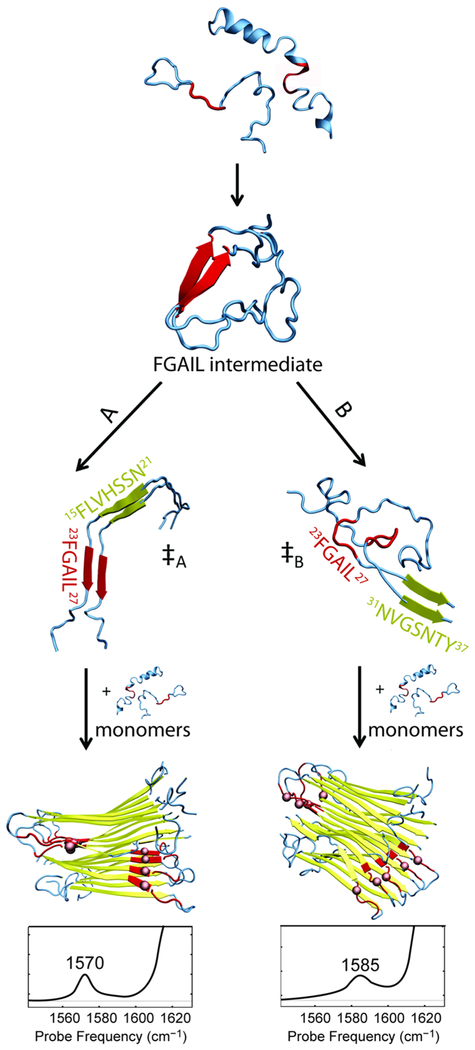
Figure 4.
Schematic mechanism of hIAPP aggregation leading to two different polymorphs. The structures were obtained from REMD simulations of dimeric hIAPP and NMR structures of the fibrils. Folding pathways proceed from disordered monomers through an intermediate with a β-sheet in the FGAIL region (residues 23–27, highlighted in red). The folding pathway diverges after the intermediate, likely via the transition state that nucleates fibril aggregation. In pathway A, the ‡A transition state has the nucleation site close to the FGAIL region (here, residues 15–21, highlighted in yellow). Eventually, fibrils form giving rise to a peak at 1570 cm−1. In pathway B, the ‡B transition state is characterized by the nucleation site far from the FGAIL region (here, residue 31–37) and the FGAIL region in the formed polymorph becomes a part of a partially disordered loop that gives rise to a peak at 1585 cm−1.