Abstract
Canonical WNT/β- catenin signaling plays pivotal roles in mammary development and tumorigenesis; and aberrant activation of this pathway is frequently observed in human breast cancer, correlating with poor outcome. However, the mechanisms underlying WNT-mediated mammary tumorigenesis remain incompletely understood. Here, we used mouse mammary tumor virus (MMTV)-Wnt1 transgenic mice, which develop aggressive mammary adenomas, to examine whether Limb-Bud-and-Heart (LBH) - a WNT/β-catenin target transcription co-factor overexpressed in human triple-negative breast cancers with WNT pathway hyperactivation, contributes to WNT-induced tumorigenesis. We found LBH is specifically overexpressed in basal epithelial tumor cells of MMTV-WNT1 mammary tumors reminiscent of its’ basal cell-restricted expression pattern in the normal postnatal mammary gland. To determine the role of LBH in mammary tumorigenesis, we crossed MMTV-Wnt1 mice with epithelial-specific Keratin 14/K14-Cre;LbhloxP knockout mice. Mammary glands from virgin LBH-deficient MMTV-Wnt1 mice exhibited reduced hyperplasia, cell proliferation and increased apoptosis. Importantly, LBH inactivation in mammary epithelium significantly delayed tumor onset in MMTV-Wnt1 transgenic mice, with a median tumor-free survival of 32.5 weeks compared to 22.5 weeks in control LBH wild type MMTV-Wnt1 mice (p<0.05). This data provides the first evidence that LBH plays an essential role in WNT-induced mammary tumorigenesis by promoting hyperplastic growth and tumor formation.
Keywords: Breast cancer, WNT/β-catenin signaling, Limb-Bud-and-Heart, mammary tumorigenesis, mouse model
INTRODUCTION
WNT signaling is vital to embryonic development and adult tissue homeostasis by controlling pattern formation, cell proliferation, differentiation, cell migration, and stem cell function [1]. WNT ligands bind to receptor complexes composed of Frizzled and LRP5/6 co-receptors and activate a canonical, β-catenin-dependent intracellular signaling pathway that results in β-catenin protein stabilization and nuclear translocation; formation of nuclear β-catenin/TCF transcriptional complexes, and induction of WNT target genes [1]. When deregulated, canonical WNT signaling causes cancer by promoting cell transformation, cancer stem cells, tumor initiation, metastatic progression, and treatment resistance [1]. However, despite the high prevalence of aberrant WNT pathway activation in many human cancers, e.g., colon, breast, pancreatic, there are no WNT inhibitory drugs currently approved in the clinic, largely due to the pleiotropic effects of WNT signaling that cause WNT inhibitory drugs to have deleterious side effects. Hence, a better understanding of the molecular mechanisms underlying WNT-driven oncogenesis is needed to devise less cytotoxic targeting strategies.
In breast cancer, Wnt/β-catenin pathway hyperactivation is primarily observed in clinically aggressive, triple negative (ER-/PR-/HER2-) basal-like breast cancers (TNBC) [2–4], predicting poor patient survival overall [5]. WNT inhibition studies, and stable knockdown of key WNT effector, β-catenin, or WNT co-receptor, LRP6, in TNBC cell lines have shown that impairing WNT signaling reduces tumor cell viability, proliferation, migration, cancer stem cells, and colony formation in vitro and attenuates tumor formation and metastasis in vivo [2, 6, 7]. Moreover, aberrant WNT pathway activation in luminal, Estrogen hormone receptor-positive (ER+) breast cancer is linked to acquisition of Tamoxifen resistance [8]. This, together with identification of Wnt1 as the first mammary oncogene activated by genomic integration of the mouse mammary tumor virus (MMTV) causes aggressive mammary adenocarcinomas in mice [9], a phenotype that is phenocopied by MMTV promoter driven-Wnt1 ligand expression in MMTV-Wnt1 transgenic mice [10], strongly suggests a critical requirement of WNT in breast carcinogenesis. However, the mechanisms underlying WNT-mediated breast carcinogenesis remain poorly understood.
We previously identified Limb Bud and Heart (LBH) [11, 12], a novel WNT/β-catenin target gene that is induced by canonical WNT signaling in epithelial tissues [13]. LBH is a highly conserved, tissue-specific transcription co-factor in vertebrates that forms its’ own protein class [11, 14]. In the postnatal mammary gland (MG), LBH is predominantly expressed in Keratin 5+/Keratin 14+ basal mammary epithelial cells that form the outer layer of the MG and comprise differentiated myoepithelial cells and a small population of mammary stem cells (MaSC), including Lrp5+ WNT-responsive MaSC [15]. Knockout studies in mice have shown that while LBH is not required for embryogenesis or adult vitality [16], it is essential for the rapid MG tissue expansion that occurs during puberty and pregnancy due to a critical requirement of LBH in basal MaSC self-renewal and maintenance [15], reminiscent of WNT/β-catenin signaling function in postnatal MG development [17, 18]. Importantly, LBH is abnormally overexpressed in MMTV-Wnt1 mammary tumors, and in human triple-negative breast cancers, correlating with WNT pathway hyperactivation [13, 19]. This, together with WNT inhibition studies, showing that endogenous LBH overexpression in TNBC cell lines is WNT-dependent [13], and a recent microarray study, identifying LBH as one of few WNT target genes rapidly and consistently induced by Wnt3a in TNBC cell lines [20], suggests a close link between LBH and WNT activation in breast cancer. Yet, the pathological roles of LBH in breast carcinogenesis and in WNT-mediated tumorigenesis have remained obscure.
By crossing MMTV-Wnt1 transgenic mice with epithelial-specific LBH knockout mice, we provide the first in vivo evidence that LBH is critically required for WNT-driven breast oncogenesis.
MATERIALS and METHODS
Mice
MMTV-Wnt1 transgenic mice in a B6SJL genetic background [B6SJL-Tg(Wnt1)1Hev/J; Stock No. 002870] [10] were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in a hemizygous state. Conditional LbhloxP knockout mice were generated in a mixed 129SvEv×C57BL/6 genetic background and genotyped, a s previously described [16]. For epithelial-specific LBH knockout (KO), LbhloxP mice were crossed with Keratin14(K14)-Cre mice [15]. Trigenic MMTV-Wnt1;K14Cre;LbhloxP/loxP KO and MMTV-Wnt1;K14Cre;Lbh+/+ wild type (WT) mice were then generated by crossing MMTV-Wnt1;K14Cre;Lbh+/loxP males with K14Cre;Lbh+/loxP females. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Miami in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry, Histology
Immunohistochemistry was performed, as described [15, 16], using 4–5 μm sections of paraffin-embedded inguinal mouse mammary glands or tumors fixed overnight in 10% formalin. Primary antibodies were to LBH (1:100; custom-made, affinity-purified) [15, 16], Keratin 5/6 (1:5,000; Covance), Ki67 (1:500; Novagen), and Cleaved-Caspase 3 (1:300; Cell Signaling), followed by incubation with HRP-coupled secondary antibodies (1:500; Invitrogen). Marker-positive cells were quantified on n=5 different areas/per animal, using ImageJ software. For histological analysis, paraffin tissue sections were stained with Hematoxylin-Eosin (H&E) or Masson’s Trichrome stain.
Mammary gland whole mount analysis
Inguinal mammary glands were fixed overnight in Carnoy’s solution (60% ethanol, 30% chloroform, and 10% glacial acetic acid), and stained with carmine alum, as described [16].
Real-time RT-PCR
Total RNA was isolated using Trizol (Invitrogen) and cDNA was synthesized using Moloney Murine Leukemia Virus reverse transcriptase (MMLV-RT) (Promega). qPCR analysis was performed, as described [13], except that 100 ng of cDNA, 2 μM of gene-specific primers and SsoFast EvaGreen Supermix (NEB) were used. Samples were assayed in triplicates and average Ct values normalized to Gapdh. For mouse Lbh and Gapdh qPCR primers, see [13].
Western Blot analysis
Mammary glands, after lymph node removal, and tumors were flash-frozen in liquid nitrogen and homogenized in RIPA buffer using a Polytron homogenizer. 30 μg of total protein extract per sample was separated by SDS-PAGE, and after semi-dry transfer onto PVDF membranes incubated with primary antibodies to LBH (1:500; [15, 16]) and β-actin (Sigma; 1:50,000), followed by incubation with secondary HRP-coupled antibodies (Santa Cruz; 1:10,000). Protein bands were detected using West Femto Super Signal kit (Pierce) on X-ray film, and quantified by densitometry and ImageJ analysis.
Statistical analyses
Values are expressed as the mean ± standard error (s.e.m.). All experiments were performed at least three times. The significance of differences between sample groups was determined with an unpaired two-tailed Student’s t-test, using Excel and Graphpad software, unless otherwise noted. P values of less than 0.05 (n≥3) were considered statistically significant.
RESULTS
LBH is overexpressed in basal epithelial tumor cells of MMTV-Wnt1 mammary tumors
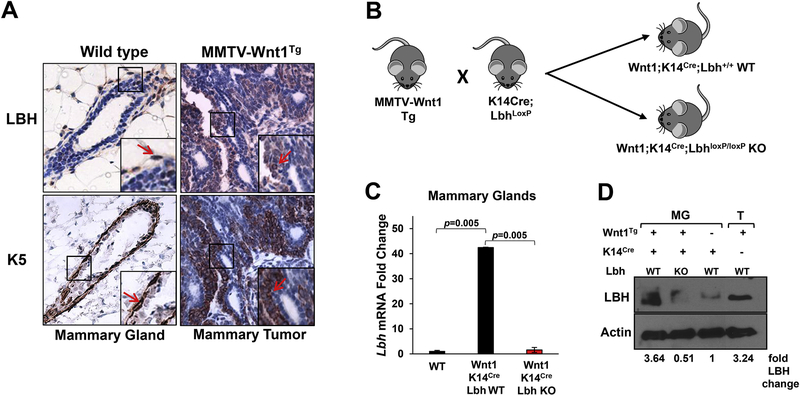
Previous RNA in situ and Western blot analyses of LBH expression in MMTV-Wnt1 transgenic mice have established that LBH is aberrantly overexpressed in MMTV-Wnt1 mammary tumors relative to normal murine mammary epithelial cells [13]. MMTV-Wnt1 mammary tumors are composed of heterogenous cell types, including luminal and basal mammary epithelial cells, tumor-associated stroma, endothelial cells, and infiltrating lymphocytes [21, 22]. To validate and extend these findings, we performed immunohistochemical analysis on tissue paraffin sections to determine LBH protein localization in these tumors. We found that LBH was predominantly expressed in epithelial tumor cells positive for the basal/progenitor cell markers, Keratin 5 and Keratin 6 (K5+/6+), but not in K5/6-negative tumor cells (Fig. 1A). Thus, LBH is expressed with a basal cell-specific expression pattern in MMTV-Wnt1 tumors similar to its basal cell-restricted expression in normal wild type (WT) MGs (Fig. 1A).
Figure 1: Elevated LBH expression in MMTV-Wnt1 mammary tumors and generation of LBH-deficient MMTV-Wnt1 transgenic mice.
(A) Immunohistochemical staining of serial tissue paraffin sections of mammary glands (MGs) from virgin wild type (WT) and mammary tumors from MMTV-Wnt1 hemizygous transgenic (Tg) female mice with antibodies specific for LBH (brown) and basal cell lineage/progenitor marker, Keratin 5/6 (K5/6) (brown-red). Blue = Hematoxylin counterstain. Right insets: Close-ups show basal cell-specific expression of LBH in normal WT mammary epithelium and in MMTV-Wnt1 Tg tumors (red arrows), as evident by co-localization with Keratin 5/6. (B) Experimental strategy for basal epithelial-specific LBH inactivation in MMTV-Wnt1 Tg mice: MMTV-Wnt1 Tg mice were interbred with conditional Keratin 14 (K14)-Cre;LbhloxP mice [15]. The F2 offspring of these crosses yielded trigenic MMTV-Wnt1Tg;K14Cre;LbhloxP/loxP knockout (KO) and control MMTV-Wnt1TG;K14Cre;Lbh+/+ mice (hereafter referred to as Wnt1;K14Cre;Lbh KO and Wnt1;K14Cre;Lbh WT, respectively). (C) Quantitative RT-PCR (qPCR) showing elevated Lbh mRNA levels in trigenic Wnt1;K14Cre;Lbh WT and efficient LBH ablation in Wnt1;K14Cre;Lbh KO MGs at 12 weeks-of-age. qPCR data was normalized to Gapdh and plotted as fold change over WT (= K14Cre;Lbh+/+). Error bars represent mean ± s.e.m. (n=2/genotype; triplicate samples; one way ANOVA). P-values as indicated. (D) Western Blot of protein extracts from 12-week-old trigenic Wnt1;K14Cre;Lbh WT and Wnt1;K14Cre;Lbh KO MGs, non-transgenic WT (= K14Cre;Lbh+/+) MGs, and MMTV-Wnt1 mammary tumors (representative examples). Quantification of LBH protein levels was performed by densitometry using Image J. Values were normalized to the Actin loading control and are shown as fold change over control K14Cre;Lbh+/+ WT MGs (lane 3).
Generation of LBH-deficient MMTV-Wnt1 transgenic mice
To study the role of LBH in WNT-driven breast tumorigenesis, we devised a gene knockout strategy to specifically inactivate LBH in basal mammary epithelial cells of MMTV-Wnt1 transgenic mice (Fig. 1B). MMTV-Wnt1 mice were crossed with conditional Keratin 14 (K14)-Cre;LbhloxP knockout mice, which, we previously showed, lack LBH expression in basal mammary epithelial cells [15]. Additionally, since K14 is expressed in bi-potent mammary epithelial progenitors during embryogenesis that give rise to both, basal and luminal cells of the pre-pubertal MG, K14Cre-mediated LBH ablation further diminishes low-level expression of LBH in alveolar luminal progenitors, whereas its’ abundant expression in MG-associated stromal cells remains unaffected [15]; thus, leading to epithelial-specific LBH inactivation. LBH-deficient MMTV-Wnt1;K14Cre;LbhloxP/loxP knockout (KO) and control MMTV-Wnt1;K14Cre;Lbh+/+ wild type (WT) mice (hereafter: trigenic Wnt1;K14Cre;Lbh WT and Wnt1;K14Cre;Lbh KO) were generated in a mixed B6SJL/129SvEv/C57BL/6 genetic background in the F2 generation (Fig. 1B) and used for all subsequent analysis. Quantitative RT-PCR (qPCR) analysis comparing Lbh mRNA expression in 12-week-old trigenic female MGs relative to age-matched WT MGs confirmed Lbh overexpression in Wnt1;K14Cre;Lbh WT and efficient LBH ablation in Wnt1;K14Cre;Lbh KO MGs (Fig. 1C). Moreover, Western Blot analysis showed a profound reduction of LBH protein levels in trigenic Wnt1;Lbh KO MGs (Fig. 1D). Thus, trigenic MMTV-Wnt1;K14Cre;LbhloxP/loxP knockout mice are an ideal model to study the consequences of LBH inactivation on Wnt1-driven mammary tumorigenesis.
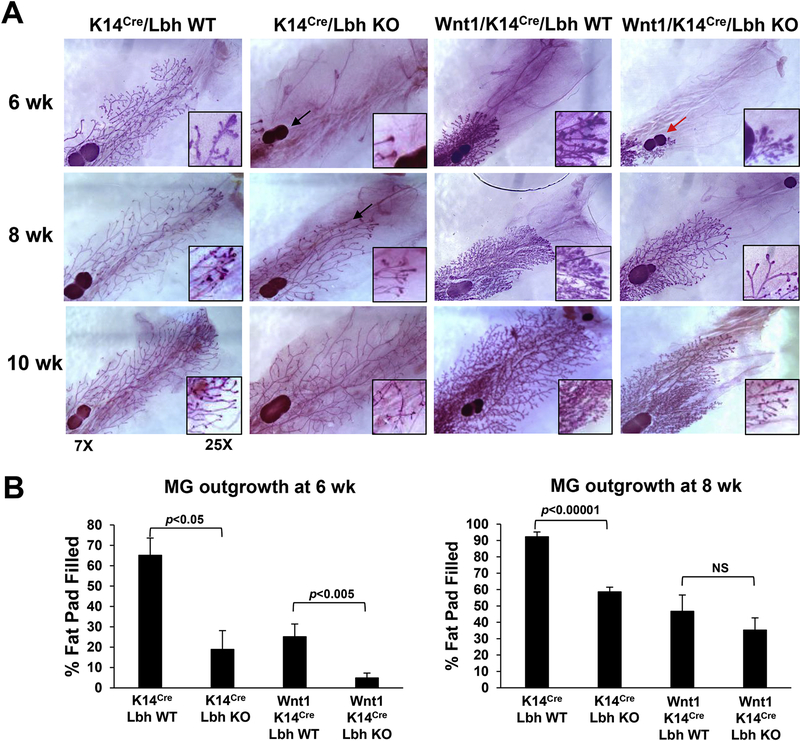
Loss of LBH affects ductal mammary gland outgrowth in MMTV-Wnt1 transgenic mice
The MMTV-Wnt1 tumor model exhibits distinct stages of mammary tumorigenesis, including hyperplasia, tumor formation, and metastasis [10]. A pre-cancerous hyperplastic phenotype is evident in MMTV-Wnt1 transgenic MGs at birth [10], associated with other morphological defects during subsequent MG development. In contrast, epithelial-specific knockout of LBH in K14Cre;LbhloxP/loxP mice delays postnatal MG outgrowth [15]. To examined the genetic interaction between LBH and WNT, we, therefore, first examined the effects of LBH deficiency on MG development in MMTV-Wnt1 mice. We assessed MG outgrowth in trigenic Wnt1;K14Cre;Lbh WT and Wnt1;K14Cre;Lbh KO in comparison with non-transgenic K14Cre;Lbh WT and K14Cre;LBH KO female mice at pubertal (6 and 8 weeks-of-age) and post-pubertal (10 and 12 weeks-of-age) stages, using carmine red wholemount analysis. This analysis confirmed a drastic reduction in ductal extension in non-transgenic K14-Cre;Lbh KO relative to age-matched K14-Cre;Lbh WT MGs (Fig. 2A,B), consistent with previous data [15]; and a hyperplastic, but stunted growth in trigenic Wnt1;Lbh WT glands at pubertal stages, similar to parental MMTV-Wnt1 MGs [10]. While the majority of non-transgenic K14-Cre;Lbh WT MGs filled the entire fat pad by 8 weeks-of-age, non-transgenic K14Cre;Lbh KO MGs and trigenic Wnt1;K14Cre;Lbh WT MGs reached complete fat pad penetrance only after 10 weeks (Figs. 2A,B; 3A; Supplementary Fig. S1); [15]. LBH-deficiency in MMTV-Wnt1;K14-Cre;Lbh KO mice also significantly attenuated pubertal MG outgrowth when compared with control MMTV-Wnt1;K14-Cre;Lbh WT mice (Fig. 2A,B; p<0.05 at 6 weeks-of-age); however this difference disappeared at the end of puberty (8 weeks) (p=0.598; Fig. 2B; see also Supplementary Fig. S1; p=0.26 for 10 weeks), whereas the delay in ductal outgrowth persisted in the absence of Wnt1 transgene in K14Cre;Lbh KO mice. Thus, epithelial LBH loss retards pubertal ductal MG outgrowth in MMTV-Wnt1 transgenic mice, suggesting LBH is required downstream of WNT at specific stages of MG development.
Figure 2: Mammary gland development in LBH-deficient MMTV-Wnt1 transgenic mice.
(A) Representative whole-mount images of carmine-red stained mammary glands (MG) from female mice of the indicated genotypes & ages in weeks (wk) (n=3/genotype/age). Note, the outgrowth defects caused by LBH deficiency in non-transgenic K14Cre;Lbh KO (black arrows) and trigenic MMTV-Wnt1;K14Cre-Lbh KO MGs (red arrow). (B) Quantification of pubertal MG outgrowth at 6 and 8 wk (distance between lymph node and the distal tips of the growing ducts). Data represent mean ± s.e.m. (n=3 mice per genotype/developmental stage/two inguinal MGs per animal); P-values (Student’s t-test) are indicated; NS = not significant.
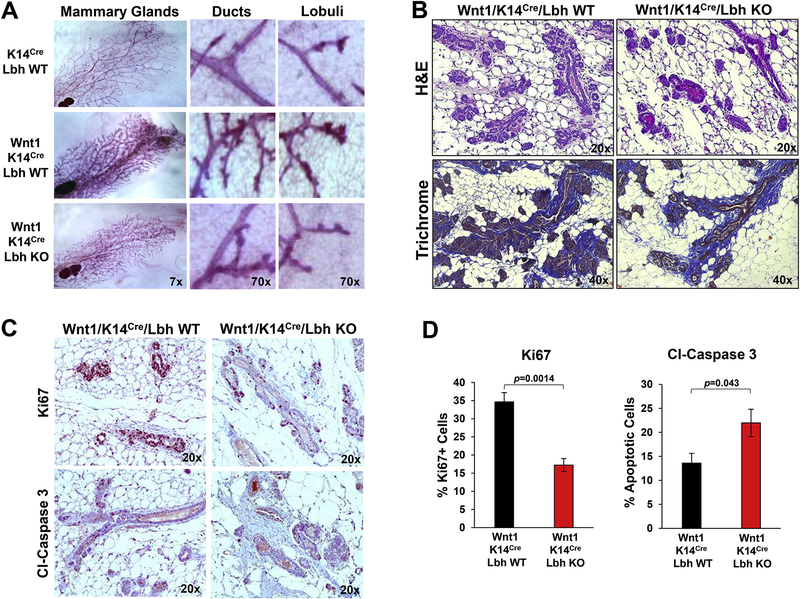
Figure 3: LBH loss attenuates Wnt1-induced mammary hyperplasia.
(A) Representative whole-mount images and close-ups of carmine-red stained mammary glands (MG) from 12 week-old virgin female mice of the indicated genotypes. (B) Representative images of Hematoxylin-Eosin (H&E)- and Masson’s Trichrome-stained MG tissue sections. Different magnifications in (A) and (B) as indicated. Note, the reduced epithelial hyperplasia in Wnt1/K14Cre/Lbh KO relative to control Wnt1/K14Cre/Lbh WT MGs, while no differences in collagen content (blue) of the peri-epithelial stroma were observed. (C,D) Immunohistochemical staining of serial MG tissue sections from 12-week old virgin female mice with anti-Ki67 and anti-Cleaved (Cl) Caspase 3 antibodies. (C) Representative images (20x magnification). (D) Quantification of Ki67- and Cl-Caspase 3-positive (+) cells in (C) using Image J software. Data represent mean ± s.e.m. (n=3 mice per genotype/5 individual areas each; Student’s t-test). P-values as indicated.
Loss of LBH attenuates Wnt1-induced mammary gland hyperplasia
Notably, epithelial LBH loss resulted in a profound reduction of the hyperplastic phenotype that is characteristic of MMTV-Wnt1 MGs throughout development. While trigenic Wnt1;K14-Cre;Lbh WT MGs exhibited extensive hyperbranching at all stages examined, hyperbranching was visibly diminished when LBH was missing (Figs. 2A, 3). This was most prominent in mature MGs at 12 weeks when ductal outgrowth had reached completion (Fig. 3A), though, we note that LBH ablation did not completely restore a wild type MG phenotype in MMTV-Wnt1 mice (Figs. 2A, 3A). These results demonstrate that LBH, in large part, is required for MMTV-Wnt1 induced mammary hyperplasia.
Hematoxylin-Eosin (H&E) staining of mammary tissue sections from 12 week-old females further revealed that LBH-deficient MMTV-Wnt1;K14Cre;LbhloxP/loxP mammary fat pads contained significantly less epithelial tissue than control MMTV-Wnt1;K14-Cre;Lbh+/+ WT mice. In contrast, loss of epithelial LBH had little effect on the MG-associated stroma, as both, hyperplastic mammary epithelium in control MMTV-Wnt1;K14Cre;Lbh WT and MMTV-Wnt1;K14Cre;Lbh KO glands were embedded in extensive stromal tissue, similar to parental MMTV-Wnt1 MGs [22], as assessed by Masson-Trichrome staining (Fig. 2B). To determine potential causes for the reduced epithelial contribution in LBH-deficient MMTV-Wnt1 MGs, we immune-stained MG tissue sections with antibodies for markers of cell proliferation (Ki67) and apoptosis (Cleaved-Caspase 3). As shown in Fig. 3C,D, MMTV-Wnt1;K14Cre;Lbh KO glands exhibited a two-fold reduction in Ki67+ mammary epithelial cells (p=0.0014). Conversely, expression of Cleaved-Caspase 3, was significantly increased in MMTV-Wnt1 mammary epithelium upon LBH KO (p=0.043; Fig. 3C,D). Collectively, these data demonstrate that LBH ablation significantly decreases Wnt1-induced pre-cancerous hyperplasia by negatively impacting proliferation, while inducing apoptosis of mammary epithelial cells.
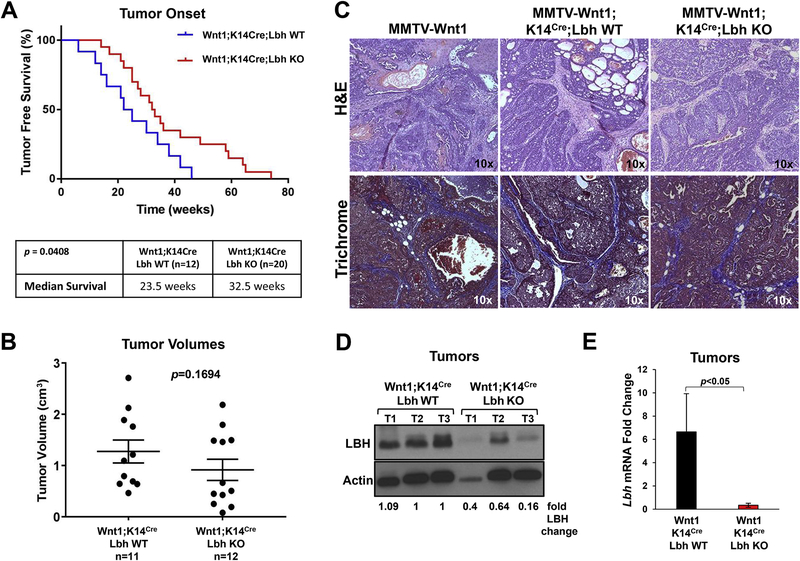
Loss of LBH delays tumor onset in MMTV-Wnt1 transgenic mice
Female MMTV-Wnt1 transgenic mice routinely develop primary mammary tumors within 3 months to 1 year [10]. To test if LBH is required for Wnt1-induced mammary tumorigenesis, virgin trigenic MMTV-Wnt1;K14-Cre;Lbh WT and MMTV-Wnt1;K14-Cre;Lbh KO female mice were aged and monitored weekly for tumor formation. We found that LBH deficiency significantly delayed tumor formation in MMTV-Wnt1;K14-Cre;Lbh KO mice, with a median time of onset of 32.5 compared to 23.5 weeks in MMTV-Wnt1;K14-Cre;Lbh WT mice (Fig. 4A; p=0.0408). Thus, loss of epithelial LBH delayed Wnt1-driven tumor development by nine weeks. Moreover, while tumor formation reached 100% penetrance in trigenic Wnt1;K14Cre;Lbh WT mice in a year’s time, the penetrance of tumor formation in trigenic Wnt1;K14Cre;Lbh KO mice was only 72% (15/n=20) during the same time period, reinforcing the notion that LBH is required for Wnt1-mediated carcinogenesis.
Figure 4: Loss of LBH delays tumor onset in MMTV-Wnt1 transgenic mice.
(A) Kaplan-Meier analysis of tumor-free survival. Cohorts of virgin female MMTV-Wnt1;K14Cre;Lbh wild type (WT) (n=11) and MMTV-Wnt1;K14Cre;Lbh knockout (KO) (n=20) mice were palpated weekly for tumor formation and biweekly, once tumors had formed. Table (bottom) shows significant differences in the median survival between the two study groups (p=0.0408; log-rank test). (B) Mann-Whitney analysis comparing tumor volumes in the same study groups at tumor onset: n=11 for MMTV-Wnt1;K14Cre;Lbh WT and n=12 for MMTV-Wnt1;K14Cre;Lbh KO. P-value as indicated. (C) Representative H&E- and Masson’s Trichrome-stained mammary tumor sections (magnification: 10x) from trigenic MMTV-Wnt1;K14Cre;Lbh WT and MMTV-Wnt1;K14Cre;Lbh KO mice vs. parental MMTV-Wnt1 transgenic mice. (D) Western Blot analysis of LBH expression in Wnt1;K14Cre;Lbh WT and Wnt1;K14Cre;Lbh KO tumors (T) (n=3 each). Actin served as loading and normalization control for the densiometric quantification of the fold changes of LBH protein levels relative to control tumors (T1, T2; lanes 2, 3). (D) qPCR quantification of Lbh mRNA levels in Wnt1;K14Cre;Lbh KO vs. Wnt1;K14Cre;Lbh WT mammary tumors (n=3/genotype; triplicate samples; one way ANOVA). Data normalized to Gapdh represent the mean ± s.e.m.; p<0.05.
Interestingly, tumor volumes at tumor onset (Fig. 4B; p=0.1694), and the time of sacrifice (three weeks after first tumor palpitation) (Supplementary Fig. S2A; p=0.688), were not significantly changed upon loss of LBH. Moreover, unlike at pre-neoplastic stages (Fig. 3C,D), there was no difference in the number of Ki67+ proliferating cells or Cleaved-Caspase 3+ pre-apoptotic cells in MMTV-Wnt1;K14Cre tumors in the presence or absence of LBH (Supplementary Fig. S2B). Thus, loss of LBH reduces tumor onset, without affecting tumor growth rates.
Histological analysis of tumor sections, furthermore, showed no major changes in tumor morphology upon epithelial LBH KO (Fig. 4C). Both, trigenic Wnt1;K14Cre;Lbh KO and Wnt1;K14Cre;Lbh WT mammary tumors were alveolar adenocarcinomas with mixed papillary, cribriform, and adenosquamous patterns, interspersed with blood lakes and collagen-rich, activated stroma (Fig. 4C), similar to parental MMTV-Wnt1 mice [10, 22]. However, trigenic Wnt1;LBH KO tumors, in addition, exhibited more foci with solid growth (Fig. 4C). Future marker analysis is necessary to further evaluate these minor morphological changes. Western blot and qPCR analysis confirmed that LBH levels were significantly decreased in Wnt1;K14Cre;Lbh KO relative to control Wnt1;K14Cre;Lbh WT tumors (Fig. 4D,E); whereby residual LBH expression in Wnt1;K14Cre;Lbh KO tumors likely is due to LBH expression in non-epithelial compartments, i.e. stromal cells [15].
DISCUSSION
This in vivo study demonstrates for the first time that LBH, a poorly characterized WNT/β-catenin target transcriptional regulator [13], is an essential effector of WNT-driven mammary tumorigenesis. Since LBH is prevalently overexpressed in clinically aggressive triple negative breast cancers, correlating with WNT pathway hyperactivation [13], this finding is significant.
Initial characterization of LBH-deficient MMTV-Wnt1 mice, generated for this study, shows LBH is critically required for WNT-induced mammary hyperplasia and tumor formation. Attenuated mammary hyperplasia in LBH-deficient MMTV-Wnt1 mice at pre-neoplastic stages was accompanied by reduced cell proliferation and increased cell death, suggesting LBH promotes mammary epithelial hyperproliferation. This is in discrepancy with studies in human nasopharyngeal cancer models, indicating LBH is a tumor suppressor that induces G1/S cell cycle arrest [23]. However, the delay in early-onset tumor formation in LBH-deficient MMTV-Wnt1 mice further reinforces the idea that LBH function in WNT-driven mammary tumorigenesis is pro-oncogenic.
The phenotype of LBH-deficient MMTV-Wnt1 mice was most similar to knockout of WNT co-receptor Lrp5 in this breast cancer mouse model [24]. Both, epithelial LBH inactivation and LRP5 KO reduced mammary hyperplasia and delayed tumor onset by 9–10 weeks, but did not completely abolish Wnt1-induced tumorigenesis, or alter tumor growth rates [24]. This could be explained by the observation that chronic Wnt1 ligand expression in MMTV-Wnt1 mammary epithelium activates other, β-catenin-independent signaling pathways, i.e. hedgehog [25], that drive tumor growth. Thus, LBH inactivation closely mimics the effects of canonical WNT signaling inhibition.
Mammary hyperplasia and tumorigenesis in MMTV-Wnt1 mice result from an amplification of basal MaSC and K6+ bipotential progenitor cells that have increased self-renewal and tumor-initiating potential [17, 26]. Notably, we found LBH to be specifically expressed in K5+/K6+ basal/progenitor cells of MMTV-Wnt1 mammary tumors. Given that LBH is an essential stem cell regulator in normal MG development that promotes a self-renewing, basal MaSC state [15], it is tempting to speculate that the phenotypes in LBH-deficient MMTV-WNT1 mice may reflect a requirement of LBH in WNT-driven MaSC/progenitor cell amplification.
Future studies are needed to further elucidate the mechanistic function of LBH as an essential WNT effector in oncogenesis and address its’ currently obscure role in breast carcinogenesis. Our MMTV-Wnt1 LBH KO mouse model represents an important, clinically relevant in vivo model to further evaluate the effects of LBH inhibition in WNT-activated breast cancers, as a promising novel anti-cancer therapeutic strategy.
Supplementary Material
Highlights.
LBH is overexpressed in basal progenitor cells of MMTV-Wnt1 transgenic mammary tumors
LBH is required for WNT1-induced mammary hyperplasia and tumor formation
LBH promotes cellular proliferation and blocks apoptosis
New LBH-deficient MMTV-Wnt1 mouse model to uncover WNT mechanisms in breast cancer
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health [RO1GM113256]. We thank Dr. Xin-Hai Pei for comments on the manuscript.
2 Abbreviations used:
- ER
Estrogen Receptor alpha
- HER2
Human Epidermal Growth Factor Receptor 2
- KO
Knockout
- LBH
Limb-Bud-and-Heart protein
- MaSC
Mammary stem cells
- MG
Mammary gland
- MMTV
Murine Mammary Tumor Virus
- PR
Progesterone Receptor
- Tg
Transgene
- TNBC
Triple (ER/PR/HER2)-negative breast cancer
- WT
Wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Clevers H, Nusse R, Wnt/beta-catenin signaling and disease, Cell, 149 (2012) 1192–1205. [DOI] [PubMed] [Google Scholar]
- [2].Dimeo TA, Anderson K, Phadke P, Feng C, Perou CM, Naber S, Kuperwasser C, A Novel Lung Metastasis Signature Links Wnt Signaling with Cancer Cell Self-Renewal and Epithelial-Mesenchymal Transition in Basal-like Breast Cancer, Cancer Res, 69 (2009) 5364–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OI, Goss KH, Wnt/{beta}-Catenin Pathway Activation Is Enriched in Basal-Like Breast Cancers and Predicts Poor Outcome, Am J Pathol, 176 (2010) 2911–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS, beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation, Mod Pathol, 24 (2011) 209–231. [DOI] [PubMed] [Google Scholar]
- [5].Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, et al. , Beta-catenin, a novel prognostic marker for breast cancer: its role in cyclin D1 expression and cancer progression., Proc Natl Acad Sci U S A, 97 (2000) 4262–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xu J, Prosperi JR, Choudhury N, Olopade OI, Goss KH, beta-Catenin is required for the tumorigenic behavior of triple-negative breast cancer cells, PLoS One, 10 (2015) e0117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu CC, Prior J, Piwnica-Worms D, Bu G, LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy, Proc Natl Acad Sci U S A, 107 (2010) 5136–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Won HS, Lee KM, Oh JE, Nam EM, Lee KE, Inhibition of beta-Catenin to Overcome Endocrine Resistance in Tamoxifen-Resistant Breast Cancer Cell Line, PLoS One, 11 (2016) e0155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nusse R, Varmus HE, Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome, Cell, 31 (1982) 99–109. [DOI] [PubMed] [Google Scholar]
- [10].Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE, Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice, Cell, 55 (1988) 619–625. [DOI] [PubMed] [Google Scholar]
- [11].Briegel KJ, Joyner AL, Identification and characterization of Lbh, a novel conserved nuclear protein expressed during early limb and heart development, Dev Biol, 233 (2001) 291–304. [DOI] [PubMed] [Google Scholar]
- [12].Briegel KJ, Baldwin HS, Epstein JA, Joyner AL, Congenital heart disease reminiscent of partial trisomy 2p syndrome in mice transgenic for the transcription factor Lbh, Development, 132 (2005) 3305–3316. [DOI] [PubMed] [Google Scholar]
- [13].Rieger ME, Sims AH, Coats ER, Clarke RB, Briegel KJ, The embryonic transcription cofactor LBH is a direct target of the Wnt signaling pathway in epithelial development and in aggressive basal subtype breast cancers, Mol Cell Biol, 30 (2010) 4267–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Al-Ali H, Rieger ME, Seldeen KL, Harris TK, Farooq A, Briegel KJ, Biophysical characterization reveals structural disorder in the developmental transcriptional regulator LBH, Biochem Biophys Res Commun, 391 (2010) 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lindley LE, Curtis KM, Sanchez-Mejias A, Rieger ME, Robbins DJ, Briegel KJ, The WNT-controlled transcriptional regulator LBH is required for mammary stem cell expansion and maintenance of the basal lineage, Development, 142 (2015) 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lindley LE, Briegel KJ, Generation of mice with a conditional Lbh null allele, Genesis, 51 (2013) 491–497. [DOI] [PubMed] [Google Scholar]
- [17].Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM, The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage, PLoS One, 4 (2009) e6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zeng YA, Nusse R, Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture, Cell Stem Cell, 6 (2010) 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lamb R, Ablett MP, Spence K, Landberg G, Sims AH, Clarke RB, Wnt pathway activity in breast cancer sub-types and stem-like cells, PLoS One, 8 (2013) e67811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maubant S, Tesson B, Maire V, Ye M, Rigaill G, Gentien D, Cruzalegui F, Tucker GC, Roman-Roman S, Dubois T, Transcriptome analysis of Wnt3a-treated triple-negative breast cancer cells, PLoS One, 10 (2015) e0122333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM, Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors, Genome Biol, 8 (2007) R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim YC, Clark RJ, Ranheim EA, Alexander CM, Wnt1 expression induces short-range and long-range cell recruitments that modify mammary tumor development and are not induced by a cell-autonomous beta-catenin effector, Cancer Res, 68 (2008) 10145–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Q, Guan X, Lv J, Li X, Wang Y, Li L, Limb-bud and Heart (LBH) Functions as a Tumor Suppressor of Nasopharyngeal Carcinoma by Inducing G1/S Cell Cycle Arrest, Scientific reports, 5 (2015) 7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO, The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis, J Biol Chem, 281 (2006) 35081–35087. [DOI] [PubMed] [Google Scholar]
- [25].Teissedre B, Pinderhughes A, Incassati A, Hatsell SJ, Hiremath M, Cowin P, MMTV-Wnt1 and -DeltaN89beta-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors, PLoS ONE, 4 (2009) e4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE, Generation of a functional mammary gland from a single stem cell, Nature, 439 (2006) 84–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.