Abstract
Understanding the molecular basis of cancer has led to a revolution in how cancers are classified and treated. Subsets of patients with specific genetic lesions are benefiting from precision medicine approaches utilizing the explosion of new drugs that target growth-promoting signaling networks. Unfortunately, relatively few cancer patients benefit from these approaches to target driver oncogenes [1]. Precision medicine is largely built on the assumption that cancer cell-intrinsic factors, such as genetic mutations or epigenetic identity, are the dominant determinants of which pathways and processes are required in cells and therefore determine response to therapies. However, in many cases, the presence of particular genetic lesions is insufficient to identify patients that will respond to a drug [1]. For instance, standard cell culture models have not been able to identify the subsets of cancer patients that respond to most conventional chemotherapies [1]. Nevertheless, these chemotherapy drugs remain standard of care for many cancers and in some cases contribute to curative regimens. Emerging data suggests that beyond cell-intrinsic factors, nutrient availability in the tumor microenvironment can also influence response to drugs. These results highlight the importance of understanding the microenvironmental factors that dictate which cellular processes are essential for disease progression and ultimately how tumors respond to treatments targeting these processes.
The success of conventional chemotherapy argues that drugs targeting cell metabolism and proliferative machinery can be effective to treat cancer. More recent efforts to target cancer metabolism have focused on how different cell-intrinsic factors like oncogenic mutations rewire metabolism to require cells to use specific metabolic pathways for growth and survival [2]. There have been clinical successes from this approach, such as targeting mutant isocitrate dehydrogenases. However, the development of drugs targeting enzymes in core metabolic pathways has been challenging. Even though profound metabolic alterations are observed broadly in cancer, an inability to match the right patients with specific drugs has limited therapeutic development of new molecules targeting metabolism.
Experiments in microorganisms have demonstrated that gene function and essentiality is largely environment dependent [3]. By extension, tumor microenvironmental context may alter the essentiality of pathways in cancer cells and influence sensitivity to drugs ranging from classic chemotherapies to new, targeted agents (Figure 1). Recent studies have demonstrated that microenvironmental factors have a profound influence on cancer cell metabolism and sensitivity to drugs targeting metabolism. For example, biochemical analysis has revealed different nutrient preferences for lung and brain tumors in vivo compared to cancer cells cultured from these tumors. In cultured lung and brain cancer cells, glutamine is used as a primary source of carbon to supply the tricarboxylic acid cycle intermediates needed for growth, whereas glutamine catabolism can be less important in tumors formed from the same cells in mice [2]. Furthermore, genetic screens of cells in culture and in xenograft tumors have yielded discordant results with respect to metabolic gene essentiality [4], lending further support to the notion that cancer cells require different metabolic processes depending on their microenvironment. The microenvironment can also affect drug response. For example, inhibitors of the enzyme glutaminase slow the proliferation of most cancer cells in culture, but this has not been predictive of tumor response to glutaminase inhibitors in either patients or mouse cancer models [2]. Similarly, drugs targeting mTOR, a protein involved in nutrient sensing and metabolic regulation, are much more successful at limiting cell growth and proliferation in cultured cells than they are at slowing tumor growth in vivo [5]. Collectively, these observations argue that the tumor microenvironment affects cancer cell metabolism and can alter drug sensitivity.
Nutrients and metabolic interactions in the cancer cell microenvironment affect cancer cell phenotypes.
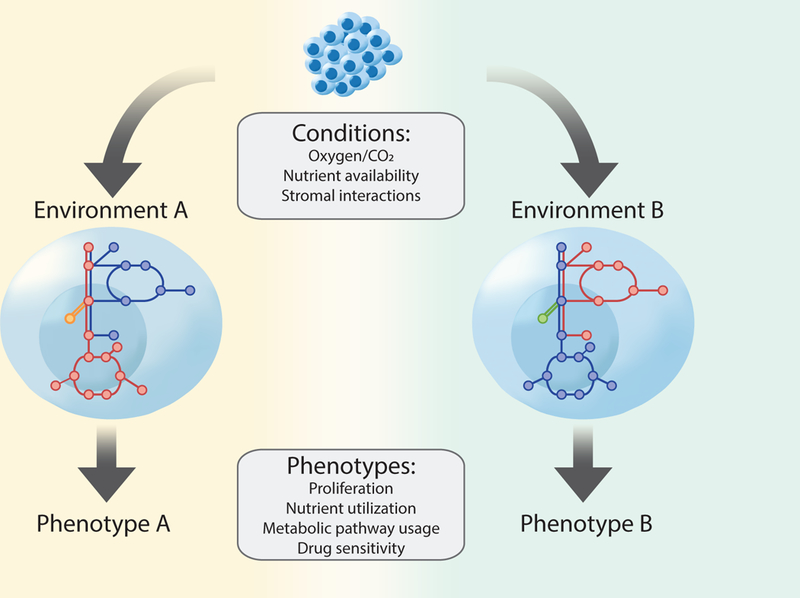
Cell-intrinsic factors, such as cell lineage and genetic mutations influence what enzymes are expressed, and thus define a metabolic network, or set of reactions the cell is capable of performing. However, how this network operates is further constrained by available nutrients and stromal interactions in the microenvironment such that nutrient availability has a major influence on cancer cell metabolic phenotypes. Thus, identical cells in different metabolic microenvironments exhibit distinct metabolic programs and variation in phenotypes, such as drug response.
Standard cell culture models of cancer do not mimic the tumor microenvironment. One major difference between the classical cell culture conditions where most drugs are initially tested and tumors is the level of available nutrients. Indeed, most culture media formulations were not intended to mimic tumor physiology, but were instead derived from experiments to identify the minimal nutrients required to grow mammalian cells [6]. Levels of oxygen, which affect cancer cell metabolism and therapy response, have long been appreciated to be non-physiologically elevated in standard culture conditions [7]. Tumor levels of other nutrients are different from that in standard culture, and this alters cell metabolism and affects response to therapies. Glioblastoma cells cultured in physiological nutrient levels rely to a lesser extent on glutamine consumption for proliferation than the same cells in standard culture media [8]. Importantly, glioblastoma tumors in mouse models in vivo produce rather than catabolize glutamine, and this difference has been attributed to artificial nutrient levels in standard culture creating a non-physiological state with respect to glutamine metabolism. Similarly, culturing lung cancer cells in physiological medium also makes cells less dependent on glutamine catabolism and reproduces the lack of glutaminase inhibitor sensitivity observed in vivo [9]. Many cell culture formulations contain high levels of the amino acid cystine, which drives glutamine catabolism and sensitivity to glutaminase inhibitors. Cancer cells in tumors are exposed to lower cystine levels, which explains at least in part why glutaminase inhibitors are less effective at slowing the growth of tumors derived from cells that are sensitive to these drugs in culture. Thus, tumor nutrient levels are an important component of the tumor microenvironment that alters metabolism and drug responses.
Environmental nutrient levels can also alter the requirement for “recycling metabolism”. In culture, intracellular protein recycling to obtain amino acids [10] and recapture of acetate from histone modifications [11] is dispensable for cancer cell proliferation, but both become required for tumor growth in vivo where many nutrients are more limiting. Catabolism of extracellular protein can be used by cells as a source of amino acids [10]. Most media formulations are protein deficient, but otherwise amino acid rich, which may limit protein catabolism. However, cells grown with limiting amino acids but physiological levels of albumin rely on extracellular protein to obtain amino acids for growth and are resistant to mTOR inhibition [5]. Thus, differences in amino acid acquisition between tumors and cells in culture may contribute to the limited efficacy of mTOR inhibitors in the clinic.
Cancer cells in tumors have access to nutrients that are not always added to standard cell culture media. For instance, the presence of uric acid, a nucleotide breakdown product found in vivo but absent from cell culture media, renders cells resistant to the pyrimidine analog and conventional chemotherapeutic drug, 5-flurouracil [12]. In another example, the addition of pyruvate to media alters the cellular redox state, which limits the ability of the anti-diabetic drug metformin to slow cancer cell proliferation [13]. The altered sensitivity to these widely used drugs illustrates that microenvironmental nutrient levels can affect metabolic pathway use and impact response to metabolism-targeted therapy.
The tumor microenvironment also contains numerous cell types that can interact metabolically with cancer cells. Non-cancer cells within a tumor can share metabolites with cancer cells, compete with cancer cells for nutrients, and provide signals that alter cancer cell metabolic pathway utilization. For example, competition between cancer cells and infiltrating lymphocytes for limiting nutrients, and secretion of metabolic by-products by cancer cells, can create an immunosuppressive microenvironment that limits the immune response to a tumor [14]. Thus, understanding the tumor microenvironment may even lead to improved therapies that control cancer via non-cell autonomous mechanisms such as immunotherapy.
Relying on in vivo models to identify cancer targets is impractical, but current scalable ex vivo models are inaccurate with respect to microenvironment. Microenvironmental factors clearly alter drug responses, and differences in nutrient levels between tumors and normal tissues could even drive targetable liabilities of cancer cells. Therefore, identifying how nutrients vary in the tumor microenvironment and efforts to model this in tractable culture systems will be crucial to identify patients likely to respond to both existing and novel drugs that target cancer processes, like metabolism. Tumor nutrient levels also fluctuate both spatially and temporally within an individual tumor, and could be affected by additional factors such as diet. This metabolic heterogeneity could be an important component of tumor heterogeneity that limits therapeutic effectiveness. Thus, defining physiological levels of nutrients and how physiology constrains cell metabolic processes could lead to a better understanding of drug responses and uncover new therapeutic opportunities. By combining knowledge of the nutritional microenvironment with models that consider other features of the tumor microenvironment, such as organoid culture, ex vivo models could be generated that might have better predictive power for drug response in patients with cancer, as well as with other diseases [15]. Ultimately, redefining culture conditions to incorporate knowledge of the tumor microenvironment could transform our understanding of cell physiology and eliminate a bottleneck in identifying new cancer therapeutics.
Acknowledgements
The authors acknowledge support from the MIT Center for Precision Cancer Medicine, the Lustgarten Foundation, the Ludwig Center at MIT, SU2C, HHMI, and the NCI (F32CA213810 to AM and R01CA168653 to MGVH). MGVH is a consultant and scientific advisory board member for Agios Pharmaceuticals and Aeglea Biotherapeutics.
References
- 1.Letai A, Functional precision cancer medicine-moving beyond pure genomics. Nat Med, 2017. 23(9): p. 1028–1035. [DOI] [PubMed] [Google Scholar]
- 2.Wolpaw AJ and Dang CV, Exploiting Metabolic Vulnerabilities of Cancer with Precision and Accuracy. Trends Cell Biol, 2018. 28(3): p. 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rancati G, et al. , Emerging and evolving concepts in gene essentiality. Nat Rev Genet, 2018. 19(1): p. 34–49. [DOI] [PubMed] [Google Scholar]
- 4.Yau EH, et al. , Genome-Wide CRISPR Screen for Essential Cell Growth Mediators in Mutant KRAS Colorectal Cancers. Cancer Res, 2017. 77(22): p. 6330–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palm W, et al. , The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell, 2015. 162(2): p. 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eagle H, Nutrition needs of mammalian cells in tissue culture. Science, 1955. 122(3168): p. 501–14. [DOI] [PubMed] [Google Scholar]
- 7.Bertout JA, Patel SA, and Simon MC, The impact of O2 availability on human cancer. Nat Rev Cancer, 2008. 8(12): p. 967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tardito S, et al. , Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat Cell Biol, 2015. 17(12): p. 1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muir A, et al. , Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaravadi R, Kimmelman AC, and White E, Recent insights into the function of autophagy in cancer. Genes Dev, 2016. 30(17): p. 1913–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schug ZT, Vande Voorde J, and Gottlieb E, The metabolic fate of acetate in cancer. Nat Rev Cancer, 2016. 16(11): p. 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantor JR, et al. , Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell, 2017. 169(2): p. 258–272 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gui DY, et al. , Environment Dictates Dependence on Mitochondrial Complex I for NAD+ and Aspartate Production and Determines Cancer Cell Sensitivity to Metformin. Cell Metab, 2016. 24(5): p. 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck MD, et al. , Metabolic Instruction of Immunity. Cell, 2017. 169(4): p. 570–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath P, et al. , Screening out irrelevant cell-based models of disease. Nat Rev Drug Discov, 2016. 15(11): p. 751–769. [DOI] [PubMed] [Google Scholar]


