Abstract
Alcohol use disorder (AUD) represents a significant and ongoing public health concern with 12-month prevalence estimates of ~5.6%. Quantitative genetic studies suggest a heritability of approximately 50% for AUD, and as a result, significant efforts have been made to identify specific variation within the genome related to the etiology of AUD. Given the limited number of replicable findings that have emerged from genome-wide linkage and candidate gene association studies, more recent efforts have focused on the use of genome-wide association studies (GWAS). These studies have demonstrated that hundreds of variants across the genome, most of small effect (R2 < 0.002), contribute to the genetic etiology of AUD. The present review describes the initial, though limited, successes of GWAS to identify loci related to risk for AUD as well as other etiologically relevant traits (e.g., alcohol consumption). In addition, “Post-GWAS” approaches that rely on GWAS data to estimate the heritability and co-heritability of traits, test causal relations between traits, and aid in gene discovery are described. Together, the described research findings illustrate the importance of molecular genetic research on AUD as we seek to better understand the mechanisms through which genetic variation leads to increased risk for AUD.
INTRODUCTION
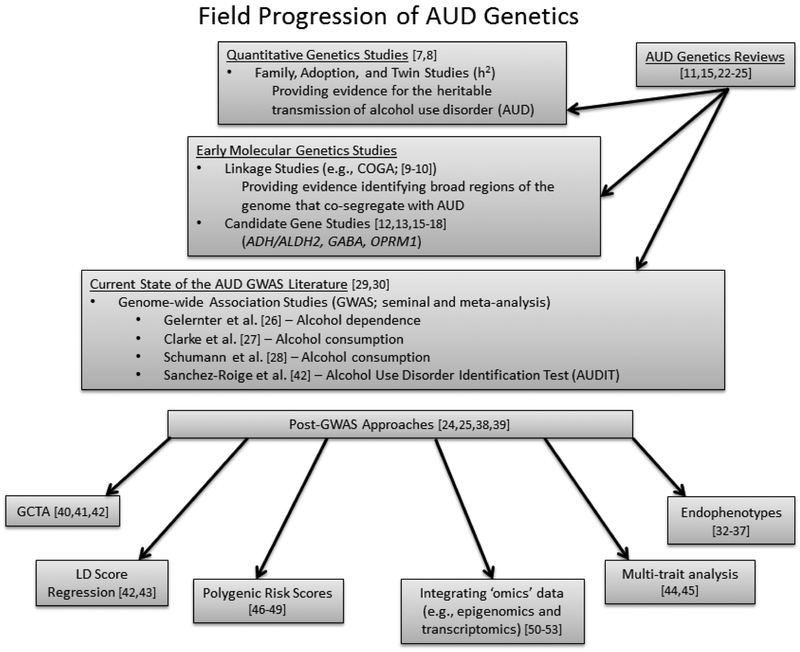
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5; [1)]) defines alcohol use disorder (AUD) as a single spectrum of problematic use and clinically significant impairment based on endorsement of 2 or more of 11 criteria assessing behavioral and physical manifestations of heavy alcohol use1. Recent estimates indicate that 5.6% of individuals meet criteria for a past year AUD [2], resulting in significant social, economic and public health costs [3,4]. In addition to the importance of environmental influences [5,6], quantitative genetic studies examining the impact of familial transmission of liability for AUD have consistently demonstrated a substantial genetic component to the disorder ([7]; see Figure 1) with a recent meta-analysis reporting a heritability of approximately 50% [8].
Figure1.
Review of AUD genetics literature.Values correspond with in-text references.
EARLY MOLECULAR GENETICS STUDIES
Given such findings, molecular genetics studies have attempted to identify specific variation within the genome related to increased risk for AUD. Early work in the field focused on genome-wide linkage and candidate gene association studies. The former relies on family-based samples to identify regions of the genome that co-segregate with the disorder of interest. For example, the Collaborative Study of the Genetics of Alcoholism (COGA) relied on a large sample of families enriched for alcohol dependence to identify regions of chromosome 4 containing the alcohol dehydrogenase (ADH) gene which encodes the ADH isozymes that metabolize alcohol into acetaldehyde and a cluster of GABA receptor genes [9–11], respectively.
Linkage studies are limited in terms of their spatial resolution, and thus, association studies that measure differences in allele frequencies between ‘case’ and ‘control’ populations were also pursued. Early association studies focused on a limited number of variants in or near genes selected a priori for their biological relevance to the trait of interest or physical location in the genome informed by prior linkage results. Though findings of associations between AUD and variants in or near alcohol metabolizing genes (e.g., ADH1B and ALDH2; [12,13]) have been some of the most commonly demonstrated effects [14], overall the linkage and candidate gene literatures are characterized by inconsistencies in replication [15], including those reported for the chromosome 4 GABA gene cluster [16], and the μ opioid receptor gene (OPRM1; [17,18]). These inconsistent findings have tempered expectations and investment in both linkage and candidate gene studies.
Notably, many of these same limitations can be applied to candidate gene studies of gene x environment interactions attempting to model the moderating effects of environmental variables on the relations between candidate gene variants and AUD risk [19]. Further, these studies may introduce additional challenges associated with accurate measurement of the environment and a lack of protections against Type I error when multiple tests are conducted in the pursuit of indirect replications (e.g., nearby variants or similar environments; [20]).
CURRENT STATE OF THE AUD GWAS LITERATURE
Recent advances in genotyping microarray technologies have allowed for genome-wide coverage at reduced cost, thus resulting in a shift from linkage and candidate gene studies to a greater focus on genome-wide association studies (GWAS) to investigate the genetic risk for AUD. Using current methods, these studies individually test for an association between a phenotype of interest and ~7,000,000 variants across the genome. Despite their promise, many GWA studies conducted thus far have resulted in inconsistent findings, partially attributable to the complex genetic architecture of AUD. More specifically, traits with complex inheritance patterns, such as AUD, tend to demonstrate high levels of polygenicity in which hundreds of variants across the genome, each exhibiting a small effect size (R2 < 0.005), contribute to the genetic etiology of that trait [21]. The ready detection of these variants is further complicated by challenges with achieving genome-wide significance thresholds (p=5.0×10−8) that account for the testing of multiple genetic variants across the genome. As a result, many published GWAS of AUD have lacked adequate power to robustly detect associations at the genome-wide level [22–25].
To address these challenges, current efforts have focused on assembling larger sample sizes via consortia-led meta-analyses of GWAS datasets to increase power. While genome-wide significant loci for the AUD diagnosis have been limited to variants in the ADH1B and ADH1C genes (e.g., [14,26]), other etiologically relevant traits have proven more successful [24,25]. For example, the largest published GWAS of alcohol consumption to date (UK Biobank, N=112,117; [27]), reported significant associations with 14 loci. Results included a replication with variants in the ADH1B and ADH1C genes (rs145452708; p=1.15×10−30), as well as novel variants in the GCKR (rs1260326; p=1.34×10−21), KLB (rs11940694; p=8.14×10−19), and CADM2 (rs9841829; p=3.36×10−10) genes. Notably, these findings replicated those from two previous GWAS of alcohol consumption [28], one of which included a large trans-ancestral sample [29], demonstrating the influence of these susceptibility loci across multiple populations. These results illustrate the power that increased sample sizes can have on detecting and replicating genetic variants involved in AUD etiology. Notably, these studies only represent those that have been published thus far, with ongoing efforts from groups such as the Psychiatric Genomics Consortium Substance Use Disorder (PGC-SUD) Working Group [30] soon to be published as well.
Despite these advances, the molecular genetic investigation of the AUD diagnosis faces multiple challenges moving forward. Perhaps the largest challenge is the way in which the AUD diagnosis is operationalized. The DSM-5 [1] currently requires the endorsement of any 2 of 11 criteria to reach the diagnostic threshold for AUD at the mild severity level. This necessarily introduces high levels of heterogeneity into the AUD phenotype, even at the moderate level (4+ symptoms), and given that the genetic influences underlying AUD may not be shared equally across all symptoms [31], likely reduces the statistical power of GWAS focusing on the AUD diagnosis.
One potential approach for addressing heterogeneity in the AUD diagnosis is examining endophenotypes that focus on specific facets of AUD. Broadly speaking, endophenotypes can be thought of as any measurable component between genotype and the disorder of interest. For example, one well-established AUD endophenotype is level of response (LR) to alcohol, defined as the extent to which a specific blood alcohol level produces responses typically associated with alcohol intake (e.g.,[32]). LR is genetically influenced, and a low LR is a significant predictor of AUD risk [33–35]. Currently, studies focusing on endophenotypes have been limited to smaller samples (e.g., [36,37]), and thus, replicable findings are limited. As sample sizes increase, endophenotypes will likely play a larger role in gene discovery and will certainly be important for understanding the mechanisms through which genetic variation leads to increased risk for AUD.
POST-GWAS APPROACHES
In addition to gene discovery, recent molecular genetics research has focused on modeling the aggregate effects of variants across the genome and leveraging other types of ‘omics’ data to further our understanding of the genetic architecture underlying AUD. Often referred to as “Post-GWAS” approaches, these methods have been used to demonstrate the highly polygenic nature of alcohol-related traits, estimate the heritability and co-heritability of traits, test causal relations between traits, and aid in gene discovery [25,38].
Recent methodological advances have made possible the estimation of single nucleotide polymorphism (SNP) -based heritability (h2SNP) and genetic correlations between traits using genotype-level data or GWAS summary statistics. These approaches provide an estimate of the additive genetic variance that can be explained by common SNPs (i.e., those with a minor allele frequency >0.01) rather than the broad-sense heritability estimates typically reported in twin studies, which can include other types of genetic effects (e.g., rare variation, epistasis; [39]). Thus, h2SNP estimates provide an indication of the upper limit of GWAS as an approach for identifying genetic variation contributing to the etiology of a trait, and are typically smaller than heritability estimates obtained from twin studies. To illustrate, Genome-wide Complex Trait Analysis (GCTA;[40]) uses individual-level genotype data to estimate the genetic similarity of each participant-pair within a sample to create a genomic similarity matrix. This matrix is then used to partition variation in a trait into an h2SNP component and a residual. One of the first such studies conducted on alcohol-related traits reported an h2SNP estimate of 16% for AUD [41]. More recently, larger GWAS examining Alcohol Use Disorder Identification Test (AUDIT) scores [42] and alcohol consumption [27] have found h2SNP estimates of 12% and 13%, respectively. An alternative method, linkage disequilibrium (LD) score regression [43], requires only GWAS summary statistics to estimate h2SNP as well as the genetic correlation between traits of interest. For example, a recent study demonstrated a positive genetic correlation (rG= 0.40) between alcohol consumption and smoking status [27]. Notably, another study showed that AUDIT scores showed a positive genetic correlation with both alcohol consumption (rG=0.68) and AUD status (rG=0.68), suggesting strong genetic overlap between these phenotypes [42]. This is of particular importance given that combined GWAS of these alcohol measures are currently underway using recently developed meta-analytic methods that capitalize on correlated traits to further increase statistical power (e.g., [44,45]).
Another commonly used method of modeling GWAS data that has shown promise in understanding the genetic architecture of complex traits is the creation of polygenic risk scores (PRSs). Briefly, PRSs are generated by selecting variants in a discovery sample that meet a predetermined significance threshold for association with a trait of interest (e.g., alcohol response). Using an independent sample, PRSs are then calculated by combining genotype data across the selected variants in an additive fashion to create an aggregate measure of genetic risk that can then be tested for a relation to the same or a second etiologically-relevant trait. Within the substance use literature, this approach has been most widely applied to tobacco use with PRS based on smoking quantity shown to predict nicotine dependence (e.g., [46]), as well as alcohol and other substance use disorders more broadly (e.g., [47]). Additionally, PRSs for variants associated with alcohol use have been found to predict AUD status (14) as well as alcohol-related problems [48,49]. Notably, the utility of the PRS approach for studying the etiology of AUD will continue to grow as GWAS sample sizes, and thus the predictive power of their PRSs, increase.
In addition to the described advances studying genetic variation in aggregate, there has also been rapid development in methods leveraging other types of ‘omics’ data (e.g., epigenomics, transcriptomics) in hopes of promoting gene discovery and aiding interpretation of GWAS findings [24]. As an example of the latter, it has become routine for researchers to explore whether an associated variant also shows a relation with gene expression by querying databases such as GTEx [50]. For example, in the GWAS of alcohol consumption described above, the authors found that the most highly associated variant in CADM2 (rs9841829) was correlated with the expression of this gene in both lung and adipose tissue, supporting a regulatory role for this variant. Similar databases cataloguing other types of ‘omics’ data can also be queried (e.g., DNA methylation, epigenetic signatures) to aid in the interpretation of significant associations.
There have also been efforts to combine different types of ‘omics’ data into a single analysis to aid in gene identification, though few such efforts have been published in the alcohol literature. As an example, PrediXcan models data from GTEx and similar gene expression databases to impute tissue-specific gene-expression based on an individual’s genotype data and uses these imputed gene-expression values to test for associations at the gene level [51]. Using this approach, a recent study demonstrated a positive association between hippocampal expression of CDK3 and delayed discounting, a devaluation of future reward often found to be associated with substance use [52]. As another example, a recent study conducted a combined analysis of methylome-wide association and GWA data in a single sample to identify an association between variants in an intronic region of CNTN4 and alcohol use [53]. Though the reported associations require replication, these studies provide important illustrations of the progression of molecular genetic investigations of alcohol-related traits.
CONCLUSIONS
Moving forward, continued efforts to integrate large GWAS datasets examining alcohol use remain critical to the detection and replication of genome-wide significant associations. These findings will further our understanding of the genetic etiology of AUD, and will also promote the advancement of “Post-GWAS” approaches seeking to better understand the mechanisms through which genetic variation leads to increased AUD risk. It is hoped that such information will ultimately lead to improved prevention and treatment efforts.
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (F31AA025516 and T32AA013526). NIAAA had no role in the writing of this report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: none
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Due to space constraints the present review will use the term AUD to refer to both DSM-5 defined alcohol use disorder and DSM-IV defined alcohol dependence. The latter required the presence of 3+ symptoms out of 7 to meet diagnostic threshold.
REFERENCES
**valuable contribution in providing a historical review of the AUD Genetics literature.
- 1.American Psychiatric Association; Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Publishing; 2013 [Google Scholar]
- 2.Hedden SL, Kennet J, Lipari R, Medley G, Tice P, Copello EAP, et al. Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. 2015;74. [Google Scholar]
- 3.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41(5):516–524. [DOI] [PubMed] [Google Scholar]
- 4.Rehm J, Gmel GE, Gmel G, Hasan OSM, Imtiaz S, Popova S, et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction. 2017;112(6):968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015. August 1;72(8):757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103(1):92–102. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008. July 1;103(7):1069–81. **This review summarizes evidence of heritability from family, adoption and twin studies of substance use disorders with an emphasis on examining distinctions between early stage substance use and the later stages of addiction as well as the validity substance-specific genetic influences.
- 8.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015. April;45(5):1061–72.This recent meta-analysis of twin and adoption studies represents the most comprehensive and most contemporary meta-analytic estimate of AUD heritability to date and explores moderations of estimates by sex, study design and assessment method.
- 9.Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci. 2002. March 19;99(6):3729–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reich T, Edenberg HJ, Goate A, Williams JT, Rice JP, Van Eerdewegh P, et al. Genome wide search for genes affecting the risk for alcohol dependence. Am J Med Genet. 1998;81(3):207–215.First large-scale genome-wide linkage analysis of alcohol dependence in a population of mostly European ancestry. Published by the Collaborative Study on the Genetics of Alcoholism , the results of this study indicated the presence of protective loci for alcohol dependence near alcohol dehydrogenase genes.
- 11.Edenberg HJ, Foroud T. REVIEW: The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006. September 1;11(3–4):386–96. ** [DOI] [PubMed] [Google Scholar]
- 12.Edenberg HJ, Xuei X, Chen H-J, Tian H, Wetherill LF, Dick DM, et al. Association of alcohol dehydrogenase genes with alcohol dependence: a comprehensive analysis. Hum Mol Genet. 2006. May 1;15(9):1539–49. [DOI] [PubMed] [Google Scholar]
- 13.Luczak SE, Glatt SJ, Wall TJ. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull. 2006;132(4):607–21.This paper demonstrated the replicability of ALDH2 protective associations with alcohol dependence in East Asian populations through the first meta-analysis of 15 such studies. Additionally, this study tested moderating effects of diagnostic assessment, recruitment strategy and gender, highlighting the importance of such variables when assessing the relation between genetic polymorphism and phenotype.
- 14.Frank J, Cichon S, Treutlein J, Ridinger M, Mattheisen M, Hoffmann P, et al. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addict Biol. 2012. January 1;17(1):171–80.This paper represents the first GWAS of alcohol dependence to provide evidence of associations near the ADH gene cluster which surpassed genome-wide significance. The study demonstrated genome-wide significance for a variant (rs1789891) located in a region between the ADH1B and ADH1C genes which is in linkage disequilibrium with the Arg272Gln variant of the ADH1C gene. The authors also conducted a polygenic score-based approach for alcohol dependence which resulted in significant differences between patients and controls.
- 15.Dick DM, Foroud T. Candidate Genes for Alcohol Dependence: A Review of Genetic Evidence From Human Studies. Alcohol Clin Exp Res. 2003;27(5):868–879. ** [DOI] [PubMed] [Google Scholar]
- 16.Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, et al. Variations in GABRA2, Encoding the α2 Subunit of the GABAA Receptor, Are Associated with Alcohol Dependence and with Brain Oscillations. Am J Hum Genet. 2004. April;74(4):705–14.This study examined the viability of brain oscillations in the beta frequency range as an endophenotype conferring further risk for development of alcohol dependence. Linkage and association analyses concluded that a cluster of GABA genes on chromosome 4 were significantly associated with both beta brain oscillations and alcohol dependence.
- 17.Chamorro A-J, Marcos M, Mirón- Canelo J-A, Pastor I, González- Sarmiento R, Laso F-J. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17(3):505–12. [DOI] [PubMed] [Google Scholar]
- 18.Ray LA, Hutchison KE. A Polymorphism of the μ-Opioid Receptor Gene (OPRM1) and Sensitivity to the Effects of Alcohol in Humans. Alcohol Clin Exp Res. 2004;28(12):1789–95.First candidate gene study to examine the association between the A118G variant of the OPRM1 gene and subjective ratings of acute alcohol intoxication, indicating that those with the G allele report higher subjective intoxication and may be more sensitive to the effects of alcohol.
- 19.Harden KP, Hill JE, Turkheimer E, Emery RE. Gene-Environment Correlation and Interaction in Peer Effects on Adolescent Alcohol and Tobacco Use. Behav Genet. 2008. July 1;38(4):339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, et al. Candidate Gene–Environment Interaction Research: Reflections and Recommendations. Perspect Psychol Sci. 2015. January;10(1):37–59. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ioannidis JPA, Trikalinos TA, Khoury MJ. Implications of Small Effect Sizes of Individual Genetic Variants on the Design and Interpretation of Genetic Association Studies of Complex Diseases. Am J Epidemiol. 2006. October 1;164(7):609–14. [DOI] [PubMed] [Google Scholar]
- 22.Edenberg HJ, Foroud T. Genetics and alcoholism. Nat Rev Gastroenterol Hepatol. 2013. August;10(8):487–94. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enoch M-A. Genetic Influences on the Development of Alcoholism. Curr Psychiatry Rep. 2013. November 1;15(11):412. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock DB, Markunas CA, Bierut LJ, Johnson EO. Human Genetics of Addiction: New Insights and Future Directions. Curr Psychiatry Rep. 2018. February 1;20(2):8. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart AB, Kranzler HR. Alcohol Dependence Genetics: Lessons Learned From Genome-Wide Association Studies (GWAS) and Post-GWAS Analyses. Alcohol Clin Exp Res. 2015. August 1;39(8):1312–27. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH, et al. Genome-wide association study of alcohol dependence: significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014. January;19(1):41–9.This study represents expanded the current literature by demonstrating the first genome-wide significant association for alcohol dependence in an African American population. Results provided additional genome-wide support for risk loci near alcohol-metabolizing enzyme genes, novel alcohol dependence genome-wide associations across both European American and African American cohorts in addition to multiple population specific associations.
- 27.Clarke T-K, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Mol Psychiatry. 2017. October;22(10):1376–84.This recent paper represents the largest genome-wide association study (GWAS) of alcohol use currently published. This study found genome-wide significant associations with alcohol consumption for 14 loci. Top associations included rs145452708 (p=1.15×10–30) found in the alcohol metabolizing genes on chromosome 4 (AHD1B/ADH1C), a signal located on chromosome 4 in the GCKR gene (rs1260326; p=1.34×10–21), an association of interest in the KLB gene (rs11940694; p=8.14×10–19), as well as CADM2 (rs9841829; p=3.36×10−10). Additional findings included a SNP-based heritability estimate of 12% for alcohol consumption, as well as a positive genetic correlation between alcohol consumption and smoking.
- 28.Schumann G, Liu C, O’Reilly P, Gao H, Song P, Xu B, et al. KLB is associated with alcohol drinking, and its gene product β-Klotho is necessary for FGF21 regulation of alcohol preference. Proc Natl Acad Sci. 2016. December 13;113(50):14372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgenson E, Thai KK, Hoffmann TJ, Sakoda LC, Kvale MN, Banda Y, et al. Genetic contributors to variation in alcohol consumption vary by race/ethnicity in a large multi-ethnic genome-wide association study. Mol Psychiatry. 2017. September;22(9):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal A, Edenberg HJ, Gelernter J. Meta-Analyses of Genome-Wide Association Data Hold New Promise for Addiction Genetics. J Stud Alcohol Drugs. 2016. September;77(5):676–80.This perspective paper reviews the ongoing efforts of the Psychiatric Genomics Consortium Substance Use Disorder (PGC-SUD) Working Group and discusses the utility of synthesizing quantitative data from multiple genome-wide association (GWA) datasets allowing for meta-analytic approaches that are well powered to identify single variant associations with substance use disorders, including AUD.
- 31.Palmer RHC, McGeary JE, Heath AC, Keller MC, Brick LA, Knopik VS. Shared Additive Genetic Influences on DSM-IV Criteria for Alcohol Dependence in Subjects of European Ancestry. Addict Abingdon Engl. 2015. December;110(12):1922–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980. March;41(3):242–9. [DOI] [PubMed] [Google Scholar]
- 33.Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29(5):1069–1081. [DOI] [PubMed] [Google Scholar]
- 34.Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI. Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol. 1996. July;57(4):368–77. [DOI] [PubMed] [Google Scholar]
- 35.Viken RJ, Rose RJ, Morzorati SL, Christian JC, Li T-K. Subjective Intoxication in Response to Alcohol Challenge: Heritability and Covariation With Personality, Breath Alcohol Level, and Drinking History. Alcohol Clin Exp Res. 2003;27(5):795–803. [DOI] [PubMed] [Google Scholar]
- 36.Enoch M-A. Genetic influences on response to alcohol and response to pharmacotherapies for alcoholism. Pharmacol Biochem Behav. 2014. August 1;123:17–24. ** [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joslyn G, Ravindranathan A, Brush G, Schuckit M, White RL. Human Variation in Alcohol Response Is Influenced by Variation in Neuronal Signaling Genes. Alcohol Clin Exp Res. 2010. May 1;34(5):800–12.This paper represents the first published genome-wide association study (GWAS) examining genetic contributions to an individual’s level of response (LR) to alcohol. No individual genome-wide significant associations with a combination of three LR to alcohol variables: the SRE (Subjective Response to the Effects of Alcohol), SHAS (Subjective High Assessment Scale), and BSA (Body Sway Assessment). Notably, a gene-set analysis found enrichment for genetic variation in neuronal signaling pathways with LR to alcohol, and has influenced future GWA studies of other alcohol response outcomes.
- 38.Salvatore JE, Han S, Farris SP, Mignogna KM, Miles MF, Agrawal A. Beyond genome-wide significance: integrative approaches to the interpretation and extension of GWAS findings for alcohol use disorder. Addict Biol [Internet]. 2018This recent review provides exhaustive coverage of Post-GWAS approaches for leveraging results from genome-wide association studies (GWAS) to investigate the genetic etiology of AUD. In this paper the authors extend extant findings from AUD GWAS by providing a discussion of approaches for studying the aggregate effects of genetic variants as well as methods for aiding interpretation in gene discovery efforts.
- 39.Yang J, Zeng J, Goddard ME, Wray NR, Visscher PM. Concepts, estimation and interpretation of SNP-based heritability. Nature Genetics. 2017. August 30;49(9):1304–10. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A Tool for Genome-wide Complex Trait Analysis. Am J Hum Genet. 2011. January 7;88(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three Mutually Informative Ways to Understand the Genetic Relationships Among Behavioral Disinhibition, Alcohol Use, Drug Use, Nicotine Use/Dependence, and Their Co-occurrence: Twin Biometry, GCTA, and Genome-Wide Scoring. Behav Genet. 2013. March 1;43(2):97–107.This paper provided the first SNP-based heritability estimates for alcohol dependence (AD) and alcohol consumption using Genome-wide Complex Trait Analysis (GCTA). SNP heritability for AD and alcohol consumption were estimated to be 16% and 19%, respectively. Additionally, the authors demonstrated the polygenic nature of alcohol dependence by modeling polygenic risk scores for AD and alcohol consumption, finding that at more inclusive p-value thresholds. aggregate genetic effects were able to explain a larger proportion of variation of alcohol use.
- 42.Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, Wit H de, Davis LK, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20,328 research participants of European ancestry. Addict Biol. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Consortium SWG of the PG, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015. March;47(3):291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, et al. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018. February;50(2):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu X, Feng T, Tayo BO, Liang J, Young JH, Franceschini N, et al. Meta-analysis of Correlated Traits via Summary Statistics from GWASs with an Application in Hypertension. Am J Hum Genet. 2015. January 8;96(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H, et al. Polygenic Risk and the Developmental Progression to Heavy, Persistent Smoking and Nicotine Dependence: Evidence From a 4-Decade Longitudinal Study. JAMA Psychiatry. 2013. May 1;70(5):534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vink JM, Hottenga JJ, Geus EJC de, Willemsen G, Neale MC, Furberg H, et al. Polygenic risk scores for smoking: predictors for alcohol and cannabis use? Addiction. 2014;109(7):1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salvatore JE, Aliev F, Edwards AC, Evans DM, Macleod J, Hickman M, et al. Polygenic Scores Predict Alcohol Problems in an Independent Sample and Show Moderation by the Environment. Genes. 2014. April 10;5(2):330–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savage JE, Salvatore JE, Aliev F, Edwards AC, Hickman M, Kendler KS, et al. Polygenic Risk Score Prediction of Alcohol Dependence Symptoms Across Population-Based and Clinically Ascertained Samples. Alcohol Clin Exp Res. 2018. March 1;42(3):520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Consortium GTEx. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science. 2015. May 8;348(6235):648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet. 2015;47(9):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Roige S, Fontanillas P, Elson SL, Pandit A, Schmidt EM, Foerster JR, et al. Genome-wide association study of delay discounting in 23,217 adult research participants of European ancestry. Nat Neurosci. 2018. January;21(1):16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark SL, Aberg KA, Nerella S, Kumar G, McClay JL, Chen W, et al. Combined whole methylome and genome-wide association study implicates CNTN4 in alcohol use. Alcohol Clin Exp Res. 2015. August;39(8):1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]