our analyses are limited to the networks chosen for analysis and the three subsequently identified functionally connected pairs (PCC-Left Frontal Pole, dlPFC-dACC, dlPFC-SMC). These are likely not the only circuits that differ in LLD or are related to depression severity, neuropsychiatric symptoms, or cognitive performance. In fact, the cognitive domains we analyzed involve additional networks beyond the scope of this report.
Conclusions
This study is among the largest to examine functional connectivity differences in LLD. Our findings support past work that LLD is characterized by differences in DMN connectivity, but also suggests that this may be a vulnerability marker unrelated to clinical presentation. In contrast, the study supports that CCN connectivity plays a role in LLD symptomology during depressive episodes, even if connectivity measures are comparable to those seen in nondepressed elders. Thus we cannot assume that clinical or cognitive symptoms are related only to circuits exhibiting clear group differences. Further work is needed to conduct dimensional analyses of both neuropsychiatric symptoms and cognitive performance in LLD and how brain aging may contribute to network alterations and influence progression of these symptoms.
Acknowledgements:
This research was supported by National Institute of Mental Health grants R01 MH102246, R21 MH099218 and K24 MH110598 and CTSA award UL1 TR002243 from the National Center for Advancing Translational Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Taylor WD (2014): Depression in the Elderly. N Engl J Med 371: 1228–1236. [DOI] [PubMed] [Google Scholar]
- 2.Tadayonnejad R, Ajilore O (2014): Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol 27: 5–12. [DOI] [PubMed] [Google Scholar]
- 3.Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I (2015): Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev 56: 330–344. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Wang Y, Ward BD, Antuono PG, Li S-J, Goveas JS (2017): Intrinsic internetwork brain dysfunction correlates with symptom dimensions in late-life depression. J Psychiatr Res 87: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron. 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner RL, Andrews-Hanna JR, Schacter DL (2008): The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- 8.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. (2009): The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A 106: 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH (2010): Neural correlates of rumination in depression. Cogn Affect Behav Neurosci 10: 470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM (2012): Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord 139: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. (2007): Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62: 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Tol M-J, Li M, Metzger CD, Hailla N, Horn DI, Li W, et al. (2013): Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med 1–13. [DOI] [PubMed] [Google Scholar]
- 14.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zilverstand A, Parvaz MA, Goldstein RZ (2016): Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage . doi: 10.1016/j.neuroimage.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strigo IA, Simmons AN, Matthews SC, Craig ADB, Paulus MP (2008): Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Arch Gen Psychiatry 65: 1275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG (2013): Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev 37: 2529–53. [DOI] [PubMed] [Google Scholar]
- 18.Ye T, Peng J, Nie B, Gao J, Liu J, Li Y, et al. (2012): Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol 81: 4035–4040. [DOI] [PubMed] [Google Scholar]
- 19.Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG (2014): The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. Neuroimage 99: 180–190. [DOI] [PubMed] [Google Scholar]
- 20.Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, et al. (2010): Automatic Mood-Congruent Amygdala Responses to Masked Facial Expressions in Major Depression. Biol Psychiatry 67: 155–160. [DOI] [PubMed] [Google Scholar]
- 21.Avery J a Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK (2014): Major Depressive Disorder Is Associated with Abnormal Interoceptive Activity and Functional Connectivity in the Insula. Biol Psychiatry 76: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horn (2010): Glutamatergic and resting state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheline YI, Price JL, Yan Z, Mintun MA (2010): Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107: 11020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery SA, Asberg M (1979): A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. (2005): The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 26.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. (1998): The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry (Vol. 59), pp 22–33. [PubMed] [Google Scholar]
- 27.Sackeim HA (2001): The definition and meaning of treatment-resistant depression. J Clin Psychiatry 62: 10–17. [PubMed] [Google Scholar]
- 28.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. (1992): Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Res 41: 237–248. [DOI] [PubMed] [Google Scholar]
- 29.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P (1995): A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br J Psychiatry 167: 99–103. [DOI] [PubMed] [Google Scholar]
- 30.Meyer TJ, Miller ML, Metzger RL, Borkovec TD (1990): Development and validation of the penn state worry questionnaire. Behav Res Ther 28: 487–495. [DOI] [PubMed] [Google Scholar]
- 31.Marin RS, Biedrzycki RC, Firinciogullari S (1991): Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38: 143–62. [DOI] [PubMed] [Google Scholar]
- 32.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989): The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 33.Nolen-Hoeksema S, Morrow J, Fredrickson BL (1993): Response styles and the duration of episodes of depressed mood. J Abnorm Psychol 102: 20–28. [DOI] [PubMed] [Google Scholar]
- 34.Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. (2006): Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry 60: 58–65. [DOI] [PubMed] [Google Scholar]
- 35.Nebes RD, Butters MA, Mulsant BH et al. (2000): Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med 30: 679–691. [DOI] [PubMed] [Google Scholar]
- 36.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH (2014): Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. J Neurophysiol 100: 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, et al. (2012): An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 59: 3774–83. [DOI] [PubMed] [Google Scholar]
- 39.Abi Zeid Daou M, Boyd BD, Donahue MJ, Albert K, Taylor WD (2017): Frontocingulate cerebral blood flow and cerebrovascular reactivity associated with antidepressant response in late-life depression. J Affect Disord 215: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreescu C, Tudorascu DL, Butters M a, Tamburo E, Patel M, Price J, et al. (2013): Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res 214: 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, Aizenstein H (2011): Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res Neuroimaging 194: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorenz R, Violante IR, Monti RP, Montana G, Hampshire A, Leech R (2018): Dissociating frontoparietal brain networks with neuroadaptive Bayesian optimization. Nat Commun 9: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eyre HA, Yang H, Leaver AM, Van Dyk K, Siddarth P, Cyr NS, et al. (2016): Altered resting-state functional connectivity in late-life depression: A cross-sectional study. J Affect Disord 189: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD, Turner JA (2013): Electroconvulsive therapy response in major depressive disorder: A pilot functional network connectivity resting state fMRI investigation. Front Psychiatry 4. doi: 10.3389/fpsyt.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS (2004): Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science (80- ). 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- 46.Gunning FM, Cheng J, Murphy CF, Kanellopoulos D, Acuna J, Hoptman MJ, et al. (2009): Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiatry 24: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Exner C, Lange C, Irle E (2009): Impaired implicit learning and reduced pre-supplementary motor cortex size in early-onset major depression with melancholic features. J Affect Disord 119: 156–162. [DOI] [PubMed] [Google Scholar]
- 48.Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG (2005): Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex 15: 229–237. [DOI] [PubMed] [Google Scholar]
- 49.Gollan JK, Connolly M, Buchanan A, Hoxha D, Rosebrock L, Cacioppo J, et al. (2015): Neural substrates of negativity bias in women with and without major depression. Biol Psychol 109: 184–191. [DOI] [PubMed] [Google Scholar]


