Abstract
Numerous pharmacotherapies have been evaluated after experimental traumatic brain injury (TBI). While amantadine (AMT) has shown potential for clinical efficacy, the few studies on its effectiveness have been mixed. It is possible that suboptimal dosing, due to the evaluation of only one dose, may be causing the discrepancies in outcomes. Hence, the goal of the current study was to conduct a dose response of AMT after TBI to determine an optimal behavioral benefit. Anesthetized adult male rats received either a cortical impact of moderate severity or sham injury and then were randomly assigned to receive once daily intraperitoneally injections of AMT (10, 20, or 40 mg/kg) or saline vehicle (VEH, 1 mL/kg) commencing 24 hr after injury for 19 days. Motor and cognitive function were assessed on post-operative days 1-5 and 14-19, respectively. There were no statistical differences among the sham groups treated with AMT or VEH so the data were pooled. AMT (20 mg/kg) facilitated beam-balance recovery and spatial learning relative to VEH-treated controls (p < 0.05). No other doses of AMT were effective. These results indicate that dosing should be carefully considered when assessing the effects of pharmacotherapies after TBI, such that potential benefits are not inadvertently missed.
Keywords: amantadine, beam-walk, controlled cortical impact, functional recovery, learning and memory, Morris water maze, traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) affects 10 million people worldwide each year [1], of which 2.5 million reside in the USA [2]. TBI-induced motor and cognitive dysfunctions are prevalent, long-lasting, and costly. It is estimated that 120,000 TBI individuals suffer from marked chronic functional impairments in the USA alone each year [3] and the economic burden due to acute medical and chronic rehabilitative services, as well as lost productivity, is $76.5 billion annually [4]. These sobering statistics underscore the need for effective therapeutic strategies for TBI. To this end, numerous studies have been conducted evaluating various pharmacotherapies in the pursuit of motor and cognitive improvement [5], but few have translated to the clinic [6].
Although amantadine (AMT), a dopamine2 receptor agonist, has shown clinical potential with no serious adverse side effects [7-11], the general conclusions from the few clinical studies conducted to date have been inconsistent due to studies reporting no salient effect relative to placebo controls [12,13]. Potential reasons for the discrepant findings may be due to the time of administration since the insult, the outcome measures utilized, or the vast differences in dosing. Similar methodological problems are observed in preclinical studies where the type of TBI (controlled cortical impact [CCI] vs. fluid percussion), severity (mild vs. moderate or severe), and dosing regimen are different [14,15].
Despite the current paucity in effective treatments for TBI, the cautious optimism for AMT as an efficacious therapy provides the impetus for its continued evaluation to ameliorate the symptoms of TBI. Hence, the goal of the current study was to conduct a dose response of AMT after CCI injury to determine an optimal behavioral benefit.
2. Materials and methods
2.1. Subjects
Sixty adult (3 months old) male Sprague-Dawley rats (Envigo RMS, Inc., Indianapolis, IN) weighing 300-325 g on the day of surgery were paired housed in ventilated polycarbonate rat cages and maintained in a temperature (21 ± 1°C) and light (on 7:00 a.m. to 7:00 p.m.) controlled environment with food and water available ad libitum. After one week of acclimatization all rats underwent a single day of beam-walk training, which consisted of 3-5 successive approximation trials to traverse the beam in under 5 sec. Following training, the rats were randomly assigned to TBI + VEH (1 mL/kg; n=10), TBI + AMT (10 mg/kg; n=10), TBI + AMT (20 mg/kg; n=10), TBI + AMT (40 mg/kg; n=10), and respective sham controls (n=5 per group). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
2.2. Surgery
TBI surgery was conducted as previously described [16,17]. Briefly, following surgical anesthesia with 4% isoflurane, a controlled cortical impact (CCI) injury of moderate severity (2.8 mm tissue deformation at 4 m/sec) was produced. Immediately after the CCI, anesthesia was discontinued and the incision was promptly sutured. The rats were subsequently extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
2.3. Acute neurological evaluation
Hind limb reflexive ability was assessed immediately after the cessation of anesthesia by gently squeezing the rats’ hind paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position three consecutive times.
2.4. Drug administration
AMT was purchased from Sigma-Aldrich (St. Louis, MO) and was prepared daily by dissolving in sterile saline, which also served as the vehicle. AMT (doses in mg/kg of 10, 20, or 40) or saline vehicle (1.0 mL/kg) was administered intraperitoneally once daily beginning 24 hrs after surgery and once daily for 19 days. The three doses of AMT and VEH were intermingled among surgical cohorts to minimize any potential variability in testing parameters.
2.5. Motor performance
Established beam-balance and beam-walk tasks were used to assess motor function. Briefly, the beam-balance task consists of placing the rat on an elevated and narrow beam (1.5 cm wide) and recording the time it remains on for a maximum of 60 sec. The beam-walk task, modified from that originally devised by Feeney and colleagues [18], consists of training/assessing rats using a negative-reinforcement paradigm to escape a bright light and white noise by traversing an elevated narrow beam (2.5 × 100 cm) and entering a darkened goal box. Performance was assessed by recording the elapsed time to traverse the beam. Rats were tested for beam-balance and beam-walk performance prior to surgery to establish a baseline measure and again on post-operative days 1-5. Testing consisted of three trials (60 sec allotted time with an inter-trial interval of 30 sec) per day on each task. If a rat was unable to traverse the beam the maximum time of 60 sec was recorded. The average daily scores for each rat were used in the statistical analyses.
2.6. Cognitive function: acquisition of spatial learning
A well-established Morris water maze (MWM) task that is sensitive to cognitive function after TBI was utilized [16,17,19]. Briefly, the pool (180 cm diameter; 60 cm high) was filled with tap water (26 ± 1°C) to a depth of 28 cm and was situated in a room with salient visual cues. The platform, a clear Plexiglas stand (10 cm diameter, 26 cm high), was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning began on post-operative day 14 and consisted of providing a block of four daily trials (4-min inter-trial interval) for five consecutive days (14-18) to locate the submerged platform (i.e., invisible to the rat). On day 19 the platform was raised 2 cm above the water surface (i.e., visible to the rat) as a control procedure to determine the contributions of non-spatial factors on cognitive performance. For each daily block of trials, the rats were placed in the pool facing the wall at each of the four possible start locations in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 s had elapsed, whichever occurred first. Rats that failed to locate the platform within the allotted time were manually guided to it. The rats remained on the platform for 30 s before being placed in a heated incubator between trials. The times of the 4 daily trials for each rat were averaged and used in the statistical analyses.
2.7. Cognitive function: probe trial (memory retention)
One day after the final acquisition training session (i.e., day 19), all rats were given a single probe trial to measure retention. Briefly, the platform was removed and the rats were given the opportunity to explore the pool for 30 sec. The percent time spent in the target quadrant was used in the statistical analysis. The data were obtained using ANY-maze video tracking software.
2.8. Data analyses
Statistical analyses were performed using StatView 5.0.1 (Abacus Concepts, Inc., Berkeley, CA) by a researcher blinded to group conditions. Only after the analyses were concluded and the code was broken did the statistician know the correct order of the groups. The motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). The acute neurological assessments, probe trial, visible platform, and swim speed were analyzed by one-factor ANOVAs. When the ANOVA showed a significant effect, the Newman-Keuls post-hoc test was implemented to determine specific group differences. The results are expressed as the mean ± standard error of the mean (S.E.M.) and are considered significant when p values were ≤ 0.05.
3. Results
3.1. No significant differences in any of the behavioral outcomes were revealed between the sham control groups regardless of treatment and thus their data were pooled into one group designated as SHAM. One rat from the TBI + AMT (40 mg/kg) was excluded due to an inability to locate the visible platform, which may be indicative of visual deficits that may impact spatial learning. Hence, the final analyses are based on 59 rats.
3.2. Acute neurological evaluation
No differences were observed among the TBI groups in hind limb withdrawal reflex after a brief paw pinch [left range = 146.5 ± 5.2 sec to 155.5 ± 4.9 sec, p > 0.05; right range = 142.1 ± 5.2 sec to 152.0 ± 4.8 sec, p > 0.05] or for righting reflex [range 349.1 ± 16.0 sec to 371.7 ± 16.9 sec, p > 0.05] following the termination of anesthesia. The lack of significant differences with these acute neurological indices suggests that all groups experienced an equivalent level of injury and anesthesia. Sham rats had reflexes that were significantly quicker than TBI rats: paw pinch [left = 20.0 ±1.2 sec; right = 15.2 ±1.1 sec] and righting reflex [125.1 ± 4.7 sec].
3.3. Motor performance: beam-balance and beam-walk
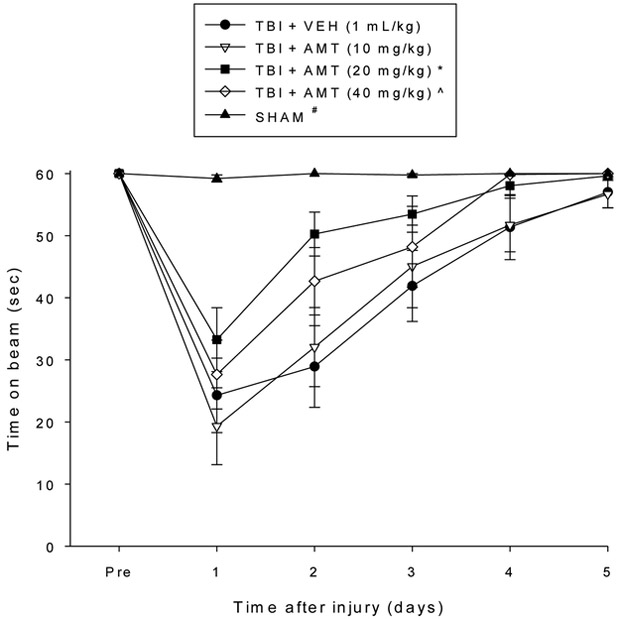
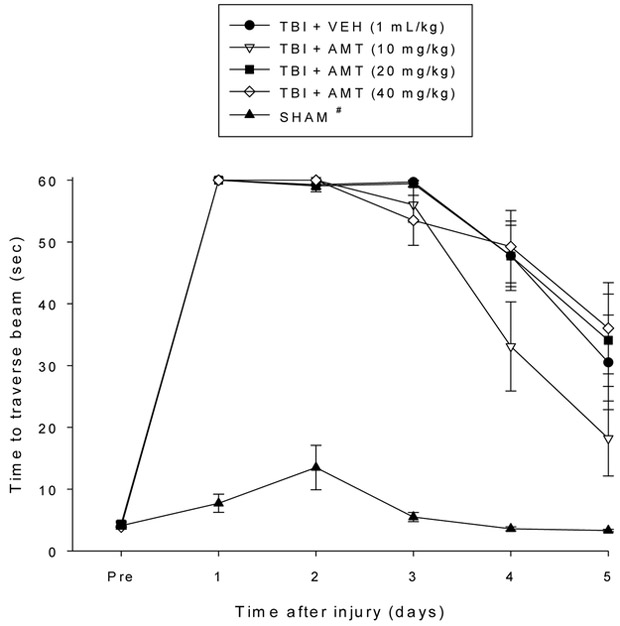
No pre-surgical differences were observed among groups as all rats were capable of balancing on the beam for the allotted 60 sec during the baseline assessments (Fig. 1). The repeated measures ANOVA revealed significant Group [F4,54 = 12.623, p < 0.0001] and Day [F5,270 = 74.645, p < 0.0001] differences, as well as a significant Group x Day interaction [F20,270 = 8.045, p < 0.0001]. Following TBI, all groups were impaired relative to the SHAM controls [p < 0.05]. Although beam-balance performance improved in all TBI groups over the five days of testing, the post-hoc analysis revealed that only the TBI + AMT (20 mg/kg) group recovered significantly faster than the TBI + VEH group [p < 0.05]. Additionally, both the TBI + AMT (20 mg/kg) and TBI + AMT (40 mg/kg) groups performed better than the TBI + AMT (10 mg/kg) group [p’s < 0.05]. No other comparisons were statistically different. Regarding the beam-walk data, no significant differences were observed among any of the groups in the time to traverse the beam prior to surgery as they all completed the task in under 5 sec (Fig. 2). However, after surgery, the repeated-measures ANOVA revealed significant Group [F4,54 = 120.581, p < 0.0001] and Day [F5,270 = 184.835, p < 0.0001] differences, as well as a significant Group x Day interaction [F20,270 = 15.147, p < 0.0001]. Like the findings for beam-balance, the post-hoc analysis revealed that the SHAM controls were significantly better than all TBI groups [p < 0.05]. No differences were revealed among the TBI groups, regardless of treatment [p > 0.05].
Fig. 1.
Mean (± S.E.M.) time (sec) balancing on an elevated narrow beam prior to, and after, TBI or SHAM injury. * p < 0.05 vs. TBI + VEH and TBI + AMT (10 mg/kg). ^ p < 0.05 vs. TBI + AMT (10 mg/kg). # p < 0.05 vs. all TBI groups, regardless of treatments.
Fig. 2.
Mean (± S.E.M.) time (sec) to travers an elevated narrow beam prior to, and after, TBI or SHAM injury. # p < 0.05 vs. all TBI groups, regardless of treatments. No differences were revealed among the TBI groups (p > 0.05).
3.4. Cognitive function: acquisition of spatial learning and memory retention
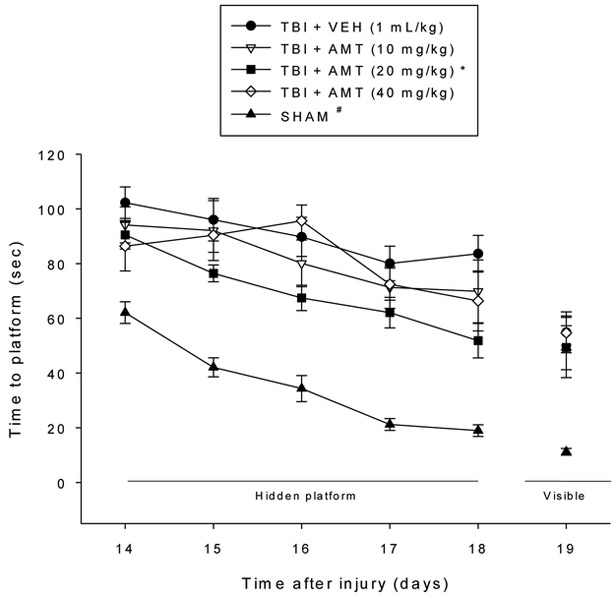
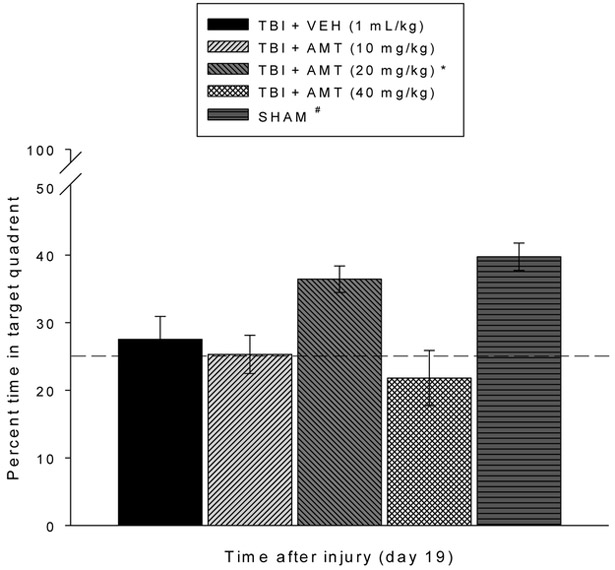
Analysis of the spatial learning data revealed significant Group [F4,54 = 50.647, p < 0.0001] and Day [F4,216 = 22.472, p < 0.0001] differences in locating the hidden platform. The post-hoc analysis revealed that the SHAM group was significantly better at learning the location of the escape platform relative to all TBI groups, regardless of treatment [p < 0.05; Fig. 3]. Among the TBI groups, the TBI + AMT (20 mg/kg) group located the escape platform significantly quicker than all other TBI groups [p’s < 0.05]. No differences were revealed between the TBI + AMT (10 mg/kg) and TBI + AMT (40 mg/kg) groups [p > 0.05], and neither differed from the TBI + VEH group [p > 0.05]. Analysis of the probe (i.e., memory retention) data revealed a significant Group effect [F4,54 = 8.276, p < 0.0001]. The post-hoc revealed that the SHAM and TBI + AMT (20 mg/kg) groups, which did not differ from one another [p > 0.05], spent a greater percentage of the allotted time in the target quadrant (39.7 ± 2.0 % and 36.4 ± 1.9 %, respectively) relative to the TBI + VEH, TBI + AMT (10 mg/kg), and TBI + AMT (40 mg/kg) groups (27.5 ± 3.4 %, 25.3 ± 2.8 %, and 21.8 ± 4.1 %, respectively) [p’s < 0.05; Fig. 4]. Lastly, no significant differences in swim speed (range = 25.6 ± 2.5 cm/sec to 31.5 ± 1.7 cm/sec) were observed among the TBI or SHAM groups [p > 0.05], but there was a difference in the time to reach the visible platform, with the SHAM group locating the platform quicker than the TBI groups, regardless of treatment [p < 0.05].
Fig. 3.
Mean (± S.E.M.) time (sec) to locate a hidden platform in the MWM. * p < 0.05 vs. TBI + VEH, TBI + AMT (10 mg/kg), and TBI + AMT (40 mg/kg). # p < 0.05 vs. all TBI groups, regardless of treatments. No differences were observed between the TBI + AMT (10 mg/kg) and TBI + AMT (40 mg/kg) groups, and neither differed from the TBI + VEH control (p > 0.05). The SHAM group located the visible platform faster than all TBI groups (p < 0.05). No other comparisons were significant (p > 0.05).
Fig. 4.
Mean (± S.E.M.) percent time spent in the target quadrant. The bar graph shows the % time that each group spent in the target quadrant and the horizontal dashed line at depicts chance (25%) level of exploration of the target quadrant. *,# p < 0.05 vs. TBI + VEH, TBI + AMT (10 mg/kg), and TBI + AMT (40 mg/kg). No differences were observed between the SHAM and TBI + AMT (20 mg/kg) groups (p > 0.05) nor among the TBI + AMT (10 mg/kg), TBI + AMT (40 mg/kg), and TBI + VEH groups (p > 0.05).
4. Discussion
The aim of this preclinical behavioral-focused study was to evaluate the efficacy of AMT, a D2 receptor agonist, on motor and cognitive outcome after controlled cortical impact (CCI) injury in adult male rats. To determine an optimal treatment strategy, three doses of AMT were evaluated. Although a previous study from our group, utilizing the same injury severity and behavioral methodology, showed that 10 mg/kg of AMT enhanced spatial learning, the effect was modest and observed only on the last day of training [14]. Hence, for this dose response experiment we included the low dose of 10 mg/kg to replicate previous data and then doubled the other two doses, which essentially provided a range from 10 mg/kg to 40 mg/kg of AMT provided once daily for 19 days. The rationale for a dose response study is that evaluating a single dose may be insufficient to detect the benefit of a potentially efficacious pharmacotherapy. Although not explicitly stated in clinical trial reports, inappropriate dosing regimens may certainly be one of many reasons for a treatment that has been found to be effective in multiple preclinical injury models and independent laboratories to be ineffective in the clinic.
The effect of AMT on motor function was modest as only beam-balance was improved and that was with only the dose of 20 mg/kg. The group receiving 40 mg/kg of AMT performed better than the group receiving 10 mg/kg, but neither improved performance relative to the VEH-treated controls. This finding is not entirely surprising as our previous study evaluating AMT also did not reveal a motor effect [14]. Moreover, using a fluid percussion brain injury model, Wang and colleagues (2014) also did not observe a benefit in motor function, despite providing much larger doses [15]. The surprise, however, comes from the fact that AMT is a D2 receptor agonist and the DA system is intimately involved in mediating motor function. Furthermore, other D2 receptor agonists, such as methylphenidate and bromocriptine have been shown to provide motor benefits using the same injury and dosing paradigm [5,20-22].
In marked contrast, the acquisition of spatial learning was robustly enhanced after treatment with 20 mg/kg of AMT, which performed better than the two other AMT groups and the VEH-treated controls. Memory retention was also significantly improved in the 20 mg/kg AMT-treatment group. That 20 mg/kg of AMT performed vastly better than the 10 mg/kg in the previous study [14] and that the lower and higher doses of AMT in the current study were ineffective underscores the need for dose response studies to determine optimal therapeutic efficacy.
Albeit the goal of this study was to evaluate behavioral outcomes with AMT treatment after TBI and not to determine mechanisms at this stage, several potential mechanisms exist. In addition to the D2 receptor agonist effects alluded to earlier that may restore DA neurotransmission, AMT may also be providing benefits by reducing inflammatory responses [23]. It is unlikely that protecting hippocampal cells is a mechanism by which AMT produces its benefits as we have previously shown that hippocampal CA1 and CA3 cell survival did not differ between AMT and VEH [14]. AMT also did not spare hippocampal neurons after fluid percussion brain injury, yet there were behavioral improvements [15].
5. Conclusion
In summary, the results suggest that AMT improves motor and cognitive performance in a dose-dependent manner after experimental TBI in adult male rats. Specifically, that only one dose (AMT, 20 mg/kg), in a relatively narrow dose response profile, produced benefits highlights the need for greater vigilance in choosing optimal doses for pharmacotherapies. Ultimately, the only way to accomplish this is to perform dose response studies, which require at a minimum three doses. Once the optimal dose has been ascertained, it can then be used in combination with other therapies, such as environmental enrichment, a preclinical model of neurorehabilitation [17,24-28], to perhaps augment the benefits and facilitate translation to the clinic.
Highlights.
Amantadine (AMT) improved motor and cognitive performance after TBI in a dose-dependent manner
That only one dose of AMT produced benefits after TBI underscores the need to carefully consider dosing when assessing the effects of pharmacotherapies after TBI, such that potential benefits are not missed
AMT did not differ from SHAM controls in memory retention, indicating its robust benefits
Acknowledgements
This work was supported, in part, by NIH grants HD069620, HD069620-S1, NS060005, NS084967 (AEK), NS094950, NS099683 (COB), the University of Pittsburgh Physicians /UPMC Academic Foundation, and the UMPC Rehabilitation Institute (COB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC, The impact of traumatic brain injuries: A global perspective, Neurorehabilitation 22 (2007) 341–355. [PubMed] [Google Scholar]
- [2].Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, Geller AI, Khoury N, Xu L, Trends in traumatic brain injury in the U.S. and the public health response: 1995-2009. J. Safety Res 43 (2012) 299–307. [DOI] [PubMed] [Google Scholar]
- [3].Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C, Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil 23 (2008) 123–131. [DOI] [PubMed] [Google Scholar]
- [4].Faul M, Xu L, Wald MM, Coronado VG, Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; (2010). [Google Scholar]
- [5].Kline AE, Leary JB, Radabaugh HL, Cheng JP, Bondi CO, Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog. Neurobiol 142 (2016) 45–67, https://doi.Org/10.1016/j.pneurobio.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Doppenberg EM, Choi SC, Bullock R, Clinical trials in traumatic brain injury: lessons for the future, J. Neurosurg. Anesthesiol 16 (2004) 87–94. [DOI] [PubMed] [Google Scholar]
- [7].Spritzer SD, Kinney CL, Candie J, Wellik KE, Hoffman-Snyder CR, Wingerchuk DM, Demaerschalk BM, Amantadine for patients with severe traumatic brain injury: a critically appraised topic, The Neurologist 19 (2015) 61–64. [DOI] [PubMed] [Google Scholar]
- [8].Ghate PS, Bhanage A, Sarkar H, Katkar A, Efficacy of amantadine in improving cognitive dysfunction in adults with severe traumatic brain injury in Indian population: a pilot study, Asian J. Neurosurg 13 (2018) 647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stelmaschuk S, Will MC, Meyers T, Amantadine to treat cognitive dysfunction in moderate to severe traumatic brain injury, J. Trauma Nursing 22 (2015) 164–203. [DOI] [PubMed] [Google Scholar]
- [10].Meythaler JM, Brunner RC, Johnson A, Novack TA, Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: a pilot doubleblind randomized trial, J. Head Trauma Rehabil 17 (2002) 300–313. [DOI] [PubMed] [Google Scholar]
- [11].Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, Eifert B, Long D, Katz DI, Cho S, Yablon SA, Luther M, Hammond FM, Nordenbo A, Novak P, Mercer W, Maurer-Karattup P, Sherer M, Placebo=controlled trial of amantadine for sever traumatic brain injury, N. Eng. J. Med 366 (2012) 819–826. [DOI] [PubMed] [Google Scholar]
- [12].Hammond FM, Sherer M, Malec JF, Zafonte RD, Dikmen S, Bogner J, Bell KR, Barber J, Temkin N, Amantadine did not positively impact cognition in chronic traumatic brain injury: a multi-site, randomized, controlled trial, J. Neurotrauma 35 (2018) 2298–2305, 10.1089/neu.2018.5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ghalaenovi H, Fattahi A, Koohpayehzadeh J, Khodadost M, Fatahi N, Taheri M, Azimi A, Rohani S, Rahatlou H, The effects of amantadine on traumatic brain injury outcome: a double-blind, randomized, controlled, clinical trial, Brain Inj 32 (2018) 1050–1055, 10.1080/02699052.2018.1476733 [DOI] [PubMed] [Google Scholar]
- [14].Dixon CE, Kraus MF, Kline AE, Ma X, Yan HQ, Griffith RG, Wolfson BM, Marion DW, Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats, Restor. Neurol. Neurosci 14 (1999) 285–294. [PubMed] [Google Scholar]
- [15].Wang T, Huang X-J, Van KC, Went GT, Nguyen JT, Lyeth BG, Amantadine improves cognitive outcome and increases neuronal survival after fluid percussion traumatic brain injury in rats, J. Neurotrauma 31 (2014) 370–377, 10.1089/neu.2013.2917 [DOI] [PubMed] [Google Scholar]
- [16].de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE, Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury, Neurorehabil. Neural Repair 25 (2011) 343–350, 10.1177/1545968310390520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Radabaugh HL, LaPorte MJ, Greene AM, Bondi CO, Lajud N, Kline AE, Refining environmental enrichment to advance rehabilitation based research after experimental traumatic brain injury, Exp. Neurol 294 (2017) 12–18, 10.1016/j.expneurol.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Feeney DM, Gonzalez A, Law WA, Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury, Science 217 (1982) 855–857. [DOI] [PubMed] [Google Scholar]
- [19].Hamm RJ, Neurobehavioral assessment of outcome following traumatic brain injury in rats: an evaluation of selected measures, J. Neurotrauma 18 (2001) 1207–1216, 10.1089/089771501317095241 [DOI] [PubMed] [Google Scholar]
- [20].Kline AE, Massucci JL, Marion DW, Dixon CE, Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact, J. Neurotrauma 19 (2002) 415–425. [DOI] [PubMed] [Google Scholar]
- [21].Kline AE, Massucci JL, Ma X, Zafonte RD, Dixon CE, Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma, J. Neurotrauma 21 (2004) 1712–1722. [DOI] [PubMed] [Google Scholar]
- [22].Kline AE, Yan HQ, Bao J, Marion DW, Dixon CE, Chronic methylphenidate treatment enhances water maze performance following traumatic brain injury in rats, Neurosci. Lett 280 (2000) 163–166. [DOI] [PubMed] [Google Scholar]
- [23].Ossola B, Schendzielorz N, Chen SH, Bird GS, Tuominen RK, Männistö PT, Hong JS, Amantadine protects dopamine neurons by a dual action: reducing activation of microglia and inducing expression of GDNF in astroglia, Neuropharmacology 61 (2011) 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bondi CO, Klitsch KC, Leary JB, Kline AE, Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury, J. Neurotrauma 31 (2014) 873–888, 10.1089/neu.2014.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bondi CO, Semple BD, Noble-Haeusslein LJ, Osier ND, Carlson SW, Dixon CE, Giza CC, Kline AE, Found in translation: Understanding the biology and behavior of experimental traumatic brain injury. Neurosci. Biobehav. Rev 58 (2015) 123–146, 10.1016/j.neubiorev.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cheng JP, Shaw KE, Monaco CM, Hoffman AN, Sozda CN, Olsen AS, Kline AE, A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits, J. Neurotrauma 29 (2012) 2684–2688, 10.1089/neu.2012.2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE, Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury, Neurorehabil. Neural Repair 25 (2011) 343–350, 10.1177/1545968310390520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Matter AM, Folweiler KA, Curatolo LM, Kline AE, Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats, Neurorehabil. Neural Repair 25 (2011) 558–564, DOI: 10.1177/1545968310397206 [DOI] [PMC free article] [PubMed] [Google Scholar]