Abstract
The syndrome of frailty for patients undergoing heart or lung transplantation has been a recent focus for perioperative clinicians due to its association with postoperative complications and poor outcomes. Patients with end stage cardiac or pulmonary failure may be under consideration for heart or lung transplantation, as well as bridging therapies such as ventricular assist device implantation or venovenous extracorporeal membrane oxygenation, respectively. Early identification of frail patients in an attempt to modify risk of postoperative morbidity and mortality has become an important area for study over the last decade. Many quantification tools and risk prediction models for frailty have been developed, but have not been extensively evaluated nor standardized in the cardiothoracic transplant candidate population. Heightened awareness of frailty, coupled with a better understanding of distinct cellular mechanisms and biomarkers apart from end-stage organ disease, may play an important role in potentially reversing frailty related to organ failure. Furthermore, the clinical management of these critically ill patients may be enhanced by waitlist and postoperative physical rehabilitation and nutritional optimization.
Keywords: Frailty, Cardiothoracic surgery, Heart transplantation, Lung transplantation, Biomarker, Critical Care, ICU, Rehabilitation, Nutrition, Heart Failure, Chronic Lung Disease, COPD, LVAD, ECMO, IABP, IGF-1, Growth Hormone, Metabolism, Sarcopenia, Cachexia
Introduction
Frailty is a multidimensional syndrome associated with subclinical dysfunction and limited physiologic reserve that may affect one or more organs.1 Optimization of the frail surgical patient undergoing heart or lung transplantation is an emerging focus for the perioperative clinician. Patients undergoing heart or lung transplantation have a particularly high incidence of frailty due to the presence of end-stage cardiopulmonary failure and the tendency to be older with more comorbidities compared to a younger and healthier cohort.2 The frail patient often displays a protracted and devastating clinical course after the inception of an inciting clinical event (Figure 1). Early identification of frailty has emerged as an important predictor for clinical progression in patients awaiting heart or lung transplantation and recent advances in clinical research have attempted to define the impact of frailty on clinical outcomes. There has been an increasing need for consistent quantification and standardized screening tools to identify frailty due to evidence suggesting that frailty is strongly associated with adverse events for such patients undergoing surgery. Furthermore, patients undergoing heart or lung transplantation may experience age-related (organ failure-independent) frailty, disease-related (organ failure-dependent) frailty, or both during the perioperative period (Figure 2).3–5 This categorization may, in part, inform on the patient’s clinical trajectory. The concept of frailty in those undergoing heart-lung transplantation en bloc, or other combinations of organ transplantation is even more challenging to understand due to the small number of patients and centers, which perform these complex operations. Therefore, discussion regarding multi-organ failure and transplantation will not be included in this review.
Figure 1.
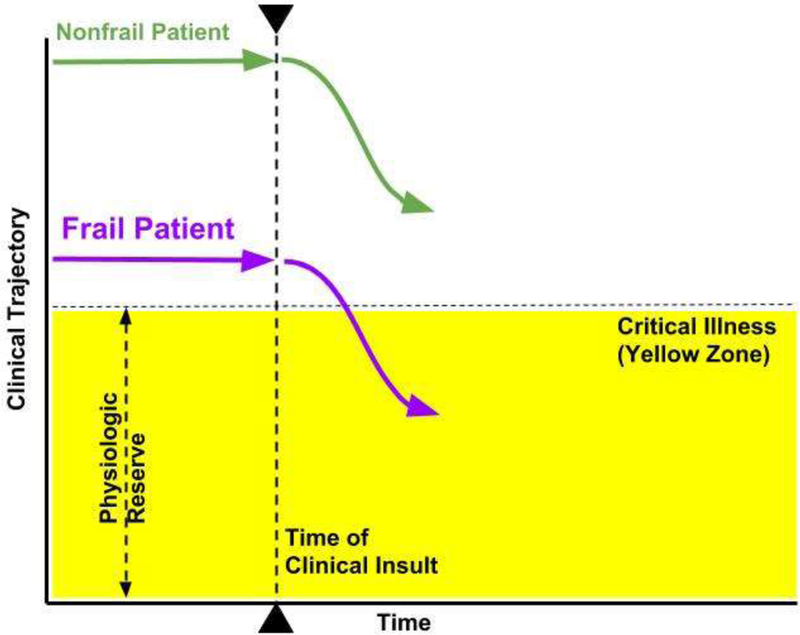
The Frailty Concept. In a simple model displaying the clinical trajectory of a frail patient compared with a nonfrail phenotype, a clinical insult such as an illness may worsen each patient’s clinical condition (dotted line with arrowheads). The frail patient, however, requires less clinical insult to rapidly decompensate below the level of intrinsic physiologic reserve and become critically ill (purple line). The nonfrail patient has more time to respond to treatment and has a stronger chance of recovery (green line) when compared with the frailty phenotype.
Figure 2.
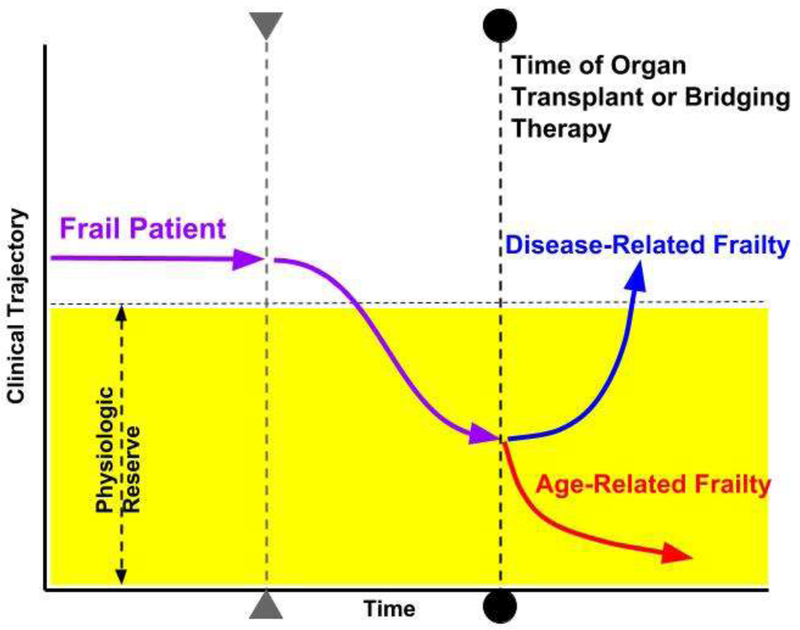
Disease-Related Frailty vs. Age-Related Frailty. Using the Frailty phenotype illustrated in Figure 1, the patient with disease-related frailty may clinically improve (blue line) after organ transplantation or implementation of bridging therapy (dotted line with circles), such as LVAD implantation or ECMO deployment. Destination therapy LVAD implantation may also display a similar improvement in clinical trajectory. Conversely, a frail patient with age-related disease may have a lower chance for improvement with organ transplantation or bridging therapy and therefore rapid decompensation may result in a higher incidence of reduce survival (red line).
The number of adult patients that undergo heart or lung transplantation continues to grow annually, with significant heterogeneity in terms of demographics and indication for transplantation.2 As the population ages, this patient group now encompasses an increasing number of elderly patients, with higher disease burden than their younger counterparts; this is associated with increased incidence of previously existing medical conditions or prior surgeries that may further intensify the risks associated with. transplantation, as well as increase perioperative and postoperative resource utilization.6 In heart or lung transplantation recipients, the fastest growing age demographic are those patients that are >65 years old.7, 8 The prevalence of frailty across medical and surgical patients according one source is approximately 5% in those over 70 years-old and escalates to 25% incidence in patients aged 85–89 years-old.9 This marginal increase from 5 to 25% despite a relatively larger increase in elder patients suggests that frailty may be less age-dependent than has been previously perceived and is rather multifactorial. This is supported by the observation that frailty occurs in younger patients with advanced, end-stage disease progression.10 Furthermore, there may be a cellular basis for the clinical presentation and frailty phenotype, which require further evaluation.
In this review, we aim to: 1) understand current definitions and categorization of frailty 2) recognize the implications of frailty on outcomes in the context of heart or lung transplantation 3) review potential cellular mechanisms and biomarkers which may represent therapeutic targets and 4) frailty modification through nutritional support, physical therapy and rehabilitation, as well as organ transplantation or mechanical support.
Age-Related and Disease-Related Frailty
Frailty may be categorized as: 1) frailty related to aging in the general population independent of end-organ disease (age-related) and 2) frailty related to chronic end-organ disease (disease-related).11 This may be an oversimplification of such a complex syndrome and the net result may often encompass an amalgam of both categories. The similar clinical presentation of both age-related and disease-related frailty identifies an important challenge for physicians when determining candidacy for organ transplantation or bridging therapies through mechanical circulatory support (e.g., Venovenous Extracorporeal Membrane Oxygenation, VV ECMO or left ventricular assist device, LVAD). However, those with disease-related frailty may have potential reversibility of their frailty symptoms after transplantation or bridging mechanical circulatory support and incur a survival benefit with meaningful quality of life after transplant (Figure 2).12 Current available frailty assessment tools, however, do a poor job of differentiating between organ failure- and age-related frailty.
As discussed, disease- and age-related frailty may coexist in the growing population of older transplant candidates. Subpopulations of lung transplant (e.g., COPD and interstitial lung disease/ILD) or heart transplant (e.g., ischemic cardiomyopathy) candidates may be more susceptible to concomitant elderly age and disease-related frailty. Conversely, disease states that present earlier in life (e.g., cystic fibrosis, congenital heart disease, or postpartum cardiomyopathy) may be categorized as disease-related frailty. Meanwhile, the ability to differentiate between disease and age-related frailty remains a challenge, particularly in a rapidly increasing population of older transplant recipients. Furthermore, some disease processes may predispose patients to a higher incidence of malnutrition due to malabsorptive states (e.g., cystic fibrosis or heart failure-associated cachexia), which can contribute to the presence of sarcopenia and weakness. Poor nutrition may increase the incidence of frailty in these subpopulations, and further confound the clinician’s ability to distinguish between age- and disease-related frailty. This distinction is of crucial importance in order to gauge the course of postoperative recovery and clinical trajectory after surgery.
Muscle Mass, Sarcopenia, and Cachexia
Sarcopenia, or muscle wasting, is an important physiologic marker of frailty. A report from the European Working Group defined sarcopenia as decreased muscle mass and the presence of at least one reduced peripheral muscle strength or function.13 Muscle strength of the upper and lower extremities may be measured using isometric and isokinetic testing. Likewise, muscle mass may be quantified by several methods, including bioelectrical impedance, measurement of skin folds, or muscle cross sectional area by computed tomography (CT) or magnetic resonance imaging (MRI).14 The specific means by which these tests are performed are beyond the scope of this review.
Cachexia, within the framework of frailty, describes muscle weakness and wasting.13 Specifically, cachexia has been described as an unintentional loss of more than 5% body weight over six months plus any three of the common associated findings (Table 1).15 Both sarcopenia and cachexia have significant impact on quality of life and clinical outcomes. For example in a cohort of 161 chronic congestive heart failure (CHF) patients separated into those with (N=39, 19.5%) and without sarcopenia (N=122, 80.5%), sarcopenic patients had increased weakness related to handgrip and quadriceps strength, reduced total peak oxygen consumption, decreased exercise time, and a reduced six-minute walk distance or test (6MWD or 6MWT).Notably, the 6MWD is a suitable index for the determination of baseline physical function related to a myriad of patient populations with chronic illness (Table 2). Further, patient performance on these tests is correlated to pre-transplant frailty measures, but not to recovery after lung transplant. In the chronic obstructive pulmonary disease (COPD) population, the 6MWD has been utilized in order to monitor therapeutic response.16 However, the 6MWD has been difficult to universally implement amongst transplant centers due to inaccuracy of a single, preoperative 6MWD in determining post-transplant survival and the resource utilization of performing serial 6-minute walk tests during the pre-transplant period.17
Table 1.
Common Criteria for Cachexia in Frailty
| Major Criteria: |
| ≥ 5% ↓ in Body Weight over prior 6 months |
| PLUS |
| Minor Criteria (any 3 of the following): |
| • Decrease in Muscle Strength |
| • Fatigue |
| • Anorexia |
| • Muscle circumference (upper arm or thigh) < 10th percentile for age and gender |
| • Abnormal biomarkers: ᴑ CRP >5.0 mg/L ᴑ IL-6 >4.0 pg/mL ᴑ Hemoglobin < 12 g/dL ᴑ Serum Albumin < 3.2 g/dl |
Abbreviations: CRP = C-reactive protein; IL-6= Interleukin-6
Table 2.
Commonly used Frailty Quantification Tools available in Heart Failure and End-Stage Lung Disease Patients
| Frailty Quantification Tool | Description | Components Included |
|---|---|---|
| Fried Frailty Phenotype 7 | Presence of 3/5: 1.Slow Gait 2.Low Physical activity 3.Unintentional Weight loss 4.Exhaustion 5Weakness |
Physical Status, Mobility, Nutritional Status, Strength, Energy |
| Frailty Deficit Index38 | 1. Symptoms 2. Clinical signs 3. Disabilities 4. Laboratory data 5. Radiographic data (>40 deficits possible) |
Physical Status, Mobility, Nutritional Status, Strength, Energy, Cognition, Mood, Social Support |
| Short Physical Performance Battery97 |
1.Walking speed 2. Sit-to-stand 3. Gait balance |
Physical Status, Mobility, Strength |
| 6 Minute Walk Distance or Test (6MWD or 6MWT)98 |
1. Distance patient can walk in six minutes. 2. Monitor oxygen saturation and clinical perception of dyspnea |
Physical Status, Mobility, Energy |
BMI= body mass index; 6MWD= 6-minute walk distance (also referred to as the 6-minute walk test, 6MWT)
Not surprisingly, the cachectic state has been found to be a strong independent risk factor for mortality in patients with CHF. Anker et al. used higher weight loss cut-off for cachexia at > 7.5% unintentional (as compared to the previously described 5% weight loss – see Table 1), documented within at least 6 consecutive months.18 Cachexia was predictive of 18-month mortality in nonsurgical, heart failure patients, independent of age, New York Heart Association (NYHA) functional class, left-ventricular ejection fraction, or peak oxygen consumption. Cachexia and concurrently reduced peak oxygen consumption identified a subset of study patients at extremely high risk for death.18 Advanced heart failure disease progression is frequently associated with anorexia and inadequate nutritional status contributes to cachexia and has been linked to poor clinical outcomes. These poor outcomes are independent of the mechanical circulatory support implantation. Nutritional status was evaluated by the Studies Investigating Comorbidities Aggravating Heart Failure investigators in an analysis of outpatient HF subjects evaluated by using the Mini-Nutritional Assessment- Short Form.19 When compared with HF patients without sarcopenia, those with muscle wasting had significantly lower values of peak oxygen consumption, 6MWD, Short Physical Performance Battery (SPPB – Table 2), and Mini-Nutritional Assessment-Short Form scores. Mini-Nutritional Assessment- Short Form remained an independent predictor of muscle wasting and mortality after adjustment for age and NYHA class.19
The absence of standardized guidelines for the quantification of sarcopenia remains an important challenge for lung transplant recipients.14, 20 Several studies have validated specific measures of muscle mass in end-stage COPD patients, which include mid-thigh cross sectional area, mid-arm circumference, and bioelectrical impedance. These measures have been linked to short- and long-term morbidity and mortality and confer better prediction of survival than body mass index (BMI).21–24 As a means of validating such methods for determining sarcopenia in the lung transplant population, a single-center, retrospective cohort of 36 lung transplant recipients utilized CT imaging performed within six months of transplant surgery and allowed for radiographic measurements of muscle mass. Specifically, muscle index was measured using appropriate Hounsfield units of radiodensity by CT scan of the cross-sectional area of skeletal muscle mass measured at the L2–L3 interspace. Lung transplant patients with a muscle index within the lower 25th percentile of the study population had an associated reduced survival (Hazard ratio, HR, for mortality = 3.83; 95% confidence interval, CI, 1.4 −10.3; p = 0.007) and longer hospital length of stay (LOS).25 A variety of techniques used to study sarcopenia across 694 lung transplant patients were highlighted in a recent systematic review.14 Muscle mass was measured by bioelectrical impedance, CT, or MRI, and/or skin fold measurement. Muscle strength was determined by quadriceps extension, handgrip evaluation, or “sit-to-stand” testing, which evaluates strength within multiple muscles of the lower extremities. Patients determined to be sarcopenic in the studies described above experienced preoperative weakness, followed by worsening postoperative weakness beginning in the early period after transplant surgery.
Frailty and Current Cardiothoracic Surgical Risk Models
The Society of Thoracic Surgeons Predicted Risk of Mortality or Major Morbidity (STS-PROMM) score has been developed to predict 30-day mortality for patients undergoing coronary artery bypass graft (CABG) surgery, single valve repairs and replacements, or both CABG and single valve operations. The STS-PROMM score has been more predictive of risk in the elderly once additional components of frailty and disability are added.26 In addition, functional testing using gait speed27 and the 6MWD28 have been shown to augment the STS-PROMM risk prediction for 30-day mortality in elderly patients. In a cohort of patients undergoing CABG surgery, isolated valve surgery, or combined operations, Lytwyn et al. found that frailty was associated with a 2- to 3.5-fold higher risk of poor functional survival at 1-year, defined as being alive and having an inadequate health-related quality of life.29 The addition of any frailty assessment (Table 2) to other traditional surgical risk scores, such as the STS-PROMM or the European System for Cardiac Operative Risk Evaluation II (EuroSCORE II), provides important incremental value in the identification of patients at risk of poor functional survival. Poor functional survival is defined as being alive at 1 year post-surgery with a health-related quality of life (QOL) score greater than 60 on the EuroQOL-Visual Analogue Scale.29
There remain challenges, however, in being able to enhance surgical outcome prediction through frailty incorporation for cardiothoracic transplant recipients. These patients are an incredibly diverse population, with many different constellations of medical pathophysiologies (e.g. pulmonary hypertension, connective tissue disorders, cystic fibrosis, adults surviving with previous congenital heart repairs, previous LVAD implantations) that undeniably influence surgical risk and outcome. Therefore, a careful scoring system should be developed and validated in these patients beyond standard available scoring systems, which can clearly distinguish frailty from disease processes. The lack of standardization for defining and quantifying frailty in heart or lung transplant candidates results in inconsistent application across centers. Such barriers related to incorporation of standardized frailty measurements in patients awaiting heart or lung transplantation is readily seen in the United States lung and heart allocation systems.
Frailty and Current Organ Allocation Systems
In the United States, the system to allocate solid organs for transplantation is regulated and maintained by the United Network for Organ Sharing (UNOS). Incorporating frailty scoring within organ allocation systems for thoracic organ transplant recipients remains a challenge due to the lack of high-level evidence support use despite several causal inference studies suggesting the association of frailty with poor post-transplant outcomes. For the lung transplant population, recipient age is a part of the lung allocation score (LAS); the LAS is a risk scoring system designed to prioritize organs for patients with the highest illness severity and likelihood of waitlist mortality (for specific components of the LAS calculator, please refer to: https://optn.transplant.hrsa.gov/resources/allocation-calculators/las-calculator/). Furthermore, recipient age alone does not predict primary graft failure after transplantation, although older recipient age has been associated with shorter survival times in the postoperative period.30 Interestingly, a shift in LAS scoring in 2005 from 1-year anticipated survival after transplantation to wait list urgency while awaiting transplantation in diseases which occur more commonly in the elderly (e.g., interstitial pulmonary fibrosis), has been in part responsible for the steady increase in lung transplant recipient age.31 Reference to frailty in the LAS calculation is subtle and includes the determination of assistance level to aide in daily activities (no assistance, partial assistance, or total assistance) and the 6MWD in meters, although these two categories could be related to the natural history of end-stage lung disease and are challenging to distinguish from the frailty syndrome. Frailty has been shown to be associated with poor outcomes in lung transplantation,20, 32 but has yet to be universally defined and incorporated in current LAS model.
At present, the United States heart allocation system does not incorporate frailty assessment into status categorization. Furthermore, the proposed update to the heart allocation system will, likewise, not include frailty assessment.33 Similar to end-stage lung disease patients on the transplant waitlist, an important barrier to utilizing frailty determination in heart failure patients awaiting transplantation is the variable application of frailty assessment in order to define, quantify, and incorporate frailty into surgical risk scoring and outcome prediction for cardiothoracic transplantation.
Frailty Assessment and Clinical Outcomes
The awareness of frailty and its association with adverse clinical events in medical and surgical patient populations has led to an increasing number of investigations, which have evaluated prevalence, associated predictors, and potential causal relationships. The prevalence of frailty among medical and surgical patients varies within the range of 4–16% among patients ≥ 65 years old.34–36 The incidence of frailty amongst lung transplant recipients approaches 28%, with an increased association with postoperative morbidity and mortality.20 Common objective frailty quantification methods have been described and are predictive of increased morbidity and mortality (Table 2). Although more than 20 scoring tools have been developed, two have demonstrated predictive validity: the Frailty Deficit Index (FDI) and the Fried Frailty Phenotype (FFP). The FDI highlights overall deficit accumulation, wherein there is lifetime accrual of deficits that result in impaired organ function (Table 2).37 Symptoms, signs, diseases, and disabilities are considered deficits that compound together in order to form this frailty index. The FDI therefore confers the likelihood that frailty is present. The FFP measures weakness, reduced speed of activities, reduced amount of activities, exhaustion, and weight loss (Table 2).9, 38 The FFP differs from the FDI in that it determines frailty by clinical measurements, which include the presence of sarcopenia, cachexia, and limited physical exertion.9 The FFP and FDI currently remain amongst the commonly utilized frailty assessment tools likely due to their rapid use and mainly objective data determination. Components common to newly-developed frailty assessment tools include not only physical status, mobility, nutritional status, and strength, but may include energy, cognition, mood, and social support.39
Patients with End-Stage Lung Disease
The previously described quantification tools have identified and quantified frailty and helped describe the relationship of frailty with poor outcomes after lung transplantation. In a prospective, single-center study (n=144), the FDI was measured based primarily on a self-reported patient questionnaire prior to lung transplantation. 32 Results showed a strong association with increased risk for death in frail patients compared with non-frail patients (unadjusted HR = 2.28 [95% CI, 1.27 – 4.16; p = 0.006] and adjusted HR for age, gender, and double- vs. single-lung transplantation = 2.24 [95% CI, 1.22 – 4.19; p = 0.0089]). This study further demonstrated reduced performance on the 6MWD (296 feet vs. 241 feet, p=0.01), increased LAS score (42 vs. 37, p=0.03), and increased number of days on the transplant wait list (169.5 days vs. 101 days, p=0.02) in frail compared to non-frail patients, respectively.32 Although the authors do not specifically address the reason for a longer transplant wait list time in frail patients, they do note that the median waitlist time was < 6 months for all study patients and that longer transplant wait times did not impact mortality in the frail patients compared with the nonfrail (HR, 1.00; 95% CI 0.99–1.00). 32 Singer et al. evaluated 395 lung transplant recipients in a multicenter, prospective study that coupled the FFP with the Short Physical Performance Battery, measured incidence of frailty, and compared frail with non-frail groups in terms of physical impairment, mortality, and postoperative complications.20 This study demonstrated that frailty as defined by FFP (score > 3) and SPPB (score < 7) correlated more strongly with exercise capacity and grip strength than with lung function, and the presence of frailty by either measure was associated with disability.20 Frail patients were preoperatively more likely to suffer greater disability and incur an increased rate of delisting or death before lung transplantation at one year using either FFP or SPPB. In fact, the HR would increase for every 1-point worsening in the score for each test (HR=1.30 [95% CI, 1.01–1.67] for FFP and 1.53 [95% CI, 1.19–1.59] for SPPB).20
Notably, poor functional status related to end stage pulmonary disease may be improved by adding VV ECMO in select patients. This avoids the complications associated with prolonged intubation, ventilator associated lung injury, and ventilator associated pneumonia. Although mechanical circulatory support historically has been prohibitive to physical rehabilitation, transplant centers that offer bridging with VV ECMO have developed programs utilizing peripheral cannulation strategies that allow for ambulation.40 This has been described in small retrospective studies, but patients with ambulatory ECMO displayed a significant reduction in the duration of mechanical ventilation as well as reduced ICU and hospital LOS when compared with their non-ambulatory counterparts,40, 41 although these studies did not specifically evaluate endpoints of frailty. A comprehensive, multidisciplinary team approach with adequate resource allocation must be utilized along with careful planning of all physical rehabilitation sessions for patients on bridging ECMO therapy in order to ensure patient safety.
Patients with Heart Failure
For advanced HF patients who are heart transplant candidates, the prevalence of frailty is a strong component of chronic disease. In a single-center study, Denfeld et al. found that the “frailty phenotype” (sarcopenia, weakness, physical exhaustion, and low physical activity) was identified in 49% of the subjects with NYHA class II-IV classifications and frailty was associated with low cardiac output HF.42 In a recent meta-analysis of 26 studies involving 6,896 HF patients worldwide, the overall estimated prevalence of frailty was 44.5% with a substantive statistical heterogeneity of prevalence across studies ranging from 22% to 77%.42 Interestingly, the average age reported was significantly different across the studies and neither average study age nor proportion of NYHA class III/IV patients in each study was significantly associated with prevalence of frailty. The authors concluded that based on these findings, other factors aside from age and severity of HF may contribute to the significant variability in frailty prevalence. In a recent, single-center study, Jha et al. specifically addressed the incidence and implications of frailty in patients with advanced HF who were referred for heart transplant and found that 33% of the 120 patients were determined to be frail. Frailty was defined as an affirmative response to ≥ 3 of the following 5 components: weak grip strength, slowed walking speed, poor appetite, physical inactivity, and exhaustion.43 During multivariate analysis, frailty was associated with other known predictors of poor outcomes, including NYHA class IV HF, lower BMI, elevated intracardiac filling pressures, lower cardiac index, anemia, hypoalbuminemia, hyperbilirubinemia/congestive hepatopathy, cognitive impairment, and depression (all p<0.05).43 Frailty was independent of covariate predictors, such as age, sex, duration of heart failure, left ventricular ejection fraction, or renal function. Frailty was an independent predictor of increased all-cause mortality: 1-year actuarial survival was 79 ± 5% in the non-frail group compared with only 54 ± 9% for the frail group (p<0.005).43 Although the study was not adequately powered to analyze the difference between mortality rates according to frailty status, frail patients that underwent heart transplantation (N=34) had a survival rate at 1-year of 52 ± 23% while 100% of non-frail patients survived this duration after heart transplantation.43
Frailty may be associated with increased risk of death for advanced HF patients undergoing LVAD for destination therapy. Dunlay et al. utilized the FDI to determine the presence and severity of frailty in order to evaluate the association with death.44 When dividing the FDI into tertiles, there was a more than 3-fold increase risk of death in patients in the highest frailty tertile compared with those who were not frail (lowest tertile), independent of age, gender, and the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) score (HR 3.08, CI 1.4– 7.5, p= 0.004).44 As older patients are increasingly considered for LVAD implantation or heart transplantation, the International Society for Heart and Lung Transplantation has introduced the frailty assessment as a consideration for heart transplant listing in their 10- year update of listing criteria.45 The guidelines specify that frailty is defined by 3 of 5 possible symptoms, including unintentional weight loss of ≥ 10 pounds within a year, sarcopenia, fatigue, slow gait, and reduced physical activity (class IIB recommendation).45 Importantly, the guideline authors confirm that the lack of standardization of frailty measures utilized in clinical practice makes it difficult to currently consider frailty as a definitive criterion for listing.45
Cognitive Frailty in Heart Failure
While most frailty assessment tools focus on physical presentation, cognitive frailty has developed recently as a clinical manifestation characterized by the simultaneous presence of both physical and cognitive impairment.46 Jha et al. showed that the rates of cognitive impairment and depression are significantly higherin frail patients with advanced HF.47 Specific sub-types of frailty within HF patients were identified and included (1) physical frailty alone, (2) cognitive frailty (physical frailty and cognitive impairment), (3) depressive frailty (physical frailty and depression), and (4) cognitive-depressive frailty (physical frailty, depression, and cognitive impairment). The same group later investigated both pre-LVAD and pre-transplant frail patients and found that those with frailty were more likely to die than the non-frail cohort while awaiting surgery (HR = 2.07, 95% C.I. 1.01–4.26, p < 0.05).43 Reduced survival at 1 year was also described amongst frail patients, compared with nonfrail patients, that underwent heart transplantation (52 ± 23% vs. 100%) or LVAD implantation as a bridge-to-transplant (58 ± 15% vs. 71 ± 14%).43 Further investigation is required to determine if cognitive frailty is a modifiable risk factor or one that persists long term after LVAD implantation.
Can frailty in Heart Failure be modified by LVAD implantation?
Isolated heart disease-related frailty is presumed to be modifiable and maybe reversed as a result of transplantation or bridging mechanical therapies for heart transplant candidates. The concept of “reversing” frailty remains poorly defined, but has been described in heart failure patients after LVAD implantation.48 The actual clinical reversibility of disease-related frailty following transplantation is not guaranteed after such major surgery, which can introduce new circumstances for clinical deterioration during the perioperative period.
Flint et al. suggested an algorithm for selection of patients with reversible frailty that undergo destination therapy LVAD placement by dividing subjects into three broad categories: (1) patients with LVAD “responsive” frailty, largely a consequence of low-cardiac output, high-filling pressure state, therefore reversible; (2) patients with primarily “LVAD-independent” frailty, largely resulting from non-cardiac conditions (lung disease, diabetes, osteoporosis, peripheral vascular disease), therefore not reversible; and (3) “LVAD-intermediate” patients with a significant component of HF-associated frailty but also possessing a considerable burden of non-cardiac comorbidities.49 An observational, prospective study evaluating changes in the FFP in 19 patients (mean age 70.6 ± 5.5 years, 72.6% male) after LVAD therapy illustrated a reduction in frailty criteria from 3.9 ± 0.9 at baseline to 2.8 ± 1.4 at 6 months after surgery (P =.003).50 Subjectively, LVAD patients reported improvements in their quality-of-life but not with their perceived mood or cognition. The authors concluded that while frailty decreased by almost 50% in this population, further study is required to evaluate and enhance frailty reversal in heart failure patients undergoing LVAD implantation.50
Cellular Mechanisms and Potential Therapeutic Targets
Predisposing genetic susceptibility, comorbidities, environmental factors, and physiologic aging influence the expression of the frailty phenotype.38, 51 Systemic inflammation, oxidative stress, mitochondrial dysfunction, and neurohormonal dysregulation drive the processes of both aging and frailty, and therefore share cellular and molecular mechanisms.38,52 Normal biologic aging involves the decline and deterioration of functional homeostatic properties at the cellular level. This loss of homeostasis results in a decreased adaptability to internal and external stressors, which then increases vulnerability to disease and hastens death.51 Cellular damage may result in (1) senescence, or proliferative arrest, resulting in slow or absent recovery (frailty) (2) transformation into neoplasms (3) repair which results in rapid recovery (non-frail) or (4) apoptosis for irreparable damage, resulting in atrophy. Cellular, organ, and overall organismal maintenance are dependent on the preservation of molecular mechanisms that are responsible for cellular repair and removal of mutations related to DNA repair, synthesis, and replication.38, 51
A common underlying feature of aging and the presentation of clinical frailty is the presence of subclinical chronic inflammation which is partially derived from an age-related decline in the immune function. Senescent cells secrete pro-inflammatory cytokines, chemokines, and proteases that are termed the senescent-associated secretory phenotype or the senescence messaging secretome.53 Some of these cellular markers have been identified as potential tools to gauge the presence and severity of frailty. In particular, serum levels of interleukin-6 (IL-6) have been significantly elevated in patients with sarcopenia.16 Inflammation may lead to mitochondrial dysfunction through deficiencies of various mitochondrial subunits resulting in decreased energy production, alterations in fatty acid metabolism, and the development of reactive oxygen species leading to oxidative stress.11,54 Neurohumoral abnormalities are detected after upregulation of inflammatory cytokines (e.g., IL-6, C-reactive protein, tumor necrosis factor [TNF]-α/cachexin), and include decreased androgen levels, abnormalities in the growth hormone (GH) and insulin growth-like factor-1 (IGF-1), leading to insulin resistance. This ultimately leads to cellular dysregulation and a metabolic imbalance favoring catabolism over anabolism.11, 55
Heart Failure
Anker et al. have evaluated the link between the frailty phenotype, biomarker expression, and chronic heart failure. Elevated levels of tumor necrosis factor (TNF-α), and its soluble receptors, particularly in chronic HF are associated with a rise in the cortisol:dehydroepiandrosterone (catabolic:anabolic) ratio and has been shown to correlate with reduced BMI and increased clinical severity of heart failure.56 Higher levels of cortisol in patients with CHF have been linked to common features of frailty, including muscle wasting,57 cachexia,18 exercise intolerance, impaired ventilatory response to exercise, and chronotropic incompetence resulting in limited functional status.58 Acquired growth hormone (GH) resistance occurs due to a reduction of tissue GH receptors as evidenced by low GH-binding protein levels, in conjunction with elevated plasma levels of growth hormone and low IGF-1 levels. This relative GH excess is present in chronic HF and has been associated with muscle wasting, loss of body weight, and impaired survival.59, 60 Additionally, a recent study has shown that implantation of a LVAD has beneficial effects on the neurohormonal and functional derangements related to frailty. In a study of 31 patients with advanced HF, LVAD implantation resulted in improvement in GH resistance accompanied by enhanced local expression of anabolic IGF-1 in skeletal muscle. Further, LVAD implantation also resulted in increased skeletal muscle cross-sectional areas as measured by CT imaging, oxidative function, and increased hand grip strength.61
There are challenges in differentiating heart failure reversal and frailty reversal since these two conditions are physiologically and phenotypically similar. Heart failure is characterized by a decline in mitochondrial biogenesis, function, and energy production, which support the concept of systemic mitochondrial cytopathy.62 Decreased energy availability results in a decline in muscle mass and muscle composition these changes in advanced HF patients are slightly different than normal aging and likely site-specific. Compared to normal aging, patients with HF exhibited a redistribution of skeletal muscle fiber composition; displaying an increased percentage of fast twitch, glycolytic, easily fatigable, type IIb fibers.63 Conversely, in the diaphragmatic muscle, there was a shift from fast to slow myosin heavy-chain isoforms with an increase in oxidative and a decrease in glycolytic capacities similar to muscle changes elicited by endurance training. This is likely attributed to the higher diaphragmatic workload of patients with advanced HF.64
Additional neurohumoral changes influence the development of muscle wasting and cachexia. The plasma levels of ghrelin, a peptide hormone secreted by the empty gastrointestinal tract, function as a neuropeptide in the central nervous system in order to signal hunger. Elevated ghrelin levels along with peripheral resistance to ghrelin have been implicated in cachectic patients with congestive HF.65 Increased ghrelin levels subsequently release GH, adrenocorticotropic hormone, cortisol, and TNF-α, resulting in a relative catabolic state.66 In a study of 12 patients, Lund et al. showed that HF may be associated with resistance to the appetite-stimulating effects of ghrelin, and that post-transplantation plasma levels may decrease to normal concentrations with the elimination of ghrelin-resistance and subsequent resolution of anorexia.67
End-Stage Lung Disease
End-stage COPD patients have served as a population from which a significant body of evidence has emerged over the past two decades surrounding inflammatory biomarkers, frailty, and outcomes. Associations have been established between end-stage COPD, frailty, and markers of chronic inflammation and endocrine dysregulation (IL-6, TNF-α, C-Reactive Protein, IGF-1).68–72 The overall resultant catabolic state may lead to activation of the renin-angiotensin-aldosterone axis and disorders of fluid balance.73 Specifically, sarcopenia and cachexia are more common in patients with COPD than matched healthy controls and these findings are independently associated with reduced functional performance, exercise capacity, and higher mortality.69, 74–76 Higher levels of these biomarkers are may predict worsening physical status, COPD exacerbations, and death. One important study in lung transplant patients that supports a reduction in circulating anabolic hormones highlights the link between the frailty phenotype and inflammatory biomarkers. Singer et al. confirmed the frailty phenotype using both FFP and SPPB, and demonstrated elevation of IL-6 and TNF receptor-1, as well as reduced levels of IGF-1 and leptin among frail patients.20 In a small cohort of lung transplant patients, the presence of IL-10, IL-8, IL-6, and chemokine ligand 2 prior to transplant were associated with subsequent development of high-grade primary graft dysfunction.77
Obesity Paradox in Adult Cardiothoracic Surgical Patients
Although obesity is a state of positive energy balance, there is a proposed link between obesity and an overall pro-inflammatory state that may alter the milieu of inflammatory cytokines produced by adipose tissue (“adipokines”, such as leptin and adiponectin). Leptin, in particular, has been implicated in the development of acute lung injury.78 A multicenter study of 512 adult lung transplant recipients with COPD or interstitial lung disease found that obesity and elevated serum leptin levels were associated with a more than two-fold increase in the risk of grade 3 (severe) primary graft dysfunction within 72 hours of transplantation.79 Interestingly, there seems to be a “U”shaped relationship between BMI and mortality after cardiac surgery.80 This apparent paradox seen in patients that are categorized as overweight or mildly obese may be due to energy stores present to aide in energy expenditure and postoperative catabolism required for healing after surgery without the chronic illness and complications associated with morbid obesity (e.g., cardiac disease, sleep apnea, diabetes, essential hypertension). Further studies are required to better understand the etiology of this relationship between BMI and outcomes.
Frailty Modification through Nutrition and Rehabilitation
Patients awaiting heart or lung transplantation may benefit greatly from improved nutrition and physical activity. “Prehabilitation” across multiple surgical populations, with a focus towards physical rehabilitation and nutrition before surgery, may improve function, enhance recovery profile, and reduce morbidity.81–83 That said, mobilizing these patients and ensuring adequate preoperative protein caloric intake represent significant challenges in this population and robust evidence surrounding frailty reversal among cardiothoracic transplant patients remains limited.
Heart Failure
In heart failure, several non-invasive therapies appear to modify the HF-associated frailty phenotype; these include nutrition, exercise training, anabolic agents (e.g. appetite stimulants, testosterone supplementation), and anti-inflammatory agents.84 Prescribing aerobic exercise training in patients with HF is a class I recommendation by the European Society of Cardiology,85 the American College of Cardiology, and the American Heart Association.86 Exercise training in patients with advanced HF has positive effects on exercise capacity and health-related quality of life.87, 88 The Heart Failure: Controlled Trial to Investigate Outcomes of Exercise Training study randomly assigned heart failure patients to either undergo a formal exercise program or to not be enrolled in this program. Patients that underwent exercise training illustrated improved peak oxygen uptake, reduced HF-related hospitalization, and lower risks for cardiovascular or all-cause mortality compared to those patients that did not.89 Exercise training has been described in patients awaiting heart transplantation and mechanically supported by a intra-aortic balloon pump (IABP) inserted through the axillary/subclavian artery and positioned antegrade into the descending thoracic aorta.90 In a series of 18 patients, the insertion of a transaxillary IABP resulted in a significant improvement in hemodynamic parameters. This improvement is consistent with IABP counterpulsation requiring strict bedrest after standard femoral arterial insertion. There was, however, a marked improvement in ambulatory capacity in patients with transaxillary IABP that yielded a 500% increase in the longest distance walked per day compared with when the patient did not have an IABP.91 Recently a minimally invasive, transaxillary arterial IABP (Nupulse®, NuPulseCV, Raleigh, NC, USA) has been developed as a durable ambulatory option for bridging counterpulsation therapy.92
After heart transplantation, exercise capacity improves dramatically compared with end-stage heart failure, but this capacity continues to be subnormal compared to age-matched healthy individuals, and peak oxygen uptake ranges from 50–70% of the general healthy population.93 Some of the factors which may explain this finding include chronotropic incompetence related to autonomic denervation of the transplanted heart, diastolic dysfunction, reduced stroke volume, reduced muscle strength, and reduced oxidative capacity or reduced capillary density.93 In a randomized trial of structured cardiac rehabilitation compared with unstructured therapy after heart transplantation, the structured group had significantly greater increases in peak oxygen consumption and workload.94 Interestingly, heart transplant recipients seem to respond to exercise by peripheral improvements of increased muscle mass and strength rather than improvements in cardiac function.93
End-Stage Lung Disease
Based on limited evidence, pulmonary rehabilitation provides appreciable physical improvement in the frail condition for end-stage lung disease patients.95–97 Exercise capacity, as measured by the 6MWD and physical training, can be preserved after transplant even in the presence of end stage lung disease. Groups with improved 6MWD prior to transplant have illustrated improved physical performance after transplantation.95 In a recent systematic review involving 1,306 patients, pulmonary rehabilitation illustrated significant improvement on 6MWD performance and quality-of-life.98
Sarcopenia has been most studied in end-stage COPD patients and in one recent systematic review of 622 patients, sarcopenia was identified in 43 elder patients (6.9%) and pulmonary rehabilitation resulted in increased muscle mass.99
Summary
There is a high prevalence of frailty in patients with heart failure and end-stage lung disease awaiting transplantation, and frailty remains an important predictor of waitlist and post-transplant morbidity and mortality. Standardized guidelines for quantification and risk stratification, however, are lacking to comparatively evaluate evidence among studies and this void has served as a significant barrier to the routine incorporation of frailty scoring during organ allocation. Frailty categorization encompasses patients that may have age-related and/or disease-related illnesses. The ability to identify modifiable disease-related frailty may be particularly important for risk prediction after transplantation. Interestingly, the biologic basis of clinical frailty derives from the process of aging, which similarly includes cellular senescence, apoptosis, and mitochondrial dysfunction. This cellular dysregulaton influences metabolic derangements that favor catabolic predominance within the presence of a chronic inflammatory state. Important objectives for future investigations include the development of a process by which disease-related and age-related phenotypes are readily distinguished and evaluated during treatment-associated risk modification. This therapeutic process may entail serial evaluations of targeted, cellular biomarkers. For now, physical rehabilitation and nutritional optimization remain important components to reduce currently available measurements of frailty for the cardiothoracic organ transplant candidate and postoperative transplant recipient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No relevant disclosures for authors BAB, AN, LDS, PEW, JNS, CBP, MAD, RNS; KG receives grant support from the NIH (NIGMS 5T32GM00860)
References
- 1.Morley JE, Vellas B, van Kan GA et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quarterly Data Report [ https://www.ishlt.org/registries/quarterlyDataReport.asp]
- 3.Sepehri A, Beggs T, Hassan A et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg 2014; 148:3110–7. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa H, Tanemoto K. Frailty in cardiothoracic surgery: systematic review of the literature. Gen Thorac Cardiovasc Surg 2015; 63:425–33. [DOI] [PubMed] [Google Scholar]
- 5.Beckert AK, Huisingh-Scheetz M, Thompson K et al. Screening for Frailty in Thoracic Surgical Patients. Ann Thorac Surg 2017; 103:956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maxwell BG, Mooney JJ, Lee PH et al. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med 2015; 191:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund LH, Edwards LB, Dipchand AI et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016; 35:1158–69. [DOI] [PubMed] [Google Scholar]
- 8.Yusen RD, Christie JD, Edwards LB et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant 2013; 32:965–78. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–56. [DOI] [PubMed] [Google Scholar]
- 10.Bagshaw M, Majumdar SR, Rolfson DB et al. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care 2016; 20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldwater DS, Pinney SP. Frailty in Advanced Heart Failure: A Consequence of Aging or a Separate Entity? Clin Med Insights Cardiol 2015; 9:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyce E Frailty in Advanced Heart Failure. Heart Fail Clin 2016; 12:363–74. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozenberg D, Wickerson L, Singer LG et al. Sarcopenia in lung transplantation: a systematic review. J Heart Lung Transplant 2014; 33:1203–12. [DOI] [PubMed] [Google Scholar]
- 15.Evans WJ, Morley JE, Argiles J et al. Cachexia: a new definition. Clin Nutr 2008; 27:793–9. [DOI] [PubMed] [Google Scholar]
- 16.Fulster S, Tacke M, Sandek A et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J 2013; 34:512–9. [DOI] [PubMed] [Google Scholar]
- 17.Castleberry AW, Englum BR, Snyder LD et al. The utility of preoperative six-minute-walk distance in lung transplantation. Am J Respir Crit Care Med 2015; 192:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anker SD, Ponikowski P, Varney S et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349:1050–3. [DOI] [PubMed] [Google Scholar]
- 19.Saitoh M, Dos Santos MR, Ebner N et al. Nutritional status and its effects on muscle wasting in patients with chronic heart failure: insights from Studies Investigating Co-morbidities Aggravating Heart Failure. Wien Klin Wochenschr 2016; 128:497–504. [DOI] [PubMed] [Google Scholar]
- 20.Singer JP, Diamond JM, Gries CJ et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respir Crit Care Med 2015; 192:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swallow EB, Reyes D, Hopkinson NS et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marquis K, Debigare R, Lacasse Y et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166:809–13. [DOI] [PubMed] [Google Scholar]
- 23.Soler-Cataluna JJ, Sanchez-Sanchez L, Martinez-Garcia MA et al. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 2005; 128:2108–15. [DOI] [PubMed] [Google Scholar]
- 24.Slinde F, Gronberg A, Engstrom CP et al. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med 2005; 99:1004–9. [DOI] [PubMed] [Google Scholar]
- 25.Kelm DJ, Bonnes SL, Jensen MD et al. Pre-transplant wasting (as measured by muscle index) is a novel prognostic indicator in lung transplantation. Clin Transplant 2016; 30:247–55. [DOI] [PubMed] [Google Scholar]
- 26.Afilalo J, Mottillo S, Eisenberg MJ et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes 2012; 5:222–8. [DOI] [PubMed] [Google Scholar]
- 27.Afilalo J, Eisenberg MJ, Morin JF et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol 2010; 56:1668–76. [DOI] [PubMed] [Google Scholar]
- 28.de Arenaza DP, Pepper J, Lees B et al. Preoperative 6-minute walk test adds prognostic information to Euroscore in patients undergoing aortic valve replacement. Heart 2010; 96:113–7. [DOI] [PubMed] [Google Scholar]
- 29.Lytwyn J, Stammers AN, Kehler DS et al. The impact of frailty on functional survival in patients 1 year after cardiac surgery. J Thorac Cardiovasc Surg 2017; 154:1990–9. [DOI] [PubMed] [Google Scholar]
- 30.Diamond JM, Lee JC, Kawut SM et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013; 187:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghu G, Chen SY, Yeh WS et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med 2014; 2:566–72. [DOI] [PubMed] [Google Scholar]
- 32.Wilson ME, Vakil AP, Kandel P et al. Pretransplant frailty is associated with decreased survival after lung transplantation. J Heart Lung Transplant 2016; 35:173–8. [DOI] [PubMed] [Google Scholar]
- 33.Meyer DM, Rogers JG, Edwards LB et al. The future direction of the adult heart allocation system in the United States. Am J Transplant 2015; 15:44–54. [DOI] [PubMed] [Google Scholar]
- 34.Cawthon PM, Marshall LM, Michael Y et al. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc 2007; 55:1216–23. [DOI] [PubMed] [Google Scholar]
- 35.Collard RM, Boter H, Schoevers RA et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–92. [DOI] [PubMed] [Google Scholar]
- 36.Woods NF, LaCroix AZ, Gray SL et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc 2005; 53:1321–30. [DOI] [PubMed] [Google Scholar]
- 37.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62:722–7. [DOI] [PubMed] [Google Scholar]
- 38.Exterkate L, Slegtenhorst BR, Kelm M et al. Frailty and Transplantation. Transplantation 2016; 100:727–33. [DOI] [PubMed] [Google Scholar]
- 39.de Vries NM, Staal JB, van Ravensberg CD et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011; 10:104–14. [DOI] [PubMed] [Google Scholar]
- 40.Abrams D, Javidfar J, Farrand E et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014; 18:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehder KJ, Turner DA, Hartwig MG et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013; 58:1291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denfeld QE, Winters-Stone K, Mudd JO et al. Frequency of and Significance of Physical Frailty in Patients With Heart Failure. Am J Cardiol 2017; 119:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jha SR, Hannu MK, Chang S et al. The Prevalence and Prognostic Significance of Frailty in Patients With Advanced Heart Failure Referred for Heart Transplantation. Transplantation 2016; 100:429–36. [DOI] [PubMed] [Google Scholar]
- 44.Dunlay SM, Park SJ, Joyce LD et al. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant 2014; 33:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehra MR, Canter CE, Hannan MM et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016; 35:1–23. [DOI] [PubMed] [Google Scholar]
- 46.Kelaiditi E, Cesari M, Canevelli M et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging 2013; 17:726–34. [DOI] [PubMed] [Google Scholar]
- 47.Jha SR, Hannu MK, Gore K et al. Cognitive impairment improves the predictive validity of physical frailty for mortality in patients with advanced heart failure referred for heart transplantation. J Heart Lung Transplant 2016; 35:1092–100. [DOI] [PubMed] [Google Scholar]
- 48.Jha SR, Hannu MK, Newton PJ et al. Reversibility of Frailty After Bridge-to-Transplant Ventricular Assist Device Implantation or Heart Transplantation. Transplant Direct 2017; 3:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flint KM, Matlock DD, Lindenfeld J et al. Frailty and the selection of patients for destination therapy left ventricular assist device. Circ Heart Fail 2012; 5:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maurer MS, Horn E, Reyentovich A et al. Can a Left Ventricular Assist Device in Individuals with Advanced Systolic Heart Failure Improve or Reverse Frailty? J Am Geriatr Soc 2017; 65:2383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedarko NS. The biology of aging and frailty. Clin Geriatr Med 2011; 27:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erusalimsky JD, Grillari J, Grune T et al. In Search of ‘Omics’-Based Biomarkers to Predict Risk of Frailty and Its Consequences in Older Individuals: The FRAILOMIC Initiative. Gerontology 2016; 62:182–90. [DOI] [PubMed] [Google Scholar]
- 53.Tchkonia T, Zhu Y, van Deursen J et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puthucheary ZA, Astin R, McPhail MJW et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax 2018; [DOI] [PubMed]
- 55.Shinmura K Cardiac Senescence, Heart Failure, and Frailty: A Triangle in Elderly People. Keio J Med 2016; 65:25–32. [DOI] [PubMed] [Google Scholar]
- 56.Anker SD, Clark AL, Kemp M et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol 1997; 30:997–1001. [DOI] [PubMed] [Google Scholar]
- 57.Anker SD, Ponikowski PP, Clark AL et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J 1999; 20:683–93. [DOI] [PubMed] [Google Scholar]
- 58.Agapitou V, Dimopoulos S, Kapelios C et al. Hormonal imbalance in relation to exercise intolerance and ventilatory inefficiency in chronic heart failure. J Heart Lung Transplant 2013; 32:431–6. [DOI] [PubMed] [Google Scholar]
- 59.Anker SD, Volterrani M, Pflaum CD et al. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol 2001; 38:443–52. [DOI] [PubMed] [Google Scholar]
- 60.Junnila RK, List EO, Berryman DE et al. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 2013; 9:366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khawaja T, Chokshi A, Ji R et al. Ventricular assist device implantation improves skeletal muscle function, oxidative capacity, and growth hormone/insulin-like growth factor-1 axis signaling in patients with advanced heart failure. J Cachexia Sarcopenia Muscle 2014; 5:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosca MG, Hoppel CL. Mitochondrial dysfunction in heart failure. Heart Fail Rev 2013; 18:607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancini DM, Coyle E, Coggan A et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation 1989; 80:1338–46. [DOI] [PubMed] [Google Scholar]
- 64.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation 1997; 95:910–6. [DOI] [PubMed] [Google Scholar]
- 65.Nagaya N, Uematsu M, Kojima M et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation 2001; 104:2034–8. [DOI] [PubMed] [Google Scholar]
- 66.Nagaya N, Miyatake K, Uematsu M et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab 2001; 86:5854–9. [DOI] [PubMed] [Google Scholar]
- 67.Lund LH, Williams JJ, Freda P et al. Ghrelin resistance occurs in severe heart failure and resolves after heart transplantation. Eur J Heart Fail 2009; 11:789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breyer MK, Rutten EP, Locantore NW et al. Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur J Clin Invest 2012; 42:983–91. [DOI] [PubMed] [Google Scholar]
- 69.Schols AM, Buurman WA, Staal van den Brekel AJ et al. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax 1996; 51:819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gan WQ, Man SF, Senthilselvan A et al. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karadag F, Karul AB, Cildag O et al. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung 2008; 186:403–9. [DOI] [PubMed] [Google Scholar]
- 72.Thomsen M, Ingebrigtsen TS, Marott JL et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. JAMA 2013; 309:2353–61. [DOI] [PubMed] [Google Scholar]
- 73.Laghi F, Adiguzel N, Tobin MJ. Endocrinological derangements in COPD. Eur Respir J 2009; 34:975–96. [DOI] [PubMed] [Google Scholar]
- 74.Sergi G, Coin A, Marin S et al. Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respir Med 2006; 100:1918–24. [DOI] [PubMed] [Google Scholar]
- 75.Schols AM, Broekhuizen R, Weling-Scheepers CA et al. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82:53–9. [DOI] [PubMed] [Google Scholar]
- 76.Greening NJ, Harvey-Dunstan TC, Chaplin EJ et al. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med 2015; 192:810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allen JG, Lee MT, Weiss ES et al. Preoperative recipient cytokine levels are associated with early lung allograft dysfunction. Ann Thorac Surg 2012; 93:1843–9. [DOI] [PubMed] [Google Scholar]
- 78.Bellmeyer A, Martino JM, Chandel NS et al. Leptin resistance protects mice from hyperoxia- induced acute lung injury. Am J Respir Crit Care Med 2007; 175:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lederer DJ, Kawut SM, Wickersham N et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 2011; 184:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mariscalco G, Wozniak MJ, Dawson AG et al. Body Mass Index and Mortality Among Adults Undergoing Cardiac Surgery: A Nationwide Study With a Systematic Review and Meta-Analysis. Circulation 2017; 135:850–63. [DOI] [PubMed] [Google Scholar]
- 81.West MA, Wischmeyer PE, Grocott MPW. Prehabilitation and Nutritional Support to Improve Perioperative Outcomes. Curr Anesthesiol Rep 2017; 7:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heyland DK, Montalvo M, MacDonald S et al. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg 2001; 44:102–11. [PubMed] [Google Scholar]
- 83.Klein S, Kinney J, Jeejeebhoy K et al. Nutrition support in clinical practice: review of published data and recommendations for future research directions. Summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr 1997; 66:683–706. [DOI] [PubMed] [Google Scholar]
- 84.von Haehling S, Ebner N, Dos Santos MR et al. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol 2017; 14:323–41. [DOI] [PubMed] [Google Scholar]
- 85.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18:891–975. [DOI] [PubMed] [Google Scholar]
- 86.Yancy CW, Jessup M, Bozkurt B et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016; 68:1476–88. [DOI] [PubMed] [Google Scholar]
- 87.Rees K, Taylor RS, Singh S et al. Exercise based rehabilitation for heart failure. Cochrane Database Syst Rev 2004:CD003331. [DOI] [PMC free article] [PubMed]
- 88.van Tol BA, Huijsmans RJ, Kroon DW et al. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail 2006; 8:841–50. [DOI] [PubMed] [Google Scholar]
- 89.Swank AM, Horton J, Fleg JL et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 2012; 5:579–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Estep JD, Cordero-Reyes AM, Bhimaraj A et al. Percutaneous placement of an intra-aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long-term support as bridge to heart transplantation. JACC Heart Fail 2013; 1:382–8. [DOI] [PubMed] [Google Scholar]
- 91.Umakanthan R, Hoff SJ, Solenkova N et al. Benefits of ambulatory axillary intra-aortic balloon pump for circulatory support as bridge to heart transplant. J Thorac Cardiovasc Surg 2012; 143:1193–7. [DOI] [PubMed] [Google Scholar]
- 92.Jeevanandam V, Song T, Onsager D et al. The first-in-human experience with a minimally invasive, ambulatory, counterpulsation heart assist system for advanced congestive heart failure. J Heart Lung Transplant 2018; 37:1–6. [DOI] [PubMed] [Google Scholar]
- 93.Nytroen K, Gullestad L. Exercise after heart transplantation: An overview. World J Transplant 2013; 3:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kobashigawa JA, Leaf DA, Lee N et al. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med 1999; 340:272–7. [DOI] [PubMed] [Google Scholar]
- 95.Li M, Mathur S, Chowdhury NA et al. Pulmonary rehabilitation in lung transplant candidates. J Heart Lung Transplant 2013; 32:626–32. [DOI] [PubMed] [Google Scholar]
- 96.Singer JP, Lederer DJ, Baldwin MR. Frailty in Pulmonary and Critical Care Medicine. Ann Am Thorac Soc 2016; 13:1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maddocks M, Kon SS, Canavan JL et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016; 71:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffman M, Chaves G, Ribeiro-Samora GA et al. Effects of pulmonary rehabilitation in lung transplant candidates: a systematic review. BMJ Open 2017; 7:e013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones SE, Maddocks M, Kon SS et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015; 70:213–8. [DOI] [PubMed] [Google Scholar]


