Abstract
Objectives
Reduced pain thresholds have been documented in adult fibromyalgia but there are no quantitative studies of altered pain sensitivity in adolescents with juvenile fibromyalgia (JFM). The current study examined differences in pressure pain sensitivity between adolescent females with JFM and healthy controls. The relationship between levels of anxiety and pain were also examined.
Methods
A total of 34 JFM (15.4 ± 1.4 years old) and 31 controls (14.5 ± 1.3 years old) completed self-report measures of pain and anxiety. Pressure pain threshold was assessed (palm and forehead sites) with a hand-held algometer. Participants indicated the first sensation of pain and then rated the intensity of pain on a numerical rating scale.
Results
Adolescents with JFM exhibited greater sensitivity to pressure pain compared to controls. While the difference between JFM and controls was only observed at the forehead, the intensity of pain produced by the pressure algometry at both sites was significantly higher in the JFM participants compared to controls. Correlations between clinical pain and anxiety were significant for the JFM group only. No relationships were observed between anxiety and pressure pain for either group.
Discussion
This study is a first step towards investigating mechanisms of altered pain processing in adolescents with JFM. Adolescents with JFM were found be more sensitive to pressure pain than their healthy peers, which suggests a propensity for sensitization of peripheral and/or central nociceptive information often reported in adult fibromyalgia, and which does not appear to be affected by anxiety.
Keywords: Anxiety, Pressure Pain, Quantitative Sensory Testing, Juvenile Fibromyalgia
1. Introduction
Juvenile fibromyalgia (JFM) is a complex condition primarily characterized by persistent widespread musculoskeletal pain (for a recent review [1]). JFM affects between 2–6% of school-age children and adolescents, predominantly females [2, 3], and is associated with significant physical and psychosocial impairment [4]. Paralleling observations in adult fibromyalgia (FM), patients with JFM may also experience symptoms including fatigue and poor sleep. Furthermore, fibromyalgia symptoms persist in an estimated 70% of JFM patients as they transition into adulthood [5]. Explanations for the development and persistence of JFM are limited, but evidence from studies in FM and other pain cohorts suggest an enhancement of nociceptive signaling within the central nervous system (i.e., central sensitization [6]), which is commonly observed as increased sensitivity to laboratory stimuli [7, 8]. Although there is evidence that adolescents with JFM have multiple painful “tender points” based on clinical assessment [9, 10], there is currently little information from quantitative studies about potential mechanisms underlying JFM, and whether these are different than adults.
One method, Quantitative Sensory Testing (QST), is useful in the evaluation of pathophysiological mechanisms underlying the development and persistence of chronic pain. QST represents a set of non-invasive, standardized psychophysical assessments to probe the function or dysfunction of peripheral afferents (Aδ, Aβ, C) in addition to providing information about their corresponding central nociceptive pathways [11]. QST methods utilize controlled, standardized stimuli to probe sensitivity across an array of modalities including thermal and mechanical sensations. To date, the application of QST to understand the pathophysiological mechanisms including central sensitization underlying fibromyalgia has focused on adults [7, 8, 12, 13]. Although the feasibility of QST has been documented for children and adolescents without [14–17] and with [18–22] chronic pain, there are no QST studies in juvenile fibromyalgia, which limits our understanding of the extent of abnormalities in pain perception in youth with JFM. Future research is needed to determine if extrapolation from adult pain studies is relevant to adolescents with fibromyalgia.
Although comprehensive QST protocols typically include a variety of stimuli, the focus of this preliminary study was limited to the assessment of pressure pain. Pressure sensitivity is a clinically relevant feature of musculoskeletal pain, which is a cardinal feature of fibromyalgia (i.e., tenderness upon palpation). In addition, pressure pain has been shown to exhibit the greatest effect sizes in other conditions characterized by musculoskeletal pain [23] while contributing to clinical pain intensity in adult fibromyalgia patients [24]. The perception of pressure pain is typically assessed by algometry in which a blunt tip is placed against a muscle or joint until the sensation of pain is experienced. The perception of pressure pain has largely been used to evaluate pain sensitivity in adult pain patients including FM [7, 25, 26] and other co-morbid pain cohorts [23, 27–29]. Overall, patients exhibit an increased pressure pain sensitivity (i.e., reduced threshold for the elicitation of pain) compared to controls, which indicates not only local changes in sensitivity but may indicate a more generalized pain state that is mediated by central sensitization. While local sensitivity indicates peripheral nociceptive sensitization, the observation of enhanced sensitivity at “naïve” sites (i.e., sites that are non-symptomatic, uninjured, or unrelated to tender point sites) or widespread sensitivity can indicate increased excitability within the central nervous system [30].
The first aim of this study was to determine if pressure pain thresholds (PPTh) and evoked pain ratings differed between adolescent females with JFM and healthy controls (HC). JFM affects a relatively small number of adolescent males and given the potential gender differences in pain sensitivity, males were not included in this pilot study. Pressure algometry was used to measure PPTh at the palm and forehead bilaterally. Pressure pain intensity was assessed with a numerical rating scale (NRS) immediately following the first sensation of pain. It was hypothesized that adolescents with JFM would have lower pain thresholds and higher pain ratings than HC. Since anxiety symptoms are common in JFM [31] and have been shown to affect responses on both tender point examination and QST measures in adult FM [32, 33] a secondary aim of the present study was to explore whether anxiety levels were a potential confound in the measurement of pressure pain sensitivity in adolescents with JFM and HC. It was hypothesized that anxiety would be higher in adolescents with JFM and also correlated with clinical and experimental pain outcomes in this group but not in controls. The present study included both a state and trait measure of anxiety to examine which specific aspect of anxiety may be more closely linked to pain sensitivity in JFM. Previous adult studies utilizing either QST or tender point examination have yielded mixed findings pertaining to whether state or trait anxiety differences exist between those with and without widespread pain [32–35].
2. Methods
2.1. Participants
Thirty-four female adolescents with JFM were recruited from pediatric rheumatology, pain management, and psychology clinics at a children’s hospital between August 2012 and October 2014. Potential participants meeting eligibility criteria were identified by medical chart review or by referral from attending physicians [10]. Attending physicians completed a checklist of fibromyalgia criteria to confirm patients’ diagnosis of JFM. Thirty-one gender-matched HC were recruited using emails and flyers posted throughout the institution, and letters were mailed to healthy youth seen in various pediatric clinics who had previously agreed to participate as volunteers in research studies. Participants were compensated for their time and participation with a $10 gift card.
Eligible participants were: 1) female, 2) between the ages 13 to 17 years old, and 3) able to read, understand and speak English. In addition, participants were excluded if they met criteria for any of the following: 1) an untreated major psychiatric diagnosis (e.g., major depression, bipolar disorder, or psychoses); 2) a documented developmental delay; and/or 3) an acute injury to the skin on the palm of the hand or forehead. JFM participants had a confirmed diagnosis of juvenile fibromyalgia as mentioned above. Healthy participants could not have a diagnosis of or seeking treatment for a recurrent or chronic pain syndrome (e.g., migraines, abdominal pain). However, HC were not excluded if they reported intermittent pain.
2.2. Study design
During a single in-person study visit, participants completed a series of measures regarding demographic (i.e., age, race/ethnicity, family socioeconomic status), medical history (i.e., known medical or psychiatric diagnoses, current medications, and any chronic pain history in the immediate family was collected), anxiety, and clinical pain ratings. After completing the self-reported measures, participants underwent several sensory assessment trials with pressure algometry.
2.3. Self-report measures
Clinical Pain
The Brief Pain Inventory (BPI [36, 37]) is a well-validated and widely used pain assessment tool. Four items are used to assess worst, least, average, and current pain level over the past 24 hours on a 0 (No pain) to 10 (Pain as bad as you can imagine) numeric rating scale (NRS).
Anxiety
The State-Trait Anxiety Inventory (STAI [38]) is a widely used anxiety measure that consists of two 20-item subtests that measure state and trait anxiety. Items on the state (S-Anxiety; “I feel at ease,” “I feel upset”) and trait (T-Anxiety; “I am a steady person,” “I lack self-confidence”) anxiety scales are written at the 6th grade reading level and are rated using a four-point scale: 1 = Almost Never, 2 = Sometimes, 3 = Often, 4 = Almost Always. Possible STAI Total scores range from a minimum of 40 to a maximum of 160. The STAI has been used extensively in a number of chronic medical conditions, including fibromyalgia and other musculoskeletal conditions [39–41]. In the present study, Cronbach’s alpha was 0.92 for State Anxiety and 0.94 for Trait Anxiety.
2.4. Pressure pain assessment
Pressure pain thresholds (PPTh) were assessed in participants with a hand-held algometer (Algomed, Medoc) connected to a laptop computer, which provides real-time visual and auditory feedback to ensure the rate of applied pressure is consistent across applications. For the assessment, the algometer was applied bilaterally to the palm of the hand (i.e., thenar eminence of the thumb) and above the supraorbital ridge of the forehead (i.e., arch of the eyebrow). Selection of the two testing sites was based on discussions with a pediatric neurologist (A. Hershey) at Cincinnati Children’s Hospital Medical Center (CCHMC). Using previous studies [42, 43] as a reference, the headache team overseen by A. Hershey commonly measures pain thresholds at the hand and forehead in their pediatric migraine patients. The order of testing site was randomized using simple randomization stratified by group. This randomization strategy ensured an equal number of participants with JFM and controls would receive testing to either the palm or forehead first. For each testing trial, pressure was gradually increased at a rate of 1kg/cm2 per second until the participant felt the first sensation of pain (PPTh). A maximum pressure of 4kg/cm2 was applied for both sites. A 30-second break was provided between trials to reduce sensitization due to repeated testing. Three trials were conducted at each body site, alternating between the right and left sides. Immediately after each trial, participants were asked to rate their pain using a 0 “no pain” to 10 “pain as bad as it could be” Numeric Rating Scale (NRS) [44], a widely used instrument to assess pain in children 5 years of age and older [44].
2.5. Data Analysis
Considering the preliminary nature of the current pilot study, the target sample size was modest with a larger goal to gather preliminary data and establish effect sizes for future larger trials. Data analysis was performed using SPSS (v22, IBM). Demographic data was analyzed with a Chi-Square and independent t-test as appropriate. Since age was significantly different between groups, all group differences on continuous variables were adjusted for age and assessed using analysis of covariance (ANCOVA). Mean and standard deviations are provided as appropriate. Based on preliminary analyses, no differences were observed between the left and right sides of the body (all p’s > 0.05) in pain sensitivity or pain intensity ratings. Thus, pressure pain measures from the left and right sides were averaged into a single score for the forehead and palm. Pearson's correlations were used to evaluate the associations among anxiety and continuously measured study variables separately for each group, which included pressure pain measures and the four clinical pain scores from the BPI (i.e., current, least, worst, and average). Partial eta squared (ηp2) are presented as measures of effect size (ηp2 = 0.01 is considered a small effect, ηp2 = 0.06 a medium-sized effect and partial ηp2 = 0.14 a large effect [45]).
3. Results
3.1. Demographics
Based on Chi-square tests between adolescents with JFM (26 non-Hispanic white; 5 African-American) and HC (26 non-Hispanic white; 2 African-American; 6 Other including Hispanic white, Asian, and Bi-racial), no group differences were observed for race (χ2 = 0.03, p > 0.05). A greater proportion of JFM participants (61.3% versus 29.0%) reported a familial history of pain (χ2 = 6.64, p < 0.01). Finally, group differences were observed for age in which adolescents with JFM (15.42 ± 1.41 years of age) were older than the control group (14.57 ± 1.28 years of age; t = 6.64, p < 0.05). Compared to the control group (none of whom were taking any pain medication), a majority of JFM patients (73.5%, 25/34) reported taking one or more medications for pain and symptom management including non-opioid analgesics (Acetaminophen; 12%), nonsteroidal anti-inflammatory drugs (Ibuprofen, Naproxen; 40%), tricyclic antidepressants (Amitriptyline; 24%), opioid analgesics (Codeine, Percocet, Tramadol; 12%), muscle relaxants (Flexeril, Methocarbamol; 16%), anti-convulsants (Lyrica, Gabapentin, Topamax; 32%), triptans (Imitrex, Maxalt; 8%) in addition to serotonin–norepinephrine reuptake (Cymbalta, Effexor; 20%) and selective serotonin re-uptake (Lexapro, Prozac, Zoloft, Trazodone; 16%) inhibitors.
3.2. Clinical Pain
There were significant differences between JFM and HC groups for all sub-scales of the BPI (see Table 1 for group differences in key variables). Compared to the control group, participants with JFM reported greater levels of current (F = 102.28, p < 0.001, ηp2 = 0.62), least (F = 71.28, p < 0.001, ηp2 = 0.54), average (F = 101.53, p < 0.001, ηp2 = 0.62), and worst (F = 95.65, p < 0.001, ηp2 = 0.61) pain over the past 24 hours. These results are consistent with findings from an adult study which found comparable pain levels (6.5 ± 1.5) on sub-scales of the BPI in an older cohort of FM patients [46]. Interestingly, HC reported low levels of pain, which may reflect a tendency to report on acute pain experiences in individuals without chronic pain.
Table 1.
Pain outcomes and anxiety in adolescents with Juvenile fibromyalgia (JFM) and healthy controls.
| JFM (n = 34) |
Controls (n = 31) |
|
|---|---|---|
| Clinical Pain (BPI) | ||
| Current Pain*** | 5.8 2(± 1.93) | 1.39 (± 1.33) |
| Least Pain*** | 3.94 (± 1.63) | 1.23 (± 0.56) |
| Average Pain*** | 5.88(± 1.37) | 1.94 (± 1.65) |
| Worst Pain*** | 7.12 (± 1.67) | 2.22 (± 2.14) |
| Anxiety (STAI) | ||
| State*** | 42.09 (± 12.64) | 29.51 (± 9.22) |
| Trait*** | 46.21 (± 12.18) | 33.06 (± 9.17) |
Mean values (± Standard Deviations) of self-reported pain and anxiety were compared between JFM and HC. For the STAI, a score of ≥ 39 on the State scale suggests the presence of clinically significant anxiety symptoms, which is observed in the JFM cohort. Significant differences between JFM and HC are indicated as follows:
p< 0.001.
Abbreviations: Brief Pain Inventory, BPI; State-Trait Anxiety Inventory, STAI; Juvenile-onset fibromyalgia, JFM; and healthy controls, HC.
3.3. Experimental Pain
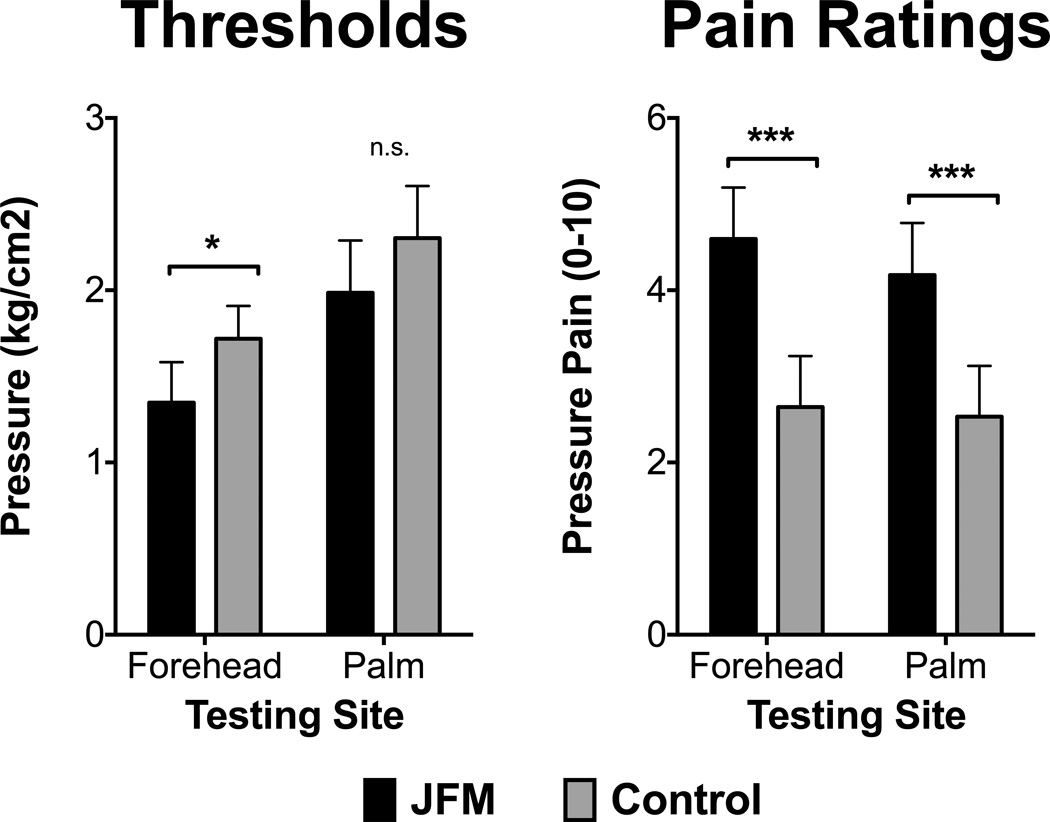
Figure 1 illustrates differences in pressure pain thresholds and the corresponding evoked pain intensity ratings between JFM and HC. Adolescents with JFM required less pressure to induce pain (i.e., lower PPTh) at the forehead (F = 5.31, p = 0.03, ηp2 = 0.08) compared to HC. Although, PPTh on the palm was also lower for adolescents with JFM, this difference was not significant (F = 1.23, p = 0.27, ηp2 = 0.02). After reaching threshold, subjective ratings of pain were significantly higher in JFM compared to HC at both sites (Forehead: F = 20.45, p < 0.001, ηp2 = 0.25; thenar: F = 14.86, p < 0.001, ηp2 = 0.20).
Figure 1. Differences in pressure pain thresholds (left panel) and numerical pain ratings associated with pain thresholds (right panel) between adolescents with Juvenile fibromyalgia (JFM) and healthy controls.
Significant differences between JFM and controls are indicated as follows: n.s., non-significant; *, p < 0.05; ***, p < 0.001.
3.4. The relationship between anxiety and pain outcomes
Table 2 presents the scores for state and trait anxiety. Similar to clinical pain outcomes, results showed that adolescents with JFM reported markedly higher levels of trait (F = 20.69, p < 0.001, ηp2 = 0.25) and state (F = 17.26, p < 0.001, ηp2 = 0.22) anxiety compared to HC. Correlations between BPI and pressure pain sensitivity variables are presented in Tables 2 and 3, respectively. In the JFM group, significant relationships were observed between state anxiety and all clinical pain ratings (Table 2, r range = 0.48 to 0.62). However, trait anxiety was only associated with pain at its worst and least over the past 24 hours. No significant associations were observed between trait and state anxiety with pressure pain (Table 3; r range = 0.10 to 0.27). Lastly, in line with our original hypotheses, anxiety was not significantly related to clinical pain ratings (Table 2; r range = 0.10 to 0.27) or pressure pain thresholds (Table 3; r range = −0.14 to 0.16) in the control group.
Table 2.
Relationships between self-reported pain measures and anxiety in adolescents with Juvenile fibromyalgia (JFM) and healthy controls.
| Current | Average | Worst | Least | |
|---|---|---|---|---|
| JFM | ||||
| Trait | 0.32 | 0.27 | 0.36* | 0.37* |
| State | 0.58*** | 0.48*** | 0.48*** | 0.62*** |
| Controls | ||||
| Trait | 0.10 | 0.19 | 0.27 | 0.11 |
| State | 0.11 | 0.10 | 0.25 | 0.19 |
Values are Pearson correlations. Significant values are indicated as follows:
, p< 0.05;
p< 0.001.
Table 3.
Relationships between experimental pain measures and anxiety in adolescents with Juvenile fibromyalgia (JFM) and healthy controls.
| Pain Ratings (NRS) | Thresholds (kg/cm2) | |||
|---|---|---|---|---|
| Forehead | Thenar | Forehead | Thenar | |
| JFM | ||||
| Trait | −0.15 | −0.09 | −0.04 | −0.18 |
| State | −0.14 | −0.00 | −0.03 | −0.23 |
| Controls | ||||
| Trait | 0.08 | 0.16 | −0.14 | −0.06 |
| State | 0.03 | 0.16 | −0.10 | −0.06 |
Values are Pearson correlations.
3.5. Exploratory Analysis related to Medication Use
Considering the potential impact of medication use, an exploratory analysis was performed to compare pressure pain thresholds and ratings among the following participants: medication-free JFM patients (n=9), JFM taking medications (n = 25), and controls (n=31). Overall, post-hoc analysis of the ANOVA revealed several interesting observations. First, the medication-free JFM patients exhibited equivalent levels of pain compared to the control group on 3 of the 4 parameters. Specifically, pain threshold and ratings at the palm were similar between medication-free JFM patients and controls. However, pain thresholds, but not pain ratings, at the forehead were similar between these groups. Second, JFM participants on medications differed from the control group on 3 of the 4 parameters such that JFM participants on medications reported higher pain ratings at the forehead and palm but lower thresholds at the forehead compared to controls. Third, differences between medication-free and medication-taking JFM patients only revealed a significant post-hoc difference for pressure thresholds at the forehead. Additionally, no differences in pain ratings were observed between the two JFM subgroups.
4. Discussion
In the current study, pressure pain sensitivity, clinical pain, and anxiety were examined in adolescents with and without JFM. Group differences were observed in several of these domains, which were characterized by small (e.g., pain thresholds at the palm), medium (e.g., pain thresholds at the forehead), and large (e.g., pain ratings at the forehead and palm, anxiety, clinical pain) effect sizes. Specifically, lower thresholds for pressure pain were observed in adolescents with JFM at the forehead but not the palm, and the effect size was small (ηp2 = 0.02). One possible explanation for this null finding could be medication use and the limited sample size, which impacts our power to detect a significant difference. However, the intensity of pain evoked at both sites was significantly higher in the JFM subjects compared to controls. Effect sizes for subjective ratings of pain were large (e.g., range of ηp2 = 0.20 to 0.25). Despite the lack of significant differences for pain thresholds at the palm, the current study provided evidentiary support of our original hypothesis that JFM would be associated with lower pain thresholds and higher pain ratings than controls. In agreement with other studies in JFM [31], self-reported anxiety was higher in adolescents with JFM and positively related to clinical pain, but self-reported anxiety was not associated with pressure pain. Though this finding is inconsistent with some studies showing relationships between experimental outcomes with psychological functioning in adult pain cohorts [32–35], critical methodological differences in patient recruitment (e.g., widespread pain; general chronic pain), experimental measures (e.g., heat pain, tender points) and psychological assessments (e.g., pain anxiety; general distress) likely contribute to these results. Additional research is needed to explore if these various patient-, experimental-, and psychological-related factors are relevant to JFM.
4.1. Pressure pain thresholds
Pain hypersensitivity is often reported in adult fibromyalgia studies to a range of experimental stimuli including pressure pain [8, 24, 26, 47]. Overall, these studies show an increased, widespread sensitivity to painful stimuli. The current study supports these observations suggesting that altered pain processing may already be present in children and adolescents with fibromyalgia in a similar fashion to adult fibromyalgia rather than altered sensitivity developing over time and culminating in greater pain sensitivity in adulthood. One observation from these studies is the association of pain sensitivity with clinical pain outcomes (e.g., intensity). For example, pressure pain thresholds are reported to be modestly correlated with intensity in musculoskeletal pain including adults with low back pain [48] and fibromyalgia [24, 49], which might be due to the selection of stimulus modality or its presentation [50]. In the current study, clinical and experimental pain was not associated (data not shown; p’s > 0.10). Since JFM patients experienced pain-related symptoms for a shorter duration than their adult counterparts, it is possible that over the course of fibromyalgia, peripheral and central changes in pain processing and modulation will develop and become more permanent due to neuroplastic changes.
Since the current study is the first to report difference in pressure pain in JFM, it is worthwhile to acknowledge the limited generalizability of our findings beyond the scope of this study. In general, previous research in pediatric populations using experimental pain paradigms for pain assessments has been established as feasible. Moreover, they provide useful information about alterations in sensory processing of pain. For example, a recent study in children with Juvenile Idiopathic Arthritis (JIA) reported greater sensitivity to a range of painful stimuli including pressure pain [19]. Clinically, pain related to JIA is typically localized to the affected joint, so the fact that pain sensitivity was observed outside these areas and during remission of symptoms suggests that widespread changes in pain processing due to central sensitization and/or reduced pain inhibition can occur in pediatric rheumatic diseases. Similar observations have been reported in a few other pediatric pain conditions (e.g., migraine [21, 51]; irritable bowel syndrome [52]) and conditions not traditionally characterized as pain-related (e.g., chronic fatigue syndrome [53]) in which evidence for localized and widespread hyperalgesia was observed. Our data support these findings in that we observed an increase in sensitivity as indicated by lower pressure pain thresholds and higher pressure pain ratings in JFM patients compared to controls [54].
4.2. Relationships with anxiety
Psychological profiles in JFM often include elevated symptoms of depression and anxiety [55, 56]. In the current study, we observed similar findings in which adolescents with JFM had higher levels of both state and trait anxiety than the HC group. Further based on theory that higher levels of anxiety might contribute to the experience of pain, we sought to better understand the relationship between anxiety and pain. Results from this study demonstrate that while anxiety was positively related to clinical pain, it was not associated with experimental pain. A potential explanation for this observation is that while anxiety is associated with clinical pain in JFM, experimental pain methods like pressure may offer a more objective measure of pain sensitivity less influenced by anxiety symptoms in adolescents. Alternatively, the assessment of anxiety through subjective measures (e.g., SCARED, RCMAS) has been shown to capture different aspects of anxiety that provide an alternative model for understanding the relationship between anxiety and pain in JFM [39, 55]. Furthermore, studies in adult fibromyalgia suggest that the relationship between anxiety and experimental pain is weakly associated [39, 55].
4.3. Limitations
Several limitations should be addressed in the current study. First, we did not assess disability or psychological factors other than anxiety (e.g., depression, catastrophizing) in the current sample of JFM that may contribute to the objective and subjective experience of pain. Second, while we did not assess other modalities, it would be informative for future studies to replicate findings in adolescents with JFM to further characterize enhanced pain sensitivity and deficits in pain modulation. In contrast to static methods (e.g., thresholds), the use of dynamic methods (e.g., temporal summation, conditioned pain modulation) should be incorporated into future research to evaluate the underlying mechanisms involved in endogenous pain modulation. Additionally, participants were not asked to abstain from medication use and those taking long-acting medications were not excluded. Due to the variety of medications (e.g., antidepressants, anticonvulsants, analgesics) used in the JFM group and the limited number of each within a specific class of medications, we were unable to directly determine the relative contribution of individual medication categories on pressure pain thresholds in JFM. However, in an effort to explore the potential effect of medication, we compared pressure pain outcomes among JFM participants taking medications, medication-free JFM participants and controls. Despite equivalent levels of clinical pain intensity, the JFM groups differed in the amount of pressure needed to induce the sensation of pain, particularly at the forehead site. This observation suggests that despite comparable levels of clinical and experimental pain intensity, medication use did not contribute to better clinical and experimental outcomes in the JFM group taking medications, which may reflect a unique phenotype. It remains unknown whether the JFM patients taking medications are more clinically complex. While the reasons supporting this idea is speculative, these patients might be characterized as having greater illness severity (e.g., disability, widespread pain), greater impairment in coping strategies (e.g., depression, catastrophizing), and/or poorer behavioral outcomes (e.g., lower physical activity, poorer sleep), which may have prompted a medical provider to prescribe medication. Considering that we did not have a large enough sample size to evaluate the effects of medications on pressure pain, the exploratory analysis should be viewed with caution. Nevertheless, it is striking that despite being treated with medications to manage their JFM symptoms, the entire group still showed higher pressure pain sensitivity than the control group.
4.4. Conclusions and Future Directions
The current study is a first step towards investigating the underlying mechanisms involved in altered pain processing and provides preliminary support for the presence of alterations in the way that painful stimulation is processed in adolescents with JFM. More specifically, adolescents with JFM appear to exhibit a hyper-sensitization marked by enhanced sensitivity and perception of pressure pain, which is consistent with findings from adults with various musculoskeletal pain conditions [28, 29] including fibromyalgia [7, 24, 25]. However, future research employing experimental assessments of endogenous pain modulation and the temporal summation of pain is necessary to confirm whether findings in JFM and adult FM are comparable. Additionally, future studies should evaluate the presence of altered somatosensory processing across a range of modalities (e.g., heat, cold, punctate) and testing parameters (e.g., detection thresholds, pain thresholds, pain suprathresholds) in a larger sample of adolescents with JFM in order to increase the generalizability of findings from the current study.
Supplementary Material
Acknowledgments
This work was partially supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health Grant K24AR056687 to S.Kashikar-Zuck
References
- 1.Kashikar-Zuck S, Ting TV. Juvenile fibromyalgia: current status of research and future developments. Nat Rev Rheumatol. 2014;10:89–96. doi: 10.1038/nrrheum.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikkelsson M, Salminen JJ, Kautiainen H. Non-specific musculoskeletal pain in preadolescents. Prevalence and 1-year persistence. Pain. 1997;73:29–35. doi: 10.1016/s0304-3959(97)00073-0. [DOI] [PubMed] [Google Scholar]
- 3.Sardini S, Ghirardini M, Betelemme L, Arpino C, Fatti F, Zanini F. Epidemiological study of a primary fibromyalgia in pediatric age. Minerva Pediatr. 1996;48:543–550. [PubMed] [Google Scholar]
- 4.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Rheum. 2007;57:474–480. doi: 10.1002/art.22615. [DOI] [PubMed] [Google Scholar]
- 5.Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW, Lovell DJ, Arnold LM. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics. 2014;133:e592–e600. doi: 10.1542/peds.2013-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep. 2011;13:513–520. doi: 10.1007/s11926-011-0206-6. [DOI] [PubMed] [Google Scholar]
- 7.Blumenstiel K, Gerhardt A, Rolke R, Bieber C, Tesarz J, Friederich HC, Eich W, Treede RD. Quantitative sensory testing profiles in chronic back pain are distinct from those in fibromyalgia. Clin J Pain. 2011;27:682–690. doi: 10.1097/AJP.0b013e3182177654. [DOI] [PubMed] [Google Scholar]
- 8.Vierck CJ, Wong F, King CD, Mauderli AP, Schmidt S, Riley JL., 3rd Characteristics of sensitization associated with chronic pain conditions. Clin J Pain. 2014;30:119–128. doi: 10.1097/AJP.0b013e318287aac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology Adult Fibromyalgia Criteria for Use in an Adolescent Female Population with Juvenile Fibromyalgia. J Pediatr. 2015;169:181–187. doi: 10.1016/j.jpeds.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28:138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014;15:61–72. doi: 10.1111/pme.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 13.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 14.Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain. 2010;149:76–88. doi: 10.1016/j.pain.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Blankenburg M, Meyer D, Hirschfeld G, Kraemer N, Hechler T, Aksu F, Krumova EK, Magerl W, Maier C, Zernikow B. Developmental and sex differences in somatosensory perception--a systematic comparison of 7-versus 14-year-olds using quantitative sensory testing. Pain. 2011;152:2625–2631. doi: 10.1016/j.pain.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Tsao JC, Seidman LC, Evans S, Lung KC, Zeltzer LK, Naliboff BD. Conditioned pain modulation in children and adolescents: effects of sex and age. J Pain. 2013;14:558–567. doi: 10.1016/j.jpain.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Walker L, Bruehl S, Hellman N, Sherman A, Rao U. Race effects on temporal summation to heat pain in youth. Pain. 2015;156:917–922. doi: 10.1097/j.pain.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob E, Chan VW, Hodge C, Zeltzer L, Zurakowski D, Sethna NF. Sensory and Thermal Quantitative Testing in Children With Sickle Cell Disease. J Pediatr Hematol Oncol. 2015;37:185–189. doi: 10.1097/MPH.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornelissen L, Donado C, Kim J, Chiel L, Zurakowski D, Logan DE, Meier P, Sethna NF, Blankenburg M, Zernikow B, Sundel RP, Berde CB. Pain hypersensitivity in juvenile idiopathic arthritis: a quantitative sensory testing study. Pediatr Rheumatol Online J. 2014;12:39. doi: 10.1186/1546-0096-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duarte MA, Goulart EM, Penna FJ. Pressure pain threshold in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2000;31:280–285. doi: 10.1097/00005176-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Zohsel K, Hohmeister J, Oelkers-Ax R, Flor H, Hermann C. Quantitative sensory testing in children with migraine: preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain. 2006;123:10–18. doi: 10.1016/j.pain.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Sherman AL, Morris MC, Bruehl S, Westbrook TD, Walker LS. Heightened Temporal Summation of Pain in Patients with Functional Gastrointestinal Disorders and History of Trauma. Ann Behav Med. 2015;49:785–792. doi: 10.1007/s12160-015-9712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Mulkey F, Rothwell R, Maixner W. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. J Pain. 2011;12:T61–T74. doi: 10.1016/j.jpain.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staud R, Weyl EE, Price DD, Robinson ME. Mechanical and heat hyperalgesia highly predict clinical pain intensity in patients with chronic musculoskeletal pain syndromes. J Pain. 2012;13:725–735. doi: 10.1016/j.jpain.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petzke F, Harris RE, Williams DA, Clauw DJ, Gracely RH. Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain. 2005;9:325–335. doi: 10.1016/j.ejpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 27.As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet Gynecol. 2013;122:1047–1055. doi: 10.1097/AOG.0b013e3182a7e1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King CD, Sibille KT, Goodin BR, Cruz-Almeida Y, Glover TL, Bartley E, Riley JL, Herbert MS, Sotolongo A, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1243–1252. doi: 10.1016/j.joca.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain. 2014;155:2134–2143. doi: 10.1016/j.pain.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Treede RD, Rolke R, Andrews K, Magerl W. Pain elicited by blunt pressure: neurobiological basis and clinical relevance. Pain. 2002;98:235–240. doi: 10.1016/S0304-3959(02)00203-8. [DOI] [PubMed] [Google Scholar]
- 31.Kashikar-Zuck S, Parkins IS, Graham TB, Lynch AM, Passo M, Johnston M, Schikler KN, Hashkes PJ, Banez G, Richards MM. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain. 2008;24:620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petzke F, Gracely RH, Park KM, Ambrose K, Clauw DJ. What do tender points measure? Influence of distress on 4 measures of tenderness. J Rheumatol. 2003;30:567–574. [PubMed] [Google Scholar]
- 33.Terry MJ, Moeschler SM, Hoelzer BC, Hooten WM. Pain Catastrophizing and Anxiety are Associated with Heat Pain Perception in a Community Sample of Adults with Chronic Pain. Clin J Pain. 2015 doi: 10.1097/AJP.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 34.Pukall CF, Baron M, Amsel R, Khalife S, Binik YM. Tender point examination in women with vulvar vestibulitis syndrome. Clin J Pain. 2006;22:601–609. doi: 10.1097/01.ajp.0000210903.67849.af. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal VR, Macfarlane GJ, McBeth J. A high tender point count is associated with the presence of multiple idiopathic pain disorders: results from a population study. Eur J Pain. 2012;16:1195–1203. doi: 10.1002/j.1532-2149.2012.00127.x. [DOI] [PubMed] [Google Scholar]
- 36.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 37.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Spielberger CD, Gorsuch RL. Manual for the State-Trait Anxiety Inventory (Form Y) : ("self-evaluation questionnaire") Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 39.Jensen KB, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Gracely R, Ingvar M, Kosek E. Anxiety and depressive symptoms in fibromyalgia are related to poor perception of health but not to pain sensitivity or cerebral processing of pain. Arthritis Rheum. 2010;62:3488–3495. doi: 10.1002/art.27649. [DOI] [PubMed] [Google Scholar]
- 40.Conte PM, Walco GA, Kimura Y. Temperament and stress response in children with juvenile primary fibromyalgia syndrome. Arthritis Rheum. 2003;48:2923–2930. doi: 10.1002/art.11244. [DOI] [PubMed] [Google Scholar]
- 41.White KP, Nielson WR, Harth M, Ostbye T, Speechley M. Chronic widespread musculoskeletal pain with or without fibromyalgia: psychological distress in a representative community adult sample. J Rheumatol. 2002;29:588–594. [PubMed] [Google Scholar]
- 42.Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenk P, Laeubli T, Klipstein A. Validity of pressure pain thresholds in female workers with and without recurrent low back pain. Eur Spine J. 2007;16:267–275. doi: 10.1007/s00586-006-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- 46.Mease PJ, Spaeth M, Clauw DJ, Arnold LM, Bradley LA, Russell IJ, Kajdasz DK, Walker DJ, Chappell AS. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res (Hoboken) 2011;63:821–826. doi: 10.1002/acr.20449. [DOI] [PubMed] [Google Scholar]
- 47.Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain. 2007;11:202–207. doi: 10.1016/j.ejpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, Kobrine AI, Wiesel SW. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine (Phila Pa 1976) 1999;24:2035–2041. doi: 10.1097/00007632-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 49.Ge HY, Wang Y, Fernandez-de-las-Penas C, Graven-Nielsen T, Danneskiold-Samsoe B, Arendt-Nielsen L. Reproduction of overall spontaneous pain pattern by manual stimulation of active myofascial trigger points in fibromyalgia patients. Arthritis Res Ther. 2011;13:R48. doi: 10.1186/ar3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebbeck T, Moloney N, Azoory R, Hubscher M, Waller R, Gibbons R, Beales D. Clinical Ratings of Pain Sensitivity Correlate With Quantitative Measures in People With Chronic Neck Pain and Healthy Controls: Cross-Sectional Study. Phys Ther. 2015 doi: 10.2522/ptj.20140352. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Mayoralas DM, Fernandez-de-las-Penas C, Ortega-Santiago R, Ambite-Quesada S, Jimenez-Garcia R, Fernandez-Jaen A. Generalized mechanical nerve pain hypersensitivity in children with episodic tension-type headache. Pediatrics. 2010;126:e187–e194. doi: 10.1542/peds.2010-0012. [DOI] [PubMed] [Google Scholar]
- 52.Stabell N, Stubhaug A, Flaegstad T, Mayer E, Naliboff BD, Nielsen CS. Widespread hyperalgesia in adolescents with symptoms of irritable bowel syndrome: results from a large population-based study. J Pain. 2014;15:898–906. doi: 10.1016/j.jpain.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Winger A, Kvarstein G, Wyller VB, Sulheim D, Fagermoen E, Smastuen MC, Helseth S. Pain and pressure pain thresholds in adolescents with chronic fatigue syndrome and healthy controls: a cross-sectional study. BMJ Open. 2014;4:e005920. doi: 10.1136/bmjopen-2014-005920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s) Best Pract Res Clin Rheumatol. 2015;29:6–19. doi: 10.1016/j.berh.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Arnold LM, Leon T, Whalen E, Barrett J. Relationships among pain and depressive and anxiety symptoms in clinical trials of pregabalin in fibromyalgia. Psychosomatics. 2010;51:489–497. doi: 10.1176/appi.psy.51.6.489. [DOI] [PubMed] [Google Scholar]
- 56.Kashikar-Zuck S, Zafar M, Barnett KA, Aylward BS, Strotman D, Slater SK, Allen JR, Lecates SL, Kabbouche MA, Ting TV, Hershey AD, Powers SW. Quality of life and emotional functioning in youth with chronic migraine and juvenile fibromyalgia. Clin J Pain. 2013;29:1066–1072. doi: 10.1097/AJP.0b013e3182850544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.