Pulmonary arterial hypertension (PAH) is a progressive disease that, if left untreated, leads to right heart failure and ultimately death. Sustained pulmonary vasoconstriction and excessive pulmonary vascular remodeling are two of the major causes of elevated pulmonary vascular resistance (PVR) and pulmonary arterial pressure (PAP) in patients with PAH. Concentric pulmonary vascular remodeling is characterized by significant intimal, medial and adventitial thickening due to i) increased proliferation of lung vascular endothelial cells (EC), pulmonary arterial smooth muscle cells (PASMC) and fibroblasts (FB), ii) inhibited apoptosis of EC and PASMC, and iii) increased endothelial-to-mesenchymal transition (EndMT) that converts EC to the highly-proliferative myofibroblast (myoFB)1–3. Notch signaling is particularly important in vascular remodeling by promoting EC proliferation and SMC recruitment during the angiogenesis and vessel regeneration but excessive Notch signaling contributes to cell hyperproliferation in pulmonary fibrosis and cancer. Upregulated Notch receptors and ligands have been implicated in the lung vascular remodeling in PAH4,5; however, the exact role of Notch (pathogenic or beneficial) in the development of pulmonary hypertension (PH) and PAH is still unclear.
The signal transduction in Notch system is initiated by binding of Notch receptors (Notch1–4) with ligands (Jagged1, Jagged2, Delta-like1 (Dll1), Dll3, and Dll4)6,7. The ligand-receptor interaction promotes two proteolytic cleavages of Notch molecules by metalloprotease ADAM10 and γ-secretase complex consisted of presenilin, nicastrin, APH1a and psenen proteins. On the completion of two cleavages, the Notch intracellular domain (NICD) is released and translocated into the nucleus where it binds to the DNA-binding protein and converts it into a potent transcriptional activator. This complex activates the transcription of Notch targets such as Hes family genes and Hey family genes, which control cell proliferation, differentiation, and apoptosis. Though Notch signaling is well established in systemic vasculature, however, its role in pulmonary circulation remains elusive.
A study by Miyagawa et al.8 published in the current issue of Circulation Research is focused on the role of BMPR2-Notch1 signaling in lung vascular EC regeneration initiated by SMC-EC contact. The authors found that contact-mediated communication between SMC and EC initiates production of collagen IV (a BMPR2-dependent component of extracellular matrix) which results in distribution of p-JNK (c-Jun N-terminal kinase from MAPK family) from the nucleus into the cytoplasm via integrin-linked kinase (ILK) activation (Figure 1). p-JNK forms complex with caveolin 1 (Cav1) and stabilizes presenilin 1 protein by inducing compartmentalization of γ-secretase complex. Presenilin 1 releases N1ICD to induce a key regulator of glycolysis PFKFB3 which is required for histone acetylation at enhancer sites of Notch target genes involved in EC regeneration. This line of evidence is consistent with previous reports that suggest a beneficial role of Notch1 in endothelial repair. It has been previously reported that BMP and Notch cascades functionally interact in lung vascular cells9. The new finding indicates that the SMC-EC contact associated EC proliferation requires BMPR2 in both cell types and is Notch1-dependent. Regeneration of systemic vascular endothelial monolayer in response to carotid artery injury is also mediated by BMPR2-Notch1 pathway. Collectively, the results of this study indicate that BMPR2-mediated Notch activation is critical for SMC-EC-contact-driven EC proliferation and endothelial regeneration.
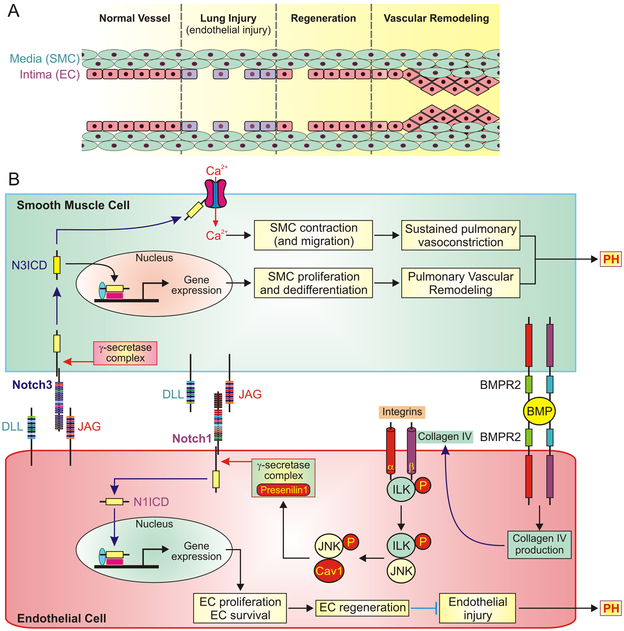
Figure 1. The proposed divergent role of Notch signaling in endothelial and smooth muscle cells.
A, The potential structural changes in endothelial monolayer of pulmonary vessel during lung vascular injury, endothelial cell (EC) regeneration and vascular remodeling. Endothelial injury results in loss of smooth muscle cell (SMC)-EC contact in the intima of injured vessels. Activated Notch pathway in EC promotes endothelial regeneration in response to lung injury but hyperactive Notch may lead to hyperproliferation of EC/SMC and concentric pulmonary artery wall thickening. B, The proposed mechanisms of Notch-mediated SMC proliferation (upper part of the panel) and EC regeneration (lower part of the panel) by EC-SMC contact and BMPR2 signaling. The mechanism involved in EC regeneration is initiated by BMPR2-dependent production of collagen IV and subsequent activation of integrin-linked kinase (ILK) and phosphorylation of the signaling molecule c-Jun N-terminal kinase (JNK) in EC. Targeting the phosphorylated JNK to caveolin 1 (Cav1) stabilizes presenilin 1 in γ-secretase complex and eventually activating Notch1. The N1ICD-associated gene expression results in EC proliferation and survival and leads to EC regeneration and endothelial repair. Re-established EC-SMC contact also activates Notch3 in SMC, especially in SMC with upregulated Notch3, and release N3ICD into the cytoplasm. N3ICD can go through the canonical pathway to stimulate gene expression involved in SMC proliferation and dedifferentiation and eventually results in pulmonary vascular remodeling. N3ICD may also functionally interact with Ca2+ channels in the plasma membrane, increases Ca2+ influx and subsequently causes SMC contraction and pulmonary vasoconstriction.
Compared with its role in regeneration program, the role of Notch signaling in PAH is more complicated. Upregulated expression of Notch1 and Notch3 have been found in human and animals with PH4,5,10,11. These studies support the hypothesis of cell-specific effects of Notch homologs in the development and progression of PAH. Notch1 has been described to be essential for hyperproliferative and apoptotic-resistant EC whereas Notch3 primarily controls vascular remodeling through regulating PASMC recruitment and proliferation. One of the characteristics of PAH is intraluminal obliteration and concentric PA wall thickening characterized by intimal, medial and adventitial thickening. Dysfunctional or injured EC could contribute to neointimal formation, intimal thickening and distal PA obliteration directly by converting to highly-proliferative mesenchymal cell phenotype via enhanced EndMT or indirectly by expressing and secreting mitogens that stimulate SMC proliferation. In human endothelial cells, Notch activation induces mesenchymal transdifferentiation via EndMT3. Interestingly, that lung epithelial stem cells require Notch signaling to activate regeneration program in response to injury but subsequent inhibition of Notch promotes differentiation towards mature epithelium12. Persistent Notch signaling prevents alveolar epithelial differentiation and lead to cystic structures in fibrosis indicating incomplete regeneration. These findings could explain, at least in part, the “switching” from beneficial activated Notch in regeneration processs to pathogenic hyperactive Notch pathway in PAH or PH in general (Figure 1).
Reduced BMPR2 signaling in the endothelium has been demonstrated as an initiating factor in the development of PAH. Both synergy and antagonism between the BMP and Notch signaling pathways in the same cell type have been demonstrated to dynamically control cellular signaling responses13. Although the authors outline the new mechanisms of crosstalk between signaling pathways in the pathogenesis of PAH, there are still mechanistic questions as to what cross-talk between impaired BMPR2 and increased Notch1 is observed in PAH patients. Therefore, it would be very informative to determine whether Notch1 is inhibited in lung vascular EC with BMPR2 mutations compared with normal control cells. In vivo experiments by Rabinovitch’s group8 demonstrated that Notch1 deficiency in EC resulted in more severe hypoxia-induced pulmonary hypertension, arousing speculation about therapeutic potential of Notch1 activators for PAH patients. In contrast, previous studies showed Notch1 increase in hypoxia/Sugen rats and hypoxia-treated human PAEC11, as well as, that Notch3−/− mice were resistant to the development of hypoxia-induced pulmonary hypertension4. Additional studies are needed to further investigate these differing findings. Based on the present report it is compelling to speculate that BMPR2 could qualify as one of the molecular modulators of Notch signaling. Hence, other molecular players need to be investigated.
The next important finding is that under the condition of SMC-EC contact, Notch1 activates 6-Phosphofructo-2-kinase PFKFB3 (a key regulator of glycolysis associated with Warburg effect) to increase energy production. Interestingly, PFKFB3 has been established as a hypoxia-inducible gene that is stimulated through HIF interaction14, but the role of PFKFB3 in the development and progression of PAH is unclear. Given that HIF activates Notch signaling under hypoxic conditions15 and has been established as a well-known transcription factor in the pathogenesis of PAH, it should be considered as a potential modulator for hyperactive Notch in PAH. The current study indicates an important role of Notch1, whether dependent or independent of BMPR2, in the pathogenesis of PAH.
The BMP/TGF-β1/SMAD pathway is a canonical pathway involved in the development of pulmonary vascular remodeling in PAH, but recent studies indicate that non-SMAD signaling pathways could be involved in the pathogenic mechanisms of BMP-related vascular diseases13. The p38/MAPK signaling is an important inflammatory pathway in vascular disease, but two other major MAPK pathways, JNK and extracellular signal-regulated kinases, also play roles in cell proliferation, differentiation, and migration. The data presented by Miyagawa et al.8 indicate that only JNK from MAPK family mediates SMC-EC-contact-driven EC proliferation. Additionally, the role of Notch ligands in BMPR2-mediated activation of Notch1 signaling was not established since the authors did not detect any changes in Notch ligands (at least Jag1 and Dll4). The diversity of Notch signaling is derived from a large number of potential receptor-ligand interactions. The well-known Notch1-Dll4 partnership is mainly involved in the regulation of angiogenesis while Dll1 activates Notch1 in vascular endothelium6. However, Dll1 expression was not investigated here. Therefore, further investigations are needed to determine the precise role of Notch ligands in endothelial regeneration.
Vascular remodeling in PAH patients includes concentric pulmonary artery (PA) wall thickening (including intimal, medial and adventitial thickening), abnormal muscularization of precapillary arterioles, formation of occlusive neointima lesions in proximal PA, and obliteration of distal PA. Endothelial injury and dysfunction are thought to be one of the initial causes for the development and progression of pulmonary vascular remodeling in PAH. Rabinovitch and her associate provide compelling evidence that i) SMC-EC contact is critical for initiating EC regeneration after lung vascular endothelial injury; ii) the BMPR2-dependent Notch1 activation in EC via SMC-EC contact is the key signaling pathway for EC regeneration and endothelial repair, and iii) loss of SMC-EC contact, by reducing the capability of EC to regenerate, may be an important pathogenic mechanism of lung vascular injury. Vascular remodeling could potentially represent a failed or abnormal regenerative process in EC, promoted at least in part by ongoing Notch activity. The striking parallels to uncompleted repair program in fibrotic lung disease, including hyperactive Notch, suggest inappropriate Notch signaling may also be a major contributor to failed or abnormal EC regeneration in PAH. During EC regeneration and endothelial repair process, re-established EC-SMC contact may stimulate SMC proliferation and contraction via activation of Notch3 in SMC4,5 causing sustained pulmonary vasoconstriction, pulmonary arterial medial hypertrophy and arteriole muscularization. The pathogenic role of EC-SMC contact or re-established EC-SMC contract during endothelial repair indicates that cell-specific Notch activation is also an important pathogenic mechanism involved in the development and progression of pulmonary vascular remodeling in PAH.
In conclusion, Notch pathway will continue to be a focus of research since better understanding of Notch signaling in pulmonary vasculature will elucidate the etiology and pathogenesis of PAH and to develop novel therapies for the disease.
Acknowledgments
We are grateful for the editorial assistance of Dr. H. Tang in preparing the manuscript.
Sources of Funding
This work was supported, in part, by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL125208 and HL135807).
Footnotes
Disclosures
None.
References
- 1.Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinovitch M Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 2012;122:4306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L1–8. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Zhang X, Leathers R, et al. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 2009;15:1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith KA, Voiriot G, Tang H, et al. Notch activation of Ca2+ signaling in the development of hypoxic pulmonary vasoconstriction and pulmonary hypertension. Am J Respir Cell Mol Biol 2015;53:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong D, Ouyang R, Li J, Chen Y, Chen P. Notch signaling in lung diseases: focus on Notch1 and Notch3. Ther Adv Respir Dis 2016;10:468–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol 2016;17:722–35. [DOI] [PubMed] [Google Scholar]
- 8.Miyagawa K, Shi M, Chen P, et al. Smooth muscle contact drives endothelial regeneration by BMPR2-Notch1 mediated metabolic and epigenetic changes. Circ Res; November 21, 2018. DOI: 10.1161/CIRCRESAHA.118.313374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurst LA, Dunmore BJ, Long L, et al. TNFalpha drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun 2017;8:14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao L, Xie L, Shi K, Zhou T, Hua Y, Liu H. Notch signaling change in pulmonary vascular remodeling in rats with pulmonary hypertension and its implication for therapeutic intervention. PLoS One 2012;7:e51514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabral S, Tian X, Kojonazarov B, et al. Notch1 signalling regulates endothelial proliferation and apoptosis in pulmonary arterial hypertension. Eur Respir J 2016;48:1137–49. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan AE, Brumwell AN, Xi Y, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 2015;517:621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia de Vinuesa A, Abdelilah-Seyfried S, Knaus P, Zwijsen A, Bailly S. BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev 2016;27:65–79. [DOI] [PubMed] [Google Scholar]
- 14.Obach M, Navarro-Sabate A, Caro J, et al. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem 2004;279:53562–70. [DOI] [PubMed] [Google Scholar]
- 15.Landor SK, Lendahl U. The interplay between the cellular hypoxic response and Notch signaling. Exp Cell Res 2017;356:146–51. [DOI] [PubMed] [Google Scholar]