Abstract
Alcohol use is associated with a variety of negative consequences, including heightened likelihood of cognitive impairment, proclivity to alcohol use disorders (AUD) and alterations in the drinker’s offspring. Children and rodents exposed to alcohol during pregnancy, or those whose fathers consumed alcohol prior to mating often exhibit neurodevelopmental, physiological, and behavioral deficits. The present study assessed cognitive function and alcohol intake in male and female rats, offspring of alcohol-exposed fathers. Adult male rats were exposed to alcohol or vehicle (0.0 or 2.0 g/kg, respectively; twice daily for two days followed by a rest day, for a total of 8 alcohol or vehicle exposure days), or were left untreated and then mated with non-manipulated females. The offspring was assessed for alcohol intake, via intraoral infusion, followed by cognitive assessment via an alternating T-maze task. The results indicated that paternal ethanol exposure, prior to breeding, resulted in offspring that consumed significantly more ethanol than vehicle or untreated controls. Furthermore, the offspring of alcohol exposed fathers exhibited a significant failure to initiate and complete the T-maze performance tests; although when they did engage in the tests they performed at the level of controls (i.e., 80% correct). The present results add to a growing body of literature suggesting that paternal pre-conception alcohol exposure can have deleterious effects on the offspring.
Keywords: paternal alcohol exposure, pregnancy, T-maze, alcohol intake
INTRODUCTION
Approximately 10% of women in the US consume alcohol during pregnancy (CDC Behavioral Risk Factor Surveillance System [BRFSS], United states 2011–2013), a behavior associated in the offspring with increased risk for alcohol use disorders (AUD) and neurobehavioral alterations, as shown by clinical studies (Baer et al., 2003; Alati et al., 2008) studies. Preclinical and clinical research have suggested similar alterations in the offspring of parents who drink alcohol (hereinafter also referred to as ethanol or EtOH) prior to copulation (Vermeulen-Smit et al., 2012; Fingersh & Homanic 2014; Yohn et. al., 2015).
Specifically, parental alcohol consumption has been correlated with both onset of alcohol use and the overall amount of alcohol consumed by adolescents (Vermeulen-Smit et. al., 2012). Children of alcoholics (COAs) show impulse control problems, which in turn may affect their ability to stop drinking (Sher et al., 1991; Zucker et al., 2006, 2011). Similarly, a review by Marquardt & Brigman (2016) reported cases showing a decrease in behavioral inhibition in rats born from ethanol-drinking parents, and Nizhnikov et al. (2014) found heightened ethanol intake in rats whose mothers had been exposed to EtOH during pregnancy. The offspring of drinkers also exhibit cognitive alterations (Pihl & Peterson, 1995). For instance, rat dams exposed to EtOH during pregnancy produced offspring with deficits in attention, working memory, spatial learning, and increased impulsivity (Yohn et. al., 2015).
The consequences of parental EtOH consumption in rodents are well studied, particularly within multigenerational phenotypes (suggesting epigenetic modifications) after alcohol exposure (Finegersh and Homanics, 2014; Nizhnikov et al., 2014; Diaz-Cenzano and Chotro, 2010; Fabio et al., 2013). Recent evidence suggests that maternal drug exposure produces behavioral, biochemical, and neuroanatomical changes in subsequent generations (Yohn et. al., 2015). For example, Nizhnikov and colleagues (2016) observed an increase in EtOH intake across 3 generations of, previously drug-naïve, offspring. Only the first breeder pairs, and among those only the dams, had been exposed to ethanol prenatally. These results suggest that epigenetic mechanisms may underlie the inheritance of alcohol abuse.
Although for years the focus has been on the effects of maternal consumption of EtOH, several studies now support the idea that fathers exposed to EtOH prior to copulation may produce deficits in the offspring (Yohn et. al., 2015, Abel & Tan, 1988; Kim et.al. 2014; Meek et al., 2007; Wozniak et al., 1991). Studies of COAs, have identified deficits in visual spatial abilities and perceptual motor skill performance, as well as learning deficits in attention and working memory, increased impulsivity, and altered reward response (Yohn et. al., 2015; Abel & Tan, 1988; Kim et.al. 2014; Meek et al., 2007; Wozniak et al., 1991) and changes in alcohol preference (Rompala et al., 2017).
The present study assessed cognitive function and EtOH intake in male and female rats, offspring of alcohol-exposed sires. Adult male rats were exposed to EtOH or vehicle (2.0 or 0.0 g/kg, respectively; twice daily for two days followed by a rest day, for a total of 8 EtOH or vehicle exposure days), or were left untreated and then mated with non-manipulated females. This protocol of paternal EtOH exposure is not as extensive as that of previous studies (Rompala et al., 2016, 2017; Finegersh et al., 2014; Kim, 2014; Ceccanti et al., 2015). The offspring was assessed for EtOH intake, via intraoral infusion, followed by cognitive assessment via an alternating T-maze task. We hypothesized similar effects as those previously found with maternal exposure, as for instance in Nizhnikov et al., 2014. Thus, we expected greater acceptance of EtOH. We also hypothesized a deficit in cognitive performance, specifically in areas of spatial learning and working memory as assessed by the alternating T-maze task. The latter maze can be solved by spatial cues (although it can also be solved via working memory and egocentric cues, Aggleton et al., 1996) integrated by the hippocampus, a structure significantly affected by developmental ethanol exposure (Gil-Mohapel et al., 2014).
METHOD
Subjects
Male and female Sprague Dawley rats (12 sires; 24 dams and 104 tested offspring; total number of rats employed = 140) born and reared in the Department of Psychology of the Southern Connecticut State University (USA) were utilized. These animals were derived from 21 litter (7 per treatment). To help prevent litter effects, no more than one male and one female were used from each litter. Subjects were kept on a 12/12-hr light/dark cycle (lights on/off at 5:00 am/pm) with constant temperature (65 +−5 degree F) and humidity (50%). At postnatal day 60 (PD 60), males were divided into three groups (1: ethanol exposed [PE], 2: water exposed [PV], and 3: untreated control (UT) groups). At 73 days of age, following exposure treatment procedures (see below), males were pair housed with untreated 2 adult females for 2 weeks. All litters were provided enrichment in the form of PVC tubing (3-inch diameter, approximately 6 inches long), and both food and water were available ad libitum except during food restriction for T-maze testing, as outlined below.
Paternal Ethanol Exposure
On PD 60, 12 sires (4 sires PE, 4 sires PV, and 4 sires UT groups) were exposed to repeated intragastric intubation of EtOH (16.8% v/v EtOH solution; volume of administrations: 0.015 ml/g; dose: 2.0 g/kg; PE), similar volumes of vehicle (tap water; PV), or remained untreated (UT). Ethanol and water were administered twice a day at 9AM and 5PM (total volume of ethanol infused was 4g/kg/day), 2 days in a row. A single “rest” day was inserted between each 2-day exposure period. This pattern of fluid administration (i.e., 2 days on, 1 day off) was repeated for a total of 11 days, resulting in a total of 8 days of exposure and 3 days of rest. Ethanol was intubated with an 8 hour-interval. This procedure models that of Kim et al., (2014). However, our length of exposure was significantly less than that of Kim et al., (2014) as they exposed their animals for 7 weeks. Our logic was to see if lower levels of paternal ethanol exposure would also have an effect on offspring.
Breeding
Following the last day of intubation, each sire (including those within the control group) was provided 2 days of rest before being placed into a cage with 2 untreated dams, for 2 weeks. Therefore, all litters were conceived between 3 (if conception happened on first day of pairing) and 14 (if conception happened on last day of pairing) days following the last intubation. IN our laboratory conception occurred 2.5 days following male -female pairing on average regardless of paternal treatment (data not shown). After removal of the male, females remained pair housed for 5 days, and then were housed individually. Births were checked daily each morning. Enrichment was removed, and litters were culled to 10 pups (at least 4, and no more than 6, pups of each sex), at PD1 to ensure proper maternal care of all pups. If a dam did not give birth within 10 days of being separated from the other female and not used in this experiment.
The litter was left undisturbed until PD14, when ethanol intake commenced. During ethanol intake procedures a total of 4 pups from each litter were utilized, and euthanized thereafter. The rest were left undisturbed until weaning, which occurred at PD21. Weanlings were pair housed with a same sex littermate in a chamber enriched with PVC tubing (IACUC requirement), and had ad libitum access to food and water until food restriction procedures, on PD38.
Ethanol Intake Testing
Ethanol intake was tested at PD 14 on 2 male and 2 female pups from each group [i.e., ethanol (PE), water (PV), and control (UT)]. The pups were removed from the dam, cannulated, and placed into chambers lined with pine shavings (4in × 4 in) and warmed by a heating pad (35.5 ±.5 °C; Kane PHM28T heating pad) for 3 hrs. Cannulation occurred as described in Nizhnikov et al., 2016. Briefly, a piece of PE-10 polyethylene tubing, 3 cm in length, flanged at one end, was attached to the back of a needle and threaded gently midline through the cheek with the flanged tip resting in an anterior position of the inside portion of the cheek.
Three hours after cannulation the pups’ bladder was voided. Each pups’ cannula was connected to a length of PE50 tubing which, in turn, connected to a 10ml syringe that was placed into a computerized rotary pump. The rats were subsequently placed into individual Plexiglas chambers (10 × 10 × 12 cm) lined with cotton, and intraoral ethanol (5%) or water was infused for 15 min. The total administration volume was equivalent to 5.5% of the subject’s preinfusion weight (averaged across litter). Specifically, 1 pup of each sex was tested from each litter on each fluid. No pup was tested twice. In other words. 1 male and 1 female were exposed to alcohol and 1 male and 1 female were exposed to water. Following infusion, pups were dried, weighed, and the cannula was removed. Percent body weight gain (BWG%) was calculated and used for subsequent analysis of paternal effects on ethanol intake.
One question that may occur at this point is the relevance of intake tests at such a young age. We have observed correlations between this testing method and adolescent drinking in two-bottle intake tests (see Ponce et al., 2008). Therefore, this model was chosen as protocol since this laboratory has over a decade of experience using it.
Alternating T-Maze Testing
Food Restriction:
Animals were housed with a peer of the same sex and of approximately same weight (i.e., within 5–10 grams weight of each other) at PD37. Starting on PD38 subjects were food restricted to 80% of their free feeding weight, as described in Anderson, Bush, & Spear (2013). At this time, and throughout the remainder of the experiment, animals were weighed daily and food- restricted, with daily food rations distributed approximately 30 to 60 min after each training or testing session. Animals in the food-restricted conditions had ad libitum access to water, but daily food allotments as follows: beginning the day prior to conditioning, they were provided with 14g of rat chow. Each day thereafter, food amount was increased as needed to allow for 5–8g of weight gain, thereby permitting maintenance of approximately 80% of the normal growth trajectory determined from the weights of free-feeding counterparts. When they reached 80% of the normal growth trajectory, they were given approximately 20g per day, with this amount increased as needed to maintain target body weight.
Training.
The T-maze apparatus had the following measurements: Starting arm 46 in. long 7 in. wide; two goal arms 22 in. long 7 in. wide. The walls were 6 in. tall and the apparatus had a clear ceiling with large slits for air movement. On PD39, 1 male and 1 female from the PE, PV, and UT groups were provided with 4 days of T-maze training, until they ran reliably to eat the reward (chocolate pellets, 1/4 of a coco puff cereal ball from General Mills) provided in the food wells. Each rat was gently placed in the start arm and could explore the maze, for a maximum of 3 min, and eat the reward in the chosen goal arm, at which time the alternate arm was blocked to prevent retracing. The second time the rat was placed into the maze, the arm originally selected by the subject was blocked, forcing the animal down the opposite arm in order to receive the food reward. Training occurred twice a day, with 4 trials per session and an inter-trial interval of approximately 30 min. The training phase was repeated 4 times, with equal number of left and right runs. To ensure that no odor cues were available, the apparatus was cleaned between trials with a damp cloth containing 3% hydrogen peroxide.
Testing.
On the fifth day (PD43), the rat was tested in 12 trial sessions, with a 60 sec delay between trials. At the start of each trial, the animal was placed in the start arm facing away from the goal arms, and then allowed to choose one goal arm (equipped with a chocolate pellet), at which time the rat was removed for 1 min. The maze was then reset by opening access to both arm entries and the reward provided only in the arm opposite to that which was originally selected. The rat was then placed back into the maze. If the rat entered the goal arm not previously selected to obtain the reward, a correct choice was scored. If the rat entered the goal arm that was chosen previously, a door was slid down, blocking the animal from exiting and, after approximately 20 sec (to ensure that it had discovered that the food well was empty), an incorrect choice was recorded. Each trial lasted no more than 2 min.
Data Analysis
The baseline body weight (g) of PV and PE sires (i.e., immediately before the commencement of the repeated intragastric intubation) was compared by a T test. To analyze the effects of the treatment on the body weight of these sires we calculated the percent body weight change between commencement and termination of the intubations. This measure was also analyzed via a T test. We did not weigh the UT group since we were attempting to minimize all of their stress.
A two-way [Paternal treatment (ethanol [PE], vehicle [PV] or untreated control [UT]) × Sex] ANOVA analyzed body weight (g) at PD14. Similar ANOVAs were used to analyze T-maze results [latency to begin moving towards the goal arms, % of correct trials performed including all trials, % of correct trials performed only counting fully completed trials in subjects exhibiting at least 4 fully completed trials, and number of non-runs]. Only four animals, randomly distributed in the groups, were not included in the analysis of % of correct trials only counting fully completed trials. These animals exhibited only 1, 2, or 3 completed trials. For the intake test, ethanol or water intake was expressed as the percentage of body weight gained and was analyzed via a three-way [Paternal treatment (PE, PV or untreated control) × Sex (male or female) × Fluid offered (ethanol or water)] ANOVA.
The loci of the significant main effects and significant interactions yielded by the ANOVAS were analyzed using Fisher’s LSD post hoc test. The partial Eta square (η2p) was calculated to estimate effect size of the significant main effects or significant interactions yielded by the ANOVAs. Across analyses, alpha level was ≤ 0.05.
Results
PE and PV sires had similar (t12 = 1.14, p≥ 0.20) body weight (g) at baseline (520.57±38.03 and 472.00±19.18), yet the percent body weight change between commencement and termination of the intubations was significantly lower (t12 = −2.68, p≤ 0.05) in PE (-2.16±0.32) than in PV sires (0.48±0.93).
The ANOVA for offspring body weight before the fluid intake test revealed a significant main effect of Paternal treatment, F2,62= 6.7, p≤.005, η2p=0.18, yet no significant main effect of Sex nor a significant Sex × Treatment interaction. The offspring, either male or female, of parents treated with ethanol (M=28.46±0.75) or vehicle (M=28.33±0.71) had significantly lower body weight (g) than control peers derived from untreated sires (M=31.77±0.77).
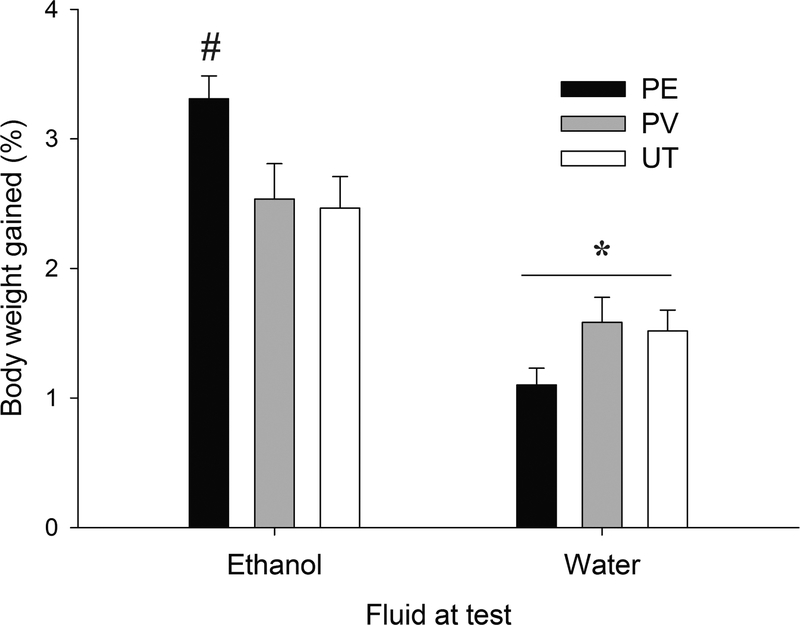
As shown in Figure 1, water intake was lower than ethanol intake yet was similar between the offspring, either male or female (Fig 1b and 1c), of PE, PV or UT sires. That was not the case for ethanol drinking, which was greater in PE rats than in PV or untreated controls (Fig. 1a). The ANOVA and subsequent post-hoc tests confirmed these impressions. The analysis yielded a significant main effect of Fluid and a significant Fluid × Paternal treatment interaction, F1,56= 31.12, p≤.001, η2p=0.52 and F2,56= 5.50, p≤.01, η2p=0.16; respectively. The post-hoc tests revealed similar water acceptance across the three paternal conditions (all p > 0.10), yet indicated significantly greater ethanol intake (%BWG) in PE vs. PV (p ≤ 0.05) or untreated (p ≤ 0.01), male or female, rats. Sex did not exert a significant main effect nor was involved in significant interactions.
Figure 1:
Paternal ethanol exposure heightens ethanol intake in the offspring. Ethanol or water intake, expressed as % body weight gained in an intraoral infusion test, in 14-day old rats, as a function of paternal treatment (ethanol [PE], vehicle [PV] or untreated control [UT]). The asterisk sign indicates that water intake was, regardless paternal treatment, significantly lower (p ≤ 0.05) than ethanol intake. The pound sign indicates that ethanol drinking was significantly greater (p ≤ 0.05) in PE rats, male or females, than in PV or untreated controls. Groups were composed by 10 or 13 animals, Vertical bar indicate SEM.
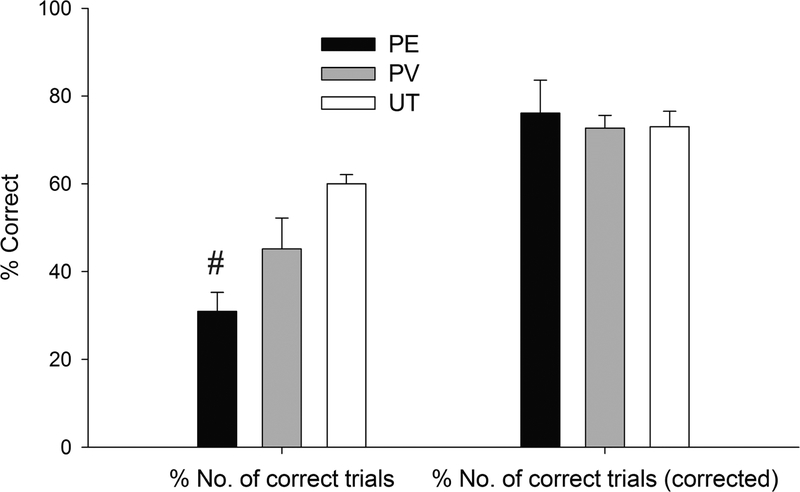
Analyses of T-maze results were as follows. The analysis for latency (s) to move into the arm towards the goal, in the first run of the test, revealed a significant main effect of Paternal treatment and a significant Sex × Paternal treatment interaction, F2,30= 3.55, p≤.05, η2p=0.19 and F2,30= 3.56, p≤.05, η2p=0.19; respectively. As indicated by the post-hoc tests, the latency was lower in UT males (M=20.40±5.29) than in same-sex PE (M=61.46±10.14) or PV (M=61.52±15.10) rats (p ≤ 0.005 and p ≤ 0.01, respectively), whereas UT, PV and PE female groups exhibited statistically similar latency (M=58.26±8.98, M=49.57±7.21 and M=65.54±5.54, respectively, all p ≥ 0.15). The ANOVA for percent number of correct trials revealed a significant main effect of Paternal treatment [F2,30= 7.61, p≤.005, η2p=0.34] and the post-hoc tests revealed significantly lower % number of correct trials in PE than in PV (p ≤ 0.05) or UT (p ≤ 0.001) rats, males or females. This pattern, suggestive of poorer performance in PE than PV or UT controls, is depicted in Figure 2. Yet the ANOVA for % number of correct trials performed after discounting the non-runs (i.e., runs in which the animal fails to move out of the starting box and only including animals that completed at least 4 out of the 12 trials, see Figure 2) failed to reveal significant main effects or significant interactions. This suggested that the apparent poorer cognitive performance of PE rats was a by-product of them failing to initiate the behaviors required in the test. To confirm this, we analyze the number of trials in which the rat actually attempted to complete the task by running through the maze (whether or not the attempt translated into a correct or incorrect set of responses) and we found a significant main effect of Treatment [F2,30= 7.72, p≤.005, η2p=0.34], with PE rats, exhibiting significantly less number of runs actually performed (males: Mean (M)=5.14±0.98; females: M=5.14±1.33) than PV (males: M=6.40±2.01; females: M=8.29±1.17) or UT (males: M=10.80±0.73; females: M=9.20±0.37) peers (p ≤ 0.05 and p ≤ 0.001, respectively). In conjunction, these results suggest that PE rats successfully acquired the task yet had expression deficits.
Figure 2:
Paternal ethanol exposure affects performance in a T-maze task. Percent number of correct trials performed and percent number of correct trials performed after correcting for or discounting the non-runs (i.e., runs in which the animal fails to move out of the starting box) in 43-day old rats, as a function of paternal treatment (ethanol [PE], vehicle [PV] or untreated control [UT]). The pound sign indicates that the percent number of correct trials was significantly lower (p ≤ 0.05) in PE than in PV or UT rats, males or females. The PE and PV groups were composed of 11 animals and the UT group was composed of 10. Vertical bar indicates SEM.
DISCUSSION
Paternal ethanol exposure, prior to copulation, has been shown to have adverse effects on the offspring, including, but not limited to, heightened drug use behavior, alterations in reward directed behaviors, and neurochemical and structural changes within the brain (Marquardt & Brigman 2016; Yohn et. al., 2015; Abel & Tan, 1988; Kim et.al. 2014; Meek et al., 2007; Wozniak et al., 1991). The present study illustrates that paternal binge ethanol exposure prior to breeding can also result in the offspring consuming significantly more EtOH than controls, and uncovers behavioral changes in a T-maze task. Having been reared and housed with drug naïve dams, it is unlikely that maternal behavior or influence contributed to the heightened ethanol consumption found in the offspring of ethanol-exposed sires. This suggests that the effects reported are most likely the result of paternal EtOH exposure.
Consistent with the results found in the ethanol intake test, previous research (Nizhnikov et al., 2016) found that paternal EtOH exposure resulted in increased EtOH intake across 3 generations. Conversely, results reported by Rompala and colleagues (2017) as well as Finegersh and Homanics (2014) showed that paternal chronic ethanol exposure produced offspring with reduced ethanol preference and intake. Several design and procedural differences may explain the apparent disparate findings. We employed rats while they used C57BL/6J or Strain 129 F1 hybrid mice. Furthermore, Rompala and colleagues (2017) utilized a two-bottle free-choice preference test and their paternal administration model was a chronic exposure to ethanol, whereas we employed an oral infusion acceptance test and a limited ethanol pre-treatment. Age of testing also differed dramatically, while they tested adults our tests were conducted on infants, perhaps resulting in the observed differences.
While the underlying mechanisms for the observed changes in subsequent drinking behavior remain unclear, one possible explanation is that the offspring of ethanol-exposed sires exhibit an altered response, either sensitization or tolerance (Finegersh & Homanics, 2014), to the reinforcing properties of EtOH (Meek et al., 2007). Glendinning et al., (2012) have also shown that prenatal ethanol exposure affects the acceptance of ethanol’s taste and that this effect is, at least partly, due to changes in TrpV1 receptors on the tongue of the offspring. This specific receptor is activated via capsaicin and so seems to mediate the sensation of spicy. They hypothesized that prenatal ethanol makes offspring more likely to engage in alcohol drinking, not only because of increased preference for ethanol’s smell and taste but by also making it less “spicy” (Glendinning et al., 2012). Furthermore, Rompala et el., (2018) has shown that small noncoding RNAs such as miRNAs and tRNA fragments are altered in sperm by ethanol. These effects may underlie the changes seen in this set of experiments. While not directly in line with the methodology used in this experiment, another set of possible explanations for the changes in behavior shown here can be found in a review by Sarkar (2016). Specifically, prenatal ethanol exposure alters (exposure to the pregnant dam) results in transgenerational changes in, among other things POMC expression. More specifically, in changes to the HPA axis. More interestingly as it pertains to this set of studies, these changes seem to be carried transgenerationally down the paternal germline (Sarkar 2016).
Alternatively, consequences beyond paternal ethanol administration may affect ethanol intake in F1 offspring. Effects of paternal experience, including chronic stress, have been suggested to alter sperm as a mechanism for epigenetic inheritance in offspring (Rompala et al., 2017).
Illustrating this point, research has shown that ethanol exposure alters the response of the hypothalamic-pituitary-adrenal (HPA) axis (Rompala et al., 2016), one of the main components of the stress response. Acute ethanol exposure over-activates the response the axis, yet chronic, and often intermittent, ethanol exposure, blunts the subsequent stress- or EtOH-mediated activation of the axis (Lee et al., 2000; Allen et al., 2016). These altered response to stress may, in turn, have resulted in greater ethanol intake in the PE offspring. Furthermore, while rats do have a blunted stress response during the first two weeks of life (Rosenfeld et al., 1992) it is still present and differs depending on ethanol dose (Pautassi et al., 2012). Furthermore, ethanol exerts anxiolytic effects, at doses as low as 0.5g/kg (Nizhnikov et al, 2014), and these effects play a significant role in driving ethanol seeking and intake. It is possible that the combination of tolerance to these effects and a hyperactive stress response may account for the heightened ethanol intake found in the PE offspring.
The PE offspring also exhibited, when compared to control counterparts, a significant increase in latency to reach the choice point in the T-maze task. The PE animal spent significantly more time than their PV or UT peers sitting in the entry arm of the maze. It is possible that these changes reflected an altered stress response in the PE offspring, which resulted in them refusing to complete the task. These findings confirm and extend existing evidence (Abel, 1989a,b; Abel & Tan, 1987; Abel & Lee, 1988), wherein offspring of EtOH exposed sires exhibited longer latencies to enter the arm areas and lower overall motor activity, in a T-maze task. Furthermore, offspring sired by ethanol exposed males have also shown increased anxiety and depression, which is consistent with likely changes in their motivation to perform the task to completion (Liang et al., 2014). Taken together, these results may suggest a manifestation of increased fearfulness as a function of paternal EtOH exposure on offspring (Abel & Lee, 1988).
In previous research, cognitive assessments utilized measurements of processing speed, navigational mapping, visual spatial abilities, attention, concentration, and memory to evaluate mental function. Such executive domain functions are usually impaired in addictive behaviors (Mallorqui-Bague et al., 2017). Several studies investigating the cognitive effects of paternal ethanol exposure found that the male offspring exhibited impaired spatial learning acquisition (Wozniak et al., 1991) and impaired working memory (Kim et al., 2014). At first glance, the present study produced results in line with previous research (i.e., cognitive testing deficit after paternal ethanol exposure). However, upon closer inspection, our results differ from previous research. Specifically, when analyzing data to discount all failures to perform the T-maze task to completion and including only animals that completed the trials a minimum of 4 times, we found no significant difference of percent correct response, or latency between groups. All groups responded at around 80% correct, well above chance. These results suggest that, when PE offspring performed the trial to completion, they performed comparably to both PV and UT groups. It is their lack of performance rather than a lack of learning that is decreasing success rate.
The present results should be considered in the context of important limitations. Our model utilized a between subjects’ design, resulting in smaller litter sizes prior to maze testing. Therefore, it is difficult to know whether PE subjects performed comparably to other groups due to the absence of cognitive impairment or from extended maternal care prior to testing. Furthermore, research has found a significant correlation between overtraining and reduced performance outcomes, and the stress associated with overtraining can alter cognitive processing (Angeli et al., 2004). In the current study, animals were subjected to 4 consecutive days of training during conditioning followed by 12 trials during testing. Therefore, stress associated with overtraining may have led to a decline of performance (i.e., increased latency and non-run trials) (Angeli et al., 2004). We also employed enrichment, therefore it is unknown how the present results translate to other breeding protocols devoid of this procedure.
The present study aimed at examining consequences of paternal ethanol exposure on the behavior of F1 offspring. The results presented here suggest a significant increase in EtOH intake, T-maze latency, and non-run trials in PE offspring. Taken together, these findings demonstrate paternal ethanol phenotype inheritance.
Therefore, future research should directly assess the underlying mechanisms associated with paternal pre-conception ethanol exposure and stress responsivity in offspring. The potential consequences of this are still unclear, however, our findings do not support the idea the PE subjects exhibit cognitive deficits as a function of paternal ethanol exposure for our specific exposure model. Future research should utilize different methodological paradigms, consider current limitations, and directly assess altered stress responsivity and associated task performance outcomes in PE offspring. Furthermore, future research should continue the work of Rompala et al., (2018) and aim to identify further epigenetic translational factors in sperm that may serve a means by which paternal effects are conveyed to future generations.
In conclusion, the present work reveals that paternal pre-conception ethanol exposure produces alteration in ethanol intake and T-maze performance in offspring. The results presented here add to a growing literature (Finegersh and Homanics 2014; Liang et la., 2014; Rompala et al., 2016; Abel and Lee, 1988; Abel 1989a,b; Wozniak et al., 1991) in understanding the consequences of paternal preconception ethanol exposure and the effects on subsequent generations.
HIGHLIGHTS.
Paternal ethanol exposure increases alcohol drinking compared to controls
Paternal ethanol affects T-maze performance compared to controls
Paternal ethanol makes animals less likely to run the T-maze to completion
When corrected for completion paternal alcohol had no effect on learning
Acknowledgments
Funding: R21 AA025163–01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abel EL (1989a) Paternal alcohol consumption: effects of age of testing and duration of paternal drinking in mice. Teratology, 40, 467–474. [DOI] [PubMed] [Google Scholar]
- Abel EL (1989b) Paternal and maternal alcohol consumption: effects on offspring in two strains of rats. Alcoholism, clinical and experimental research, 13, 533–541. [DOI] [PubMed] [Google Scholar]
- Abel EL, Lee JA, (1988). Paternal Alcohol Exposure Affects Offspring Behavior but not Body or Organ Weights in Mice. Alcoholism: Clinical and Experimental Research, 12, 349–355. [DOI] [PubMed] [Google Scholar]
- Abel EL, Tan SE (1988). Effects of paternal alcohol consumption on pregnancy outcome in Rats. Neurotoxicol Teratology. 10, 187–192 [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, Neave N (1996). The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioral Brain Research. 81, 189–98. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Carter JM, Landin JD, Varlinskaya EI, Bordner KA, Werner DF, Spear NE (2014). Agonist (U62,066E) and Antagonist (Nor-BNI) and Reduces Kappa Opioid Receptor Expression. Alcoholism: Clinical and Experimental Research, 38(6), 1630–1638. [DOI] [PubMed] [Google Scholar]
- Alati R, Clavarino A, Najman JM, O’Callaghan M, Bor W, Mamun AA, Williams GM (2008). The developmental origin of adolescent alcohol use: Findings from the Mater University Study of Pregnancy and its outcomes. Drug and Alcohol Dependence, 98(1–2), 136–143. [DOI] [PubMed] [Google Scholar]
- Allen CD, Grigoleit JS, Hong J, Bae S, Vaughan J, Lee S, (2016). Exposure to alcohol during adolescence exerts long-term effects on stress response and the adult brain stress circuits. Neuroscience, 339, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Bush PC, Spear LP (2013). Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behavioural Brain Research, 257, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli A, Minetto M, Dovio A, Paccotti P (2004). The Overtraining Syndrome in Athletes: A Stress-Related Disorder. Journal of Endocrinological Investigation, 27(6), 603–612. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD & Streissguth AP (2003) A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Archives of general psychiatry, 60, 377–385. [DOI] [PubMed] [Google Scholar]
- [BRFSS], United states 2011–2013: https://www.cdc.gov/ncbddd/fasd/data.html
- Ceccanti M, Coccurello R, Carito V, Ciafrè S, Ferraguti G, Giacovazzo G, Mancinelli R, Tirassa P, Chaldakov GN, Pascale E, Ceccanti M, Codazzo C, Fiore M (2015). Paternal alcohol exposure in mice alters brain NGF and BDNF and increases ethanol-elicited preference in male offspring. Addiction Biology, 21(4), 776–787. [DOI] [PubMed] [Google Scholar]
- Díaz-Cenzano E, Chotro MG (2010). Prenatal binge ethanol exposure on gestation days 19–20, but not on days 17–18, increases postnatal ethanol acceptance in rats. Behavioral Neuroscience, 124(3), 362–369. [DOI] [PubMed] [Google Scholar]
- Fabio MC, March SM, Molina JC, Nizhnikov ME, Spear NE & Pautassi RM (2013). Prenatal ethanol exposure increases ethanol intake and reduces c-Fos expression in infralimbic cortex of adolescent rats. Pharmacology, biochemistry, and behavior, 103, 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegersh A, Homanic GE, (2014). Paternal alcohol exposure reduces alcohol drinking and Increases behavioral sensitivity to alcohol selectively in male offspring. PLoS ONE. 9(6), e99078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Titterness AK, Patten AR, Taylor S, Ratzlaff A, Helfer J, Christie BR (2014). Prenatal ethanol exposure differentially affects hippocampal neurogenesis in the adolescent and aged brain. Neuroscience, 273, 178–88. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Simons YM, Youngentob L, Youngentob SL (2012). Fetal ethanol exposure attenuates aversive oral effects of TrpV1, but not TrpA1 agonists in rats. Experimental Biology and Medicine, 237(3), 236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Choi CS, Park JH, Joo SH, Kim SY, Ko HM, Kim KC, Jeon SJ, Park SH, Han SH, Ryu JH, Cheong JH, Han JY, Ko KN, Shin CY (2014). Chronic Exposure to Ethanol of Male Mice before Mating Produces Attention Deficit Hyperactivity Disorder-Like Phenotype along with Epigenetic Dysregulation of Dopamine Transporter Expression in Mouse Offspring. Journal of Neuroscience Research, 92(5), 658–670. [DOI] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C (2000). Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcoholism: Clinical and Experimental Research, 24, 110–122. [PubMed] [Google Scholar]
- Liang F, Diao L, Liu J, Jiang N, Zhang J, Wang H, Zhou W, Huang G, Ma D (2014) Paternal ethanol exposure and behavioral abnormities in offspring: associated alterations in imprinted gene methylation. Neuropharmacology, 81, 126–133. [DOI] [PubMed] [Google Scholar]
- Mallorquí-Bagué N, Tolosa-Sola I, Fernández-Aranda F, Granero R, Fagundo AB, LozanoMadrid M, Mestre-Bach G, Gómez-Peña M, Aymamí N, Borrás-González I, Sánchez-González J, Baño M, Del Pino-Gutiérrez A, Menchón JM, Jiménez-Murcia S (2017). Cognitive Deficits in Executive Functions and Decision-Making Impairments Cluster Gambling Disorder Sub-types. Journal of Gambling Studies, 34(1), 209–223 [DOI] [PubMed] [Google Scholar]
- Marquardt K, Brigman JL (2016). The impact of prenatal alcohol exposure on social, cognitive and affective behavioral domains: Insights from rodent models. Alcohol, 51, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek LR, Myren K, Sturm J, Burau D (2007). Acute paternal alcohol use affects offspring development and adult behavior. Physiology & Behavior, 91(1), 154–160. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Carter JM, Landin JD, Varlinskaya EI, Bordner KA, Werner DF, Spear NE (2014). Brief Prenatal Ethanol Exposure Alters Behavioral Sensitivity to the Kappa Opioid Receptor. Alcoholism: Clinical and Experimental Research, 38(6), 1630–8. [DOI] [PubMed] [Google Scholar]
- Nizhnikov ME, Popoola DO, Cameron NM (2016). Transgenerational Transmission of the Effect of Gestational Ethanol Exposure on Ethanol Use-Related Behavior. Alcoholism: Clinical and Experimental Research, 40(3), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Spear NE (2012). Ethanol-mediated appetitive conditioning in infant rats, but not corticosterone release, is dependent on route of ethanol administration. Developmental Psychobiology, 54(1), 98–104. [DOI] [PubMed] [Google Scholar]
- Pihl RO Peterson JB, (1995). Alcoholism: the role of different motivational systems. Psychiatry Neuroscience, 20(5), 372–96. [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Homanics GE (2016). Paternal Preconception Ethanol Exposure Blunts Hypothalamic-Pituitary-Adrenal Axis Responsivity and Stress-Induced Excessive Fluid Intake in Male Mice. Alcohol, 53, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Finegersh A, Slater M, Homanics GE (2017). Paternal Preconception Alcohol Exposure Imparts Intergenerational Alcohol-Related Behaviors to Male Offspring on a Pure C57BL/6J Background. Alcohol, 60, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompala GR, Mounier A, Wolfe CM, Lin Q, Lefterov I, Homanics GE. (2018). Heavy Chronic Intermittent Ethanol Exposure Alters Small Noncoding RNAs in Mouse Sperm and Epididymosomes. Frontiers in Genetics. 8, 9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P, Suchecki D, Levine S (1992). Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neuroscience Biobehavioral Reviews, 16, 553–568. [DOI] [PubMed] [Google Scholar]
- Sarkar DK (2016). Male germline transmits fetal alcohol epigenetic marks for multiple generations: a review. Addiction Biology, 21(1), 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE (1991). Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. Journal of Abnormal Psychology, 100(4), 427–448. [DOI] [PubMed] [Google Scholar]
- Vermeulen-Smit E, Koning IM, Verdurmen JE, Vorst HV, Engels RC, Vollebergh WA (2012). The influence of paternal and maternal drinking patterns within two-partner families on the initiation and development of adolescent drinking. Addictive Behaviors, 37(11), 1248–1256. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Cicero TJ, Kettinger L, Meyer ER (1991). Paternal alcohol consumption in the rat impairs spatial learning performance in male offspring. Psychopharmacology, 105(2), 289–302. [DOI] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, Blendy JA (2015). Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Progress in Biophysics and Molecular Biology, 118(1–2), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA (2006). Alcohol use and alcohol use disorders: a developmental-biopsychosocial systems formulation covering the life course In: Cicchetti D; Cohen DJ, Developmental Psychopathology. 2nd ed. Wiley; Hoboken, NJ: 2006 p. 620–656. [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT (2011). Parsing the undercontrol/disinhibition pathway to substance use disorders: a multilevel developmental problem. Child Developmental Perspective. 5, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]