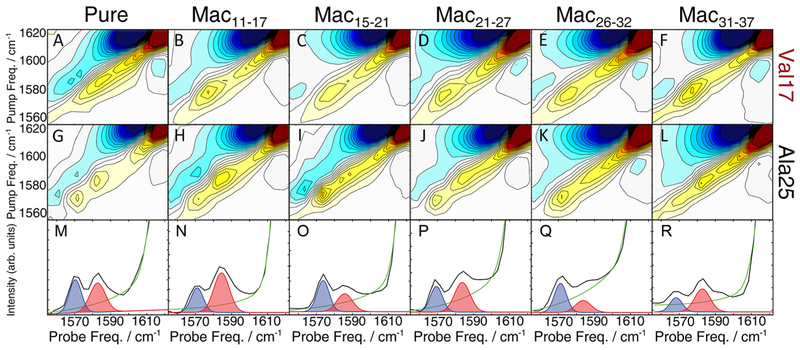
Figure 3.
Macrocyclic peptides do not change fibril structure but systematically alter the distribution of Ala25 polymorphs. 2D IR spectra of Val17-labeled hIAPP (top row) and Ala25-labeled hIAPP (middle row) are mixed with stoichiometric macrocycles. A single Val17 peak is observed regardless of which macrocycle is used to seed fibril formation. Ala25 exhibits two peaks that appear at the same frequencies in all sample preparations, but their relative intensities depend on the macrocycle used to seed fibril formation. Diagonal slices of Ala25 (bottom row) show altered ratios of the two polymorph peaks, highlighted in blue and red, which were fit with pseudo-Voigt functions. The fitting routine and parameters are given in the Supporting Information.