Abstract
A woman presented to the emergency department with sharp abdominal pain and no evidence of a food-borne illness. How would you treat the patient?
A 34-year-old African American woman presented to the emergency department (ED) after several hours of sharp lower abdominal pain and cramping followed by nausea and vomiting. The pain initially began in the periumbilical region and migrated to the bilateral lower quadrants. The patient reported no fevers, chills, diarrhea, hematemesis, or hematochezia associated with these symptoms. She also reported no unusual food exposures or sick contacts.
The patient’s medical history was notable only for hypertension; her surgical history included 2 cesarean section births several years prior to presentation. Her father was diagnosed with stomach cancer while in his 40s. The patient’s only medications were an oral contraceptive, lisinopril, and an antihistamine taken as needed for seasonal allergies. She had no history of tobacco, alcohol, or illicit drug use and no known drug allergies.
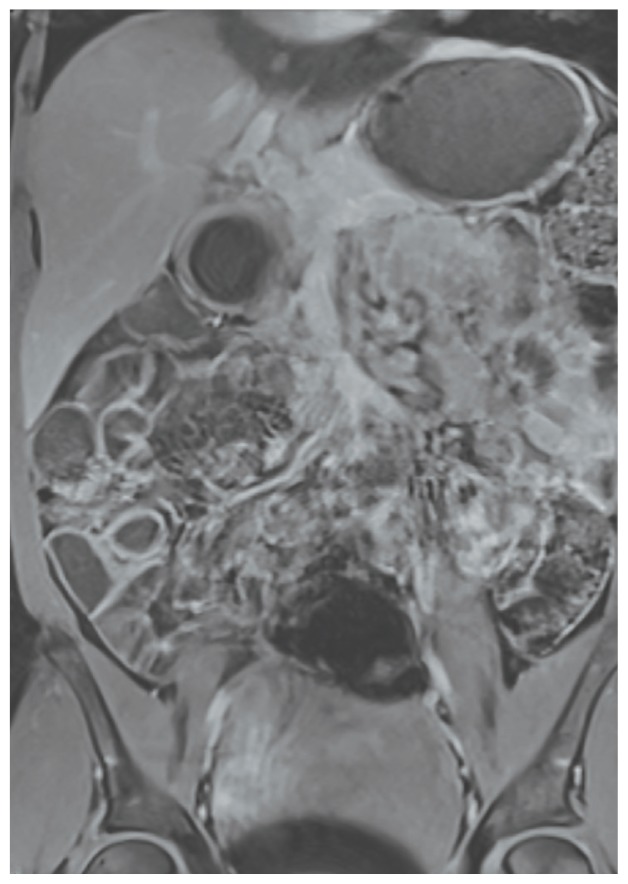
While in the ED, the patient’s physical exam revealed mild tachycardia (104 bpm on arrival, which improved with fluid resuscitation) and diffuse abdominal tenderness. Laboratory evaluation revealed a mild leukocytosis (10.7 × 103/L) but normal liver-associated enzymes, lipase, and urinalysis. A computed tomography (CT) scan of the abdomen and pelvis with oral and IV contrast revealed diffuse ileal wall thickening with significant perihepatic and perisplenic ascites with pelvic free fluid suspicious for an inflammatory vs infectious enteritis (Figure 1).
Figure 1.
Presenting Computed Tomography Showing Diffuse Ileal Wall Thickening With Perihepatic and Perisplenic Ascites
The patient was treated supportively with IV fluids, antiemetics, and pain medication. Her symptoms generally improved over several days, though she did develop loose stools that prompted infectious stool studies, which were negative for typical pathogens. Follow-up laboratory testing revealed resolution of her leukocytosis.
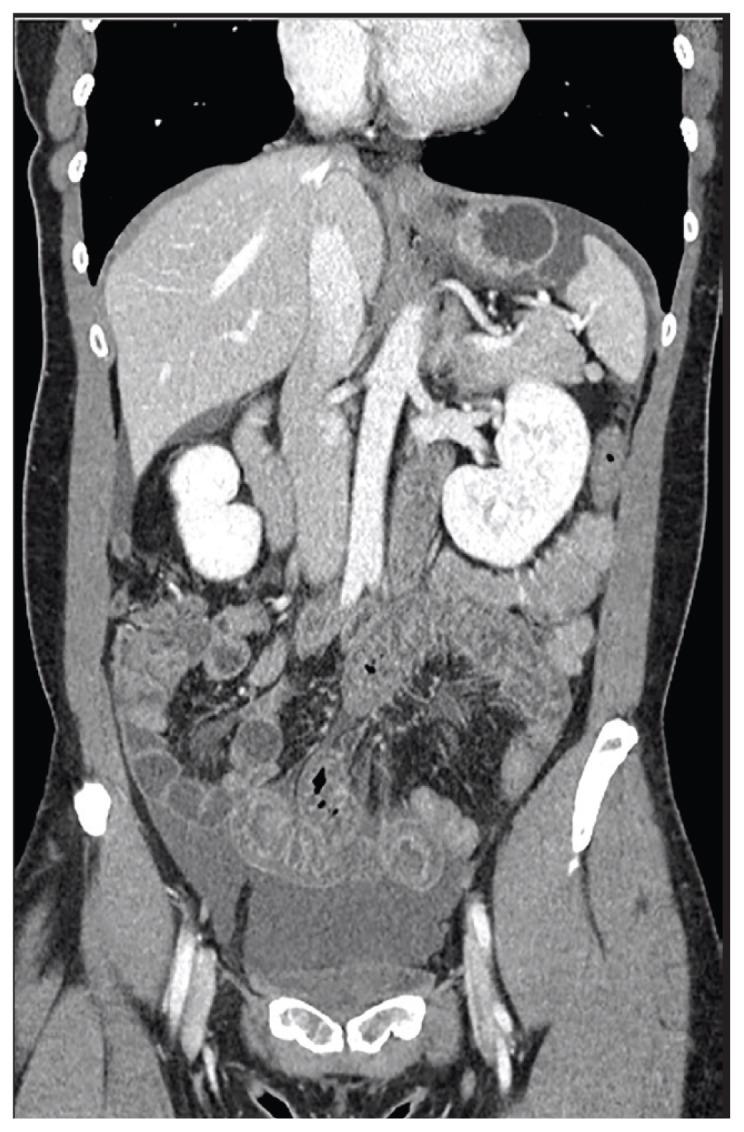
About 2 weeks later, the patient had another acute attack of abdominal pain, again associated with nausea and vomiting. Similar to her prior visit, a mild leukocytosis (10.3 × 103/L) was the only laboratory abnormality. Imaging was not repeated. She was again treated supportively, and once more, her symptoms resolved spontaneously. An upper endoscopy with push endoscopy was performed and no abnormalities were identified as far as the proximal jejunum (about 130 cm from the incisors). The mucosa of the colon and distal terminal ileum were shown to be normal on colonoscopy. Random biopsies were taken throughout the gastrointestinal tract and all showed no histologic abnormalities. A magnetic resonance enterography was performed shortly thereafter and revealed resolution of the prior bowel wall thickening (Figure 2).
Figure 2.
Normal Abdomen/Pelvis Magnetic Resonance Image Showing Resolution of Ileitis and Ascites
Six weeks after her initial presentation, the patient presented to the ED for the third time with the same symptoms. A CT scan again displayed diffuse ileal wall thickening with significant ascites; slightly worse than the image from initial presentation (Figure 3).
Figure 3.
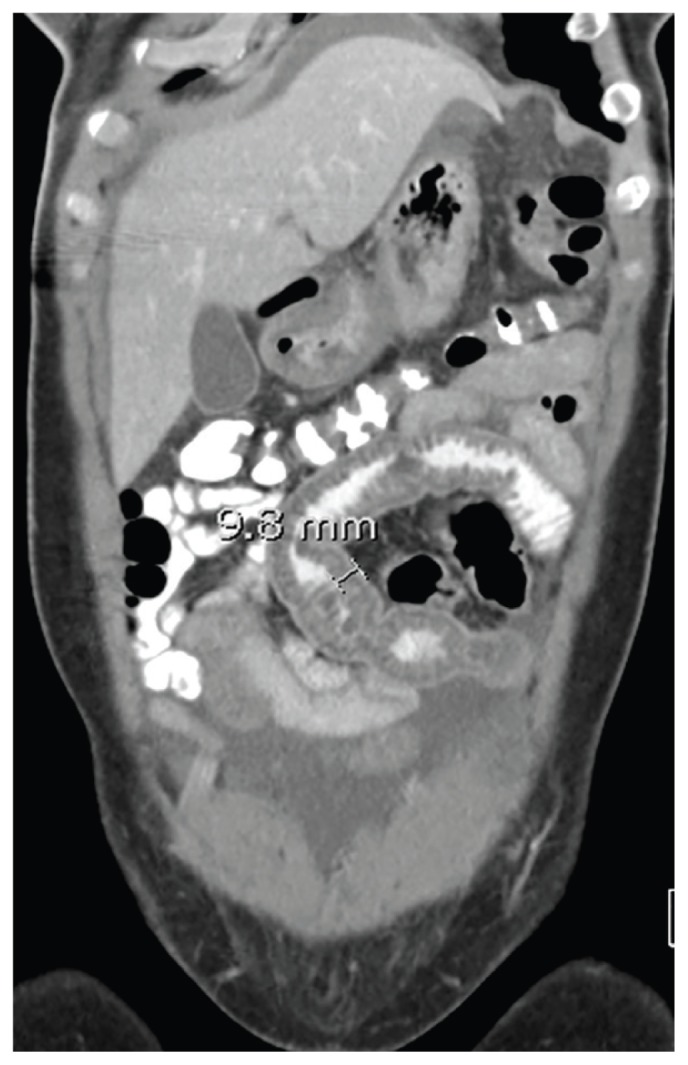
Follow-up Abdomen/Pelvis Computed Tomography Showing Diffuse Ileal Wall Thickening With Significant Ascites
■ What is your diagnosis?
■ How would you treat this patient?
DIAGNOSIS
This patient presented with recurrent episodes of diffuse small bowel wall thickening and ascites associated with diffuse abdominal pain, nausea, and vomiting. Her symptoms resolved spontaneously, correlating with normalization of bowel wall appearance on imaging studies. Initially, the patient’s symptoms were most concerning for infectious or inflammatory enteritis. Infection became lower on the differential as the patient’s symptoms continued to recur and then resolve spontaneously without antiviral or antibiotic treatment. She also had no fevers, and stools samples were negative for infectious causes of her symptoms.
Inflammatory bowel disease (IBD) was considered, but direct visualization of the small bowel and colonic mucosa was unremarkable. In addition, the sporadic nature of her symptoms did not fit the typical IBD presentation. The patient had no risk factors or history that would suggest ischemic disease, vasculitis, or radiation-induced enteritis. Hereditary angioedema and acquired C1 esterase deficiency were considered given the intermittent nature and characteristic quality of her symptoms. However, serum C4 and C1 esterase inhibitor levels returned within normal limits when measured during these episodes. Finally, visceral angioedema was considered.
Visceral angioedema may be related to medications and is specifically associated with angiotensin-converting-enzyme (ACE) inhibitors as well as β-lactams and high doses of nonsteroidal anti-inflammatory drugs (NSAIDs). Given the characteristic presentation with no other inciting cause, the patient’s lisinopril was felt to be the causative agent and was discontinued. Her symptoms resolved completely and never returned. The patient’s final diagnosis was ACE inhibitor-induced visceral angioedema.
ABOUT THIS CONDITION
Angiotensin-converting enzyme inhibitors were first introduced in the early 1980s and have been prescribed more frequently as the indications for their use has increased. Some estimate that ACE inhibitors are used by more than 40 million people worldwide.1 Angioedema has been reported to occur in 0.1% to 0.2% of patients taking ACE inhibitors and accounts for 20% to 30% of all angioedema cases presenting to EDs.2,3 However, ACE inhibitors recently have been recognized as a rare cause of angioedema of the gastrointestinal tract. One of the largest literature reviews on ACE inhibitor-induced gastrointestinal angioedema describes only 27 cases.3
Prevalence seems to be highest among middle-aged or older women, particularly among African Americans. 3 The interval between medication initiation and onset of symptoms can vary, ranging from 24 hours to 9 years.3,4 In many cases, lack of recognition of this condition early in the disease course led to costly and invasive procedures, such as abdominal laparotomy, before reaching a diagnosis. Other literature reviews report similar patient characteristics and initial disease manifestations: female predominance, often middle-aged, presenting with abdominal pain and emesis associated with bowel wall thickening and ascites on CT.4,5 Additionally, in the majority of cases, visceral angioedema occurred in the absence of oropharyngeal angioedema. Unlike allergic angioedema or NSAID-induced angioedema, ACE inhibitor-induced angioedema is not associated with urticaria.6
The exact pathway of ACE inhibitor-induced angioedema is not completely understood but is thought to be bradykinin mediated. Angiotensin-converting enzyme inhibitors decrease the degradation of bradykinin, which ultimately leads to an increase in vascular permeability and results in an increased plasma extravasation into the interstitial space of subcutaneous or submucosal tissue.1 However, many experts believe that the exclusive role of bradykinin is unlikely. Some suggest that patients with ACE inhibitor-induced angioedema are more likely to have decreased levels or defects in other enzymes such as carboxypeptidase N and aminopeptidase P, which are involved in the breakdown of bradykinin and its metabolites.6 Given the female predominance in this patient population, it also seems reasonable to consider the role of estrogens in the pathogenesis of this disease, although none have been identified to the knowledge of the authors.
Treatment of ACE inhibitor-induced angioedema is largely supportive following discontinuation of the offending medication. There have been case reports of infrequent, mild, recurrent episodes of angioedema, even after ACE inhibitor discontinuation, so these should be anticipated.7
CONCLUSIONS
Angiotensin-converting enzyme inhibitor-induced gastrointestinal angioedema is a rare condition. It generally presents as recurrent abdominal pain and nausea with CT findings of intestinal edema and ascites. It is more common among the middle-aged, women, and minorities. ACE inhibitor-induced angioedema should be kept on the differential for patients with the aforementioned characteristics, especially if infection, inflammatory bowel disease, ischemic disease, or vasculitis is deemed unlikely. Identifying this condition early can save patients from unnecessary hospitalizations, physical and emotional discomfort, and further health care costs.
Footnotes
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients
REFERENCES
- 1.Campo P, Fernandez TD, Canto G, Mayorga C. Angioedema induced by angiotensin-converting enzyme inhibitor. Curr Opin Allergy Clin Immunol. 2013;13(4):337–344. doi: 10.1097/ACI.0b013e328362b835. [DOI] [PubMed] [Google Scholar]
- 2.Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol. 2000;31(3):254–257. doi: 10.1097/00004836-200010000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Benson BC, Smith C, Laczek JT. Angiotensin converting enzyme inhibitor-induced gastrointestinal angioedema: a case series and literature review. J Clin Gastroenterol. 2013;47(10):844–849. doi: 10.1097/MCG.0b013e318299c69d. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci. 2002;324(2):106–108. doi: 10.1097/00000441-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Scheirey CD, Scholz FJ, Shortsleeve MJ, Katz DS. Angiotensin-converting enzyme inhibitor-induced small-bowel angioedema: clinical and imaging findings in 20 patients. AJR Am R Roentgenol. 2011;197(2):393–398. doi: 10.2214/AJR.10.4451. [DOI] [PubMed] [Google Scholar]
- 6.Inomata N. Recent advances in drug-induced angioedema. Allergol Int. 2012;61(4):545–557. doi: 10.2332/allergolint.12-RAI-0493. [DOI] [PubMed] [Google Scholar]
- 7.Cicardi MC, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatment. Arch Intern Med. 2004;164(8):910–913. doi: 10.1001/archinte.164.8.910. [DOI] [PubMed] [Google Scholar]