Abstract
Manganese (Mn) and iron (Fe) are trace elements that are essential for proper growth and physiological functions as both play critical role in a variety of enzymatic reactions. At high concentrations, however, they can be toxic and cause neurodegenerative disorders, particularly Parkinson-like syndromes. Nicotine, on the other hand, has been shown to have neuroprotective effects against various endogenous or exogenous toxins that selectively damage the dopaminergic cells. These cells include neuroblastoma-derived SH-SY5Y cells which express significant dopaminergic activity. However, practically no information on possible neuroprotective effects of nicotine against toxicity induced by trace elements is available. Therefore, in this study we investigated the effects of nicotine on toxicity induced by manganese or iron in these cells. Exposure of SH-SY5Y cells for 24 h to manganese (20 μM) or iron (20 μM) resulted in approximately 30% and 35% toxicity, respectively. Pretreatment with nicotine (1 μM) completely blocked the toxicities of Mn and Fe. The effects of nicotine, in turn, were blocked by selective nicotinic receptor antagonists. Thus, dihydro-beta erythroidine (DHBE), a selective alpha4-beta2 subtype antagonist and methyllycaconitine (MLA), a selective alpha7 antagonist, as well as mecamylamine, a non-selective nicotinic antagonist all dose-dependently blocked the protective effects of nicotine against both Mn and Fe. These findings provide further support for the potential utility of nicotine or nicotinic agonists in Parkinson’s disease-like neurodegenerative disorders, including those that might be precipitated by trace elements, such as Fe and Mn. Moreover, both alpha4-beta2 and alpha7 nicotinic receptor subtypes appear to mediate the neuroprotective effects of nicotine against toxicity induced by these two trace metals.
Keywords: Nicotine, Manganese, Iron, Nicotinic Receptors, Neurotoxicity, Neuroprotection, Cell Culture, Parkinson’s Disease
INTRODUCTION
Parkinson’s disease (PD), associated with loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), is the second most common progressive neurodegenerative disorder following Alzheimer’s disease (Cacabelos, 2017). Main symptoms include motor deficits characterized by akinesia, rigidity, resting tremor and postural instability (Sveinbjornsdottir, 2016). Although its exact etiology remains elusive, involvement of various neurotoxicological agents as well as excess accumulation of trace elements such as manganese and iron have been suggested (Tanner et al., 2011; Wang et al., 2016; Andrade et al., 2017; Vaccari et al., 2017). The most common treatment consists of dopamine replacement (e.g. levodopa=L-DOPA), which not only loses its full efficacy in a few years but may also induce severe dyskinesia (Picconi et al., 2017). Hence more studies on etiology as well as development of more efficacious interventions aiming at neuroprotection are urgently needed.
Manganese (Mn), an activator or cofactor for a variety of metalloenzymes, is a transition metal that is essential for normal cell growth and development (Yousefi Babadi et al., 2014; Caito and Aschner, 2015; O’Neal and Zheng, 2015; Peres et al., 2016; Andrade et al., 2017). The enzyme or co-enzymes utilizing Mn play critical role in important biological functions such as gluconeogenesis, suppression of oxidative stress (Mn-superoxide dismutase, SOD) and conversion of glutamate into glutamine (glutamine synthetase) (Aschner and Gannon 1994, Burton and Guilarte, 2009). Thus, Mn deficiency may lead to impaired reproductive function, retarded growth including skeletal abnormalities and seizures (Roth and Garrick 2003; Aschner and Aschner, 2005). Excessive exposure to Mn, on the other hand, may also cause impaired reproductive function (McMillan, 1999) as well as severe neurotoxicity leading to Parkinson’s disease-like syndrome (Peres et al 2016; Andrade et al., 2017).
Iron (Fe), like Mn is also a critical component of a variety of enzymes or co-enzymes including catalases and cytochromes that mediate cellular processes and drug metabolism. Fe is best recognized as an essential element in hemoglobin synthesis, where its deficiency is reflected in wide-spread iron-deficiency anemia (Anand and Gupta, 2018). Excess iron (or iron overload), on the other hand, may lead to conditions such as diabetes, increased infection risk as well as severe neurotoxicological symptoms including Parkinson-like syndrome (Wang et al., 2016; Zhang and Rawal, 2017; Ganz, 2018). Indeed, substantia nigra, where the selective loss of dopaminergic neurons occurs, is the primary region in the brain where iron is deposited (Dexter et al., 1989; Riederer et al., 1989].
Cellular models of substantia nigra dopamine containing neurons include neuroblastoma-derived SH-SY5Y cells. We and others have used this cell line extensively to investigate mechanism of neurotoxicity as well as potential novel neuroprotective agents (Manavalan et al., 2017, Han et al., 2018; Getachew et al., 2018; Omura et al., 2018; Saksonová et al., 2018). In this regard, protective effects of nicotine or curcumin against toxicity induced by selective dopaminergic toxins, including salsolinol and rotenone have been observed (Copeland et al., 2005; Qualls et al., 2014). Whereas rotenone is a synthetic pesticide, salsolinol is an endogenous compound that is generated from the condensation of dopamine and aldehydes and is selectively toxic to dopaminergic cells (Collins, 1988; Naoi, 2004; Segura-Aguilar and Kostrzewa, 2004). It was shown that protective effects of nicotine against salsolinol are mediated via nicotinic receptors because mecamylamine, a non-selective nicotinic antagonist, could totally block the effects of nicotine (Copeland et al., 2005). In addition, many other in-vitro and in-vivo studies have provided evidence of nicotine’s protection against toxicants that lead to neurodegenerative/neurological consequences including PD. These studies, summarized in recent reviews, include genetically modified mice and non-human primates where protective effects of nicotine against neuronal damage induced by 6-hydroxydopamine (6-OHDA), 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine (MPTP), paraquat, methamphetamine, glutamate and β-amyloid were reported (Tizabi, 2016; Tizabi and Getachew, 2017). Indeed, the findings with neuroprotective effects of nicotine has led to the suggestion of initiating pulsatile nicotine trials in patients suffering from PD (Tizabi and Getachew, 2017).
To better characterize the utility of nicotine in PD-like symptoms that might be brought about by excess exposure to trace elements such as Mn and Fe, we hereby evaluated the effects of nicotine against toxicity brought about by these two metals in SH-SY5Y cells. Moreover, we aimed to determine the involvement of specific nicotinic receptors in potential protective effects of nicotine.
MATERIALS AND METHODS
Manganese sulfate, iron sulfate, nicotine (bitartrate salt), dihydro-beta-erthroydine (DHBE). methyllycaconitine (MLA), selective antagonists for alpah4-beta2 and alpha7 nicotinic receptor subtypes, respectively, mecamylamine (MEC), a non-selective nicotinic antagonist, and other analytical reagents were purchased from Sigma Chemical Company (Sigma-Aldrich, St. Louis, MO). The SH-SY5Y human neuroblastoma cell line were purchased from American Type Culture Collection (ATCC, Manassas, VA). 3, (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was purchased from Fisher Scientific (Pittsburgh, PA).
As reported in detail previously (Manavaln et al., 2017; Getachew et al., 2018) and briefly here, SH-SY5Y cells were cultured in a 1:1 mixture of Dulbeccos Modified Eagle Medium (DMEM) and Ham’s F12 supplemented with 10 % fetal bovine serum, penicillin/streptomycin (100 IU/ml), and gentamicin (50ug/ml) at 37° C in 95% O2/5% CO2 humidified incubator. The cells were trypsinized when confluent and plated in 96 well plates (1.2 × 104 cells/well). Cells were allowed to adhere to bottom surface for 24 h. Then, fresh media containing various concentrations of Mn, Fe, nicotine and nicotinic antagonists were added to the carefully aspirated wells.
We first conducted concentration-response experiments to determine the levels of toxicity of Mn and Fe. Once a suitable toxicity level was obtained, that concentration of Mn or Fe was used in subsequent studies. This was followed by a concentration-response experiment involving nicotine and Mn or Fe. Here also, once an optimal concentration of nicotine (one that totally blocked the effects of Mn or Fe) was obtained, that concentration was used in subsequent studies involving nicotinic antagonists. Thus, various concentrations of DHBE, MLA or MEC were added 1 h prior to nicotine, which was added 1 h prior to Mn or Fe. In all cases, the control group consisted of cells that were maintained in media alone and without any drug treatment. All treatments were carried out for 24 h and the effects on cell viability were determined following the 24 h incubation. Each cell viability study was run in sextuplicate (i.e., 6 replicates) and a minimum of 4 assays were conducted for each experimental manipulation.
Determination of cell viability was done by 3, (4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) colorimetric assay according to the manufacturer’s protocol as described previously (Manavalan et al., 2017; Getachew et al., 2018). Briefly, the yellow MTT tetrazolium salt (0.5 mg/ml) was dissolved in phosphate-buffered saline (PBS) with 10 mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). 30 μl of MTT was added to each well and incubated for 3 h at 37° C. The live cells cause a reduction of the yellow salt to insoluble purple formazan crystals. The wells were then aspirated, and 50 μl of dimethyl sulfoxide (DMSO) was added to the wells to solubilize the crystals, and the plates were placed in a shaker for an hour. The plates were then read spectrophotometrically at 570 nm with a background of 630 nm in a plate reader. Cell viability was determined by subtracting the test results from the background and is presented as a percentage of the control.
Data is expressed as mean ± standard error of the mean (SEM). Statistical differences within and between treatment groups were determined by one-way analysis of variance (ANOVA) followed by post-hoc Newman–Keuls Multiple comparison test, where P < 0.05 was considered statistically significant. Data were analyzed using Graphpad Prism 3 (Graphpad Software, Inc., San Diego, CA).
RESULTS
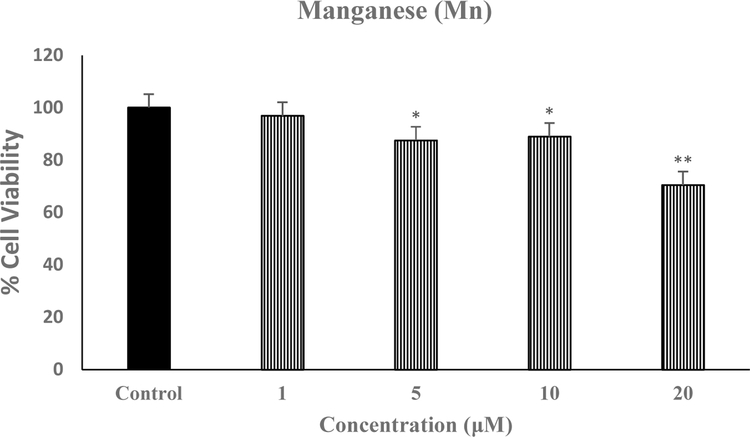
Figure 1 depicts the effect of various concentrations of Mn on SH-SY5Y viability. We used the highest concentration of 20 μM because it is equivalent to what would be a toxicological exposure in the environment (Caito and Aschner, 2015; O’Neal and Zheng, 2015). As seen, this concentration caused the highest toxicity of approximately 30% [F(4,20=16.1, p<0.01], and hence was used in all subsequent studies.
Fig. 1.
Effect of various concentrations of Mn on SH-SY5Y cell viability. Cells were treated with various concentrations of Mn for 24 h and cell viability was determined by MTT. Values are mean ± SEM. *p<0.05, **p<0.01 compared to control. N=5 per treatment
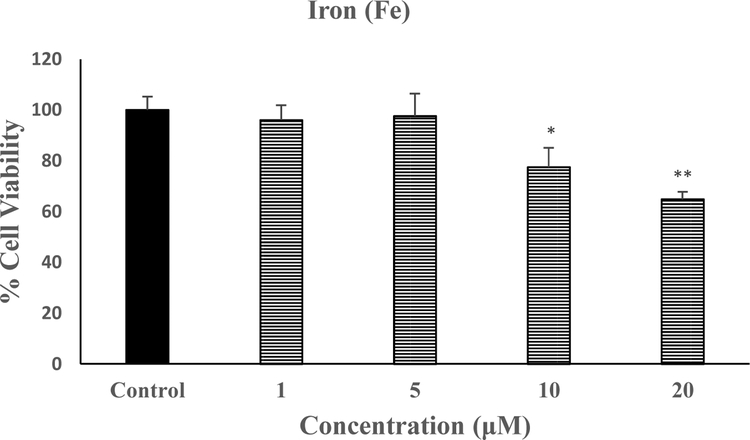
Figure 2 depicts the effect of various concentrations of Fe on SH-SY5Y viability. We used the highest concentration of 20 μM here also because it is equivalent to what would be a toxicological exposure in the environment (Dexter et al., 1989; Fine, 2000). As seen, this concentration caused the highest toxicity of approximately 35% [F(4,20=16.8, p<0.01], and hence was used in all subsequent studies.
Fig. 2.
Effect of various concentrations of Fe on SH-SY5Y cell viability. Cells were treated with various concentrations of Mn for 24 h and cell viability was determined by MTT. Values are mean ± SEM. *p<0.05, **p<0.01 compared to control. N=5 per treatment
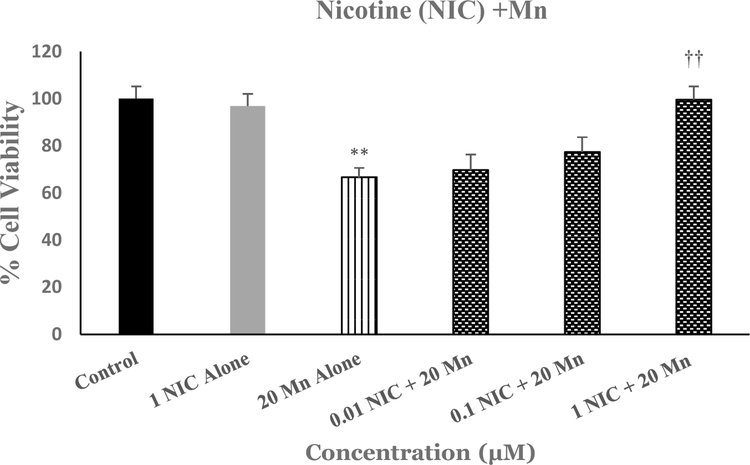
Figure 3 depicts the effects of various concentrations of nicotine against Mn-induced toxicity in SH-SY5Y cells. As seen, there was a concentration-dependent protection of Mn-induced toxicity by nicotine (NIC), where 1μM NIC completely blocked the effects of Mn [F(5,25=12.6, p<0.01]. Thus, this concentration of NIC was used in subsequent studies to investigate the effects of nicotinic antagonists.
Fig.3.
Effect of various concentrations of NIC on Mn-induced toxicity in SH-SY5Y cells. Cells were treated with various concentrations of NIC 1h prior to Mn (20 uM) and cell viability was determined by MTT 24 h later. Values are mean ± SEM. **p<0.01 compared to control. ††p<0.01 compared to Mn alone. N=5 per treatment
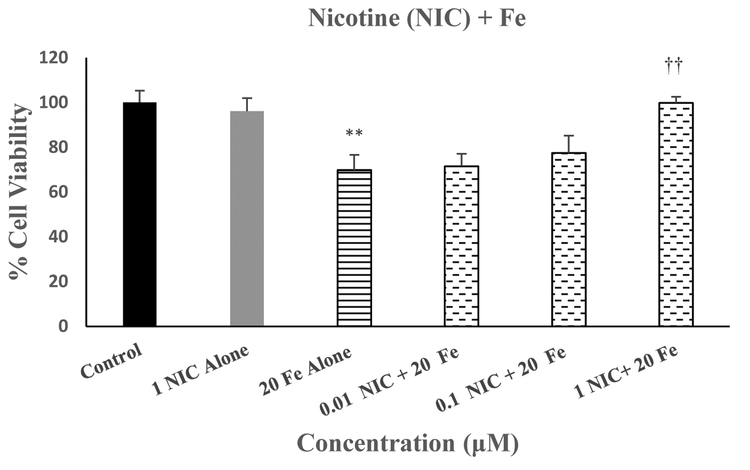
Figure 4 depicts the effects of various concentrations of nicotine against Fe-induced toxicity in SH-SY5Y cells. As seen, there was a concentration-dependent protection of Fe-induced toxicity by nicotine (NIC), where 1μM NIC completely blocked the effects of Fe [F(5,25=12.8, p<0.01]. Thus, this concentration of NIC was used in subsequent studies to investigate the effects of nicotinic antagonists.
Fig 4.
Effect of various concentrations of NIC on Fe-induced toxicity in SH-SY5Y cells. Cells were treated with various concentrations of NIC 1h prior to Fe (20 uM) and cell viability was determined by MTT 24 h later. Values are mean ± SEM. **p<0.01 compared to control. ††p<0.01 compared to Mn alone. N=5 per treatment
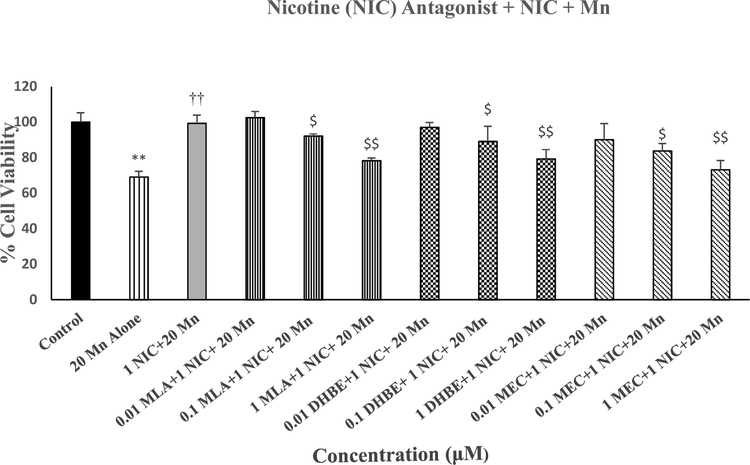
Figure 5 depicts the effects of various concentrations of MLA (selective alpha7 antagonist); DHBE (selective alpha4-beta2 antagonist) and MEC (non-selective nicotinic antagonist) against protective effects of NIC on Mn-induced toxicity in SH-SY5Y cells. All three antagonists concentration-dependently blocked the effects of NIC, where 1μM of each antagonist completely abolished the effects of NIC [F(11,48=9.3, p<0.01]. None of the antagonists by themselves had any effect on cell viability (data not shown).
Fig 5.
Effects of various concentrations of MLA, DHBE and MEC against protective effects of NIC on Mn-induced toxicity in SH-SY5Y cells. Cells were treated with various concentrations of the antagonists 1 h prior to NIC (1 uM), which was applied 1 h prior to Mn (20 uM) and cell viability was determined by MTT 24 h later. Values are mean ± SEM. **p<0.01 compared to control. ††p<0.01 compared to Mn alone.$p<0.05,$ $p<0.01 compared to NIC+Mn. N=5 per treatment
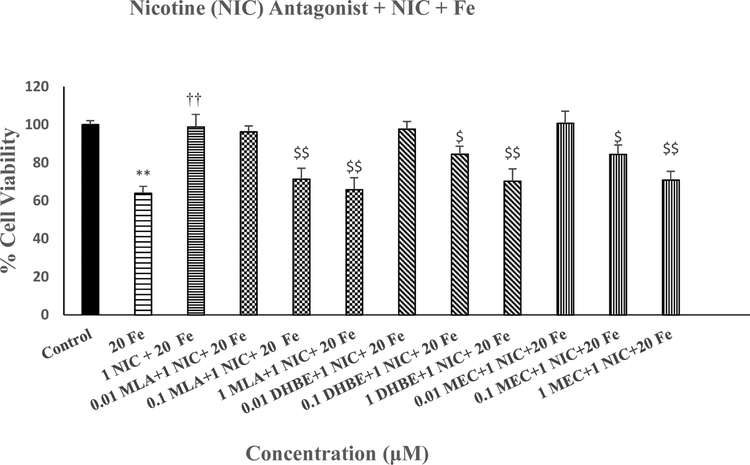
Figure 6 depicts the effects of various concentrations of MLA, DHBE and MEC against protective effects of NIC on Fe-induced toxicity in SH-SY5Y cells. All three antagonists concentration-dependently blocked the effects of NIC, where 1μM of each antagonist completely abolished the effects of NIC [F(11,48=10.1, p<0.01]. None of the antagonists by themselves had any effect on cell viability (data not shown).
Fig 6.
Effects of various concentrations of MLA, DHBE and MEC against protective effects of NIC on Fe-induced toxicity in SH-SY5Y cells. Cells were treated with various concentrations of the antagonists 1 h prior to NIC (1 uM), which was applied 1 h prior to Fe (20 uM) and cell viability was determined by MTT 24 h later. Values are mean ± SEM. **p<0.01 compared to control. ††p<0.01 compared to Fe alone.$p<0.05,$ $p<0.01 compared to NIC+Fe. N=5 per treatment.
DISCUSSION
The results of this study provide novel evidence on protective effects of nicotine against trace elements-induced toxicity in SH-SY5Y cells, which further support the potential utility of nicotinic intervention in PD. This contention is based on the ability of nicotine to block the damage that is induced by toxicological concentrations of manganese and iron, two essential elements, implicated in pathophysiology of PD or PD-like symptoms. The protective effects of nicotine against these two elements was observed in SH-SY5Y cells, which express nicotinic receptors (Gould et al., 1992; Lukas et al., 1993; Elnagar et al., 2018) and are commonly used as a model of substantia nigra dopaminergic neurons that are primarily affected in PD (Brown et al., 2013; Manavalan et al., 2017, Getachew et al., 2018).
Indeed, a number of studies have identified the presence of nicotinic cholinergic receptors in the substantia nigra. These include earlier experiments utilizing autoradiography (Clarke and Pert 1985) and immunohistochemistry (Göldner et al, 1997; Sorenson et el, 1998). More recently potential roles of these receptors in this area (Fature et al, 2014) as well as in nigrostriatal terminals (Inden et al, 2016) have been provided. In addition, it has been suggested that nicotine, by inhibiting astrocytes activation in the substantia nigra pars compacta (SNpc), can protect dopaminergic neurons against degeneration (Jurado-Coronel et al, 2016). Interestingly, both DHBE and MLA, selective antagonists to nicotinic receptor subtypes, alpha4-beta2 and alpha7, respectively, appear to equally mediate the effects of nicotine. This is because both of these antagonists at equivalent concentrations, totally blocked the effect of nicotine. Further confirmation of both receptor involvement was provided by results of mecamylamine, a nonselective nicotinic receptor antagonist, which also equi-potently blocked nicotine’s protective effects.
It is of relevance to note that nicotine’s main targets are nicotinic receptors that belong to the ionotropic class of receptors, which directly regulate the opening of a cation channel in the neuronal membrane. The endogenous ligand for these receptor is the important neurotransmitter acetylcholine (ACh), hence these receptors are commonly referred to as nAChRs. Currently, 11 neuronal nAChR subunits: alpha2-alpha7, alpha 9, alpha 10, beta2-beta4, which assemble into pentameric complexes and provide subtype diversity and distinct anatomical, physiological, and pharmacological characteristics have been identified (Changeux et al., 1998; Dani, 2015). The most predominant and extensively studied subtype in the brain is formed from α4 and β2 subunits and is commonly referred to as high-affinity binding site because of its high binding affinity to nicotine and ACh. The other major class with a high affinity for α–bungarotoxin but low affinity for nicotine is formed from α7 homomeric subunits and is commonly referred to as low-affinity binding site. These receptors have been directly implicated not only in nicotine addiction (Tizabi et al., 2002, 2007; Meyerhoff et al., 2006; Picciotto and Mineur, 2014; Liu and Li, 2018), but also in a variety of central functions such as cognitive and attentional processes (Tizabi, 2007; Proulx et al., 2014), mood regulation (Hurley and Tizabi, 2013; Lewis and Picciotto, 2013), pain (Campbell et al., 2006; Bufalo et al., 2014), neuronal plasticity (Serres and Carney, 2006; Antonelli et al., 2012; Barreto et al., 2015; Fuenzalida et al., 2016) and neuronal protection (Das and Tizabi 2009; Dineley et al. 2015; Quik et al., 2015; Tizabi and Getachew, 2017). Although in some cases a specific receptor subtype might be mediating the action of nicotine (e.g. beta2 containing subunit for its addictive property) (Picciotto and Mineur, 2014; Miller et al., 2018; Liu and Li., 2018) or alpha7 for its antipsychotic effects (Freedman, 2014; Wallace and Bertrand, 2015; Wadenberg et al., 2017; Jones, 2018), here, the protective effects of nicotine against Mn and Fe toxicity, appear to be mediated by both alpha4-beta2 and alpha7 subunits. This finding is similar to observation of nicotine protection against glutamate or beta-amyloid-induced toxicity in cell culture, where both of these nicotinic receptor subtypes were implicated (Kihara et al., 1998; Akaike et al., 2010; Yu et al., 2011).
Interestingly, both Mn and Fe appear to target primarily the basal ganglia, which includes the globus pallidus and substantia nigra, the latter being the primary site of damage leading to PD (Erikson and Aschner, 2003; Rajagopalan et al., 2016). Thus, manganism associated with increased Mn levels, particularly in basal ganglia, may manifest PD-like symptoms (Peres et al., 2016; Andrade et al., 2017). Although the pathological mechanisms associated with Mn neurotoxicity are poorly understood, several reports have implicated oxidative stress as well as inflammatory processes as important contributory factors (Dusek et al., 2015; Santos et al., 2015). Similarly, iron-induced neurodegenerative diseases including PD has been at least partially ascribed to increases in oxidative stress as well as neuroinflammatory factors (Salvador et al., 2011; Dusek et al., 2015). On the other hand, it has been hypothesized that neuroprotective effects of nicotine are also likely to involve suppression of oxidative stress and pro-inflammatory cytokines (Chatterjee et al., 2012; Hurley and Tizabi, 2013; Barreto et al., 2015; Perez, 2015; Tizabi, 2016). Hence, it may be suggested that similar mechanisms play a role in protective effects of nicotine against trace metal induced toxicity. Curiously, it has been shown that in regard to damage induced by other metals such as copper, nicotine’s protective mechanism may involve regulation of metal homeostasis. Thus, it has been demonstrated that copper-induced beta-amyloidosis can be attenuated by nicotine via reduction of this metal level in both in-vivo and in-vitro studies (Zhang et al., 2006). Therefore, it would be of significant interest to determine whether nicotine may have a similar effect on Mn and/or Fe. Another important undertaking would be to determine whether the combination of trace metals including tin, copper, zinc, etc. with Mn and/or iron would exacerbate the neurodegenerative condition (Lee et al., 2006; Andrade et al., 2017) and whether nicotine would be equally effective.
It should also be noted that death/survival signaling mechanisms have been implicated in the action of nicotine (Wei et al., 2011). Thus, a role for ERK-MAPK and JNK (Dineley et al, 2001; Xue et al, 2014; Li et al, 2015), a role for caspase pathways (Ramlochansingh et al, 2011; Lu et al, 2017), and more recently a role for Wnt/β-catenin signaling (Liu et al., 2017) in protective effects of nicotine have been provided.
In summary, the results of this study provide evidence for neuroprotective effects of nicotine against toxicity induced by Mn or Fe in a cellular model of PD. Moreover, both high and low affinity nicotinic receptors (i.e., alpha4-beta2 and alpha7 subtypes) appear to mediate the effects of nicotine. Thus, utility of nicotine or nicotinic agonists in trace element-induced Parkinson-like syndrome may be suggested.
Highlights:
Manganese (Mn) caused a concentration-dependent toxicity in SH-SY5Y cells
Iron (Fe) caused a concentration-dependent toxicity in SH-SY5Y cells
Nicotine (1 μM) completely blocked the toxicities of both Mn an Fe
Nicotine effects, in turn were blocked by selective nicotinic antagonists
Nicotine or nicotinic agonist may counter Mn and Fe induced toxicity
Acknowledgement:
Supported by: NIH/NIAAA R03AA022479 (YT), NIA/NIH 1R25AG047843–01 (ABC), NIEHS R01ES10653 (MA).
Abbreviations:
- ACh
acetylcholine
- DHBE
dihydro-beta-erthroydine
- Fe
iron
- L-DOPA
levodopa
- MEC
mecamylamine
- MLA
methyllycaconitine
- Mn
manganese
- MPTP
1-methyl-4-phenyl-1,2,3,6tetrahydropyridine
- MTT
3, (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NIC
nicotine
- PD
Parkinson’s disease
- SNpc
substantia nigra pars compacta
- 6-OHDA
6hydroxydopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40(1–2):211–6. [DOI] [PubMed] [Google Scholar]
- Anand IS, Gupta P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation. 2018;138(1):80–98. [DOI] [PubMed] [Google Scholar]
- Andrade VM, Aschner M, Marreilha Dos Santos AP. Neurotoxicity of Metal Mixtures. Adv Neurobiol. 2017;18:227–265. [DOI] [PubMed] [Google Scholar]
- Antonelli MC, Guillemin GJ, Raisman-Vozari R, Del-Bel EA, Aschner M, Collins MA, et al. New strategies in neuroprotection and neurorepair. Neurotoxicity research. 2012;21(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Molecular Aspects of Medicine. 2005;26(4–5):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferrin-dependent transport mechanisms. Brain Research Bulletin. 1994;33(3):345–349. [DOI] [PubMed] [Google Scholar]
- Barreto GE, Iarkov A, Moran VE. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front Aging Neurosci. 2015; 6:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Tamas A, Reglodi D, Tizabi Y. PACAP protects against inflammatory-mediated toxicity in dopaminergic SH-SY5Y cells: implication for Parkinson’s disease. Neurotox Res. 2014;26(3):230–9. [DOI] [PubMed] [Google Scholar]
- Bufalo AD, Cesario A, Salinaro G, Fini M, Russo P. Alpha9 Alpha10 Nicotinic Acetylcholine Receptors as Target for the Treatment of Chronic Pain. Curr Pharm Des. 2014; 20(38):6042–7. [DOI] [PubMed] [Google Scholar]
- Burton NC, Guilarte TR. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environmental Health Perspectives. 2009;117(3):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R Parkinson’s Disease: From Pathogenesis to Pharmacogenomics. Int J Mol. 2017; Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S, Aschner M. Neurotoxicity of metals. Handb Clin Neurol. 2015;131:169–89. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Antinociceptive effects of alcohol and nicotine: involvement of the opioid system. Brain Res. 2006;30;1097(1):71–77. [DOI] [PubMed] [Google Scholar]
- Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Léna C, Le Novère N, Marubio L, Picciotto M, Zoli M. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Res Rev. 1998;26(2–3):198–216. [DOI] [PubMed] [Google Scholar]
- Chatterjee PK, Yeboah MM, Dowling O, Xue X, Powell SR, Al-Abed Y, Metz CN. Nicotinic acetylcholine receptor agonists attenuate septic acute kidney injury in mice by suppressing inflammation and proteasome activity. PLoS One. 2012;7(5):e35361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348(2):355–8. [DOI] [PubMed] [Google Scholar]
- Collins MA. Acetaldehyde and its condensation products as markers in alcoholism. Recent Dev Alcohol. 1988;6:387–403. [DOI] [PubMed] [Google Scholar]
- Copeland RL Jr, Leggett YA, Kanaan YM, Taylor RE, Tizabi Y. Neuroprotective effects of nicotine against salsolinol-induced cytotoxicity: implications for Parkinson’s disease. Neurotoxicity Res. 2005;8(3–4):289–293. [DOI] [PubMed] [Google Scholar]
- Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol. 2015; 124:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinolinduced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16(3):194–204. [DOI] [PubMed] [Google Scholar]
- Dexter DT et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52, 1830–1836 (1989). [DOI] [PubMed] [Google Scholar]
- Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. 2015;36(2):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. Beta-amyloid activates the mitogen-activated protein kinase cascade via hippocampal alpha7 nicotinic acetylcholine receptors: In vitro and in vivo mechanisms related to Alzheimer’s disease. J Neurosci. 2001;21(12):4125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek P, Roos PM, Litwin T, Schneider SA, Flaten TP, Aaseth J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J Trace Elem Med Biol. 2015;31:193–203. [DOI] [PubMed] [Google Scholar]
- Elnagar MR, Walls AB, Helal GK, Hamada FM, Thomsen MS, Jensen AA. Functional characterization of α7 nicotinic acetylcholine and NMDA receptor signaling in SHSY5Y neuroblastoma cells in an ERK phosphorylation assay. Eur J Pharmacol. 2018;826:106–113. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochemistry International. 2003;43(4–5):475–480. [DOI] [PubMed] [Google Scholar]
- Fine JS. Iron poisoning. Curr Probl Pediatr. 2000;30(3):71–90. [DOI] [PubMed] [Google Scholar]
- Faure P, Tolu S, Valverde S, Naudé J. Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience 2014;282:86–100. [DOI] [PubMed] [Google Scholar]
- Freedman R α7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med. 2014;65:245–61. [DOI] [PubMed] [Google Scholar]
- Fuenzalida M, Pérez MÁ, Arias HR. Role of nicotinic and muscarinic receptors on synaptic plasticity and neurological diseases. Curr Pharm Des. 2016;22(14):2004–14. [DOI] [PubMed] [Google Scholar]
- Ganz T Iron and infection. Int J Hematol. 2018;107(1):7–15. [DOI] [PubMed] [Google Scholar]
- Getachew B, Hudson T, Heinbockel T, Csoka AB, Tizabi Y. Protective effects of donepezil against alcohol-induced toxicity in cell culture: role of caspase-3. Neurotox Res. 2018;34(3):757762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göldner FM, Dineley KT, Patrick JW. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit alpha6 to dopaminergic neurons in the substantia nigra and ventral tegmental area. Neuroreport. 1997;8(12):2739–42. [DOI] [PubMed] [Google Scholar]
- Gould J, Reeve HL, Vaughan PF, Peers C. Nicotinic acetylcholine receptors in human neuroblastoma (SH-SY5Y) cells. Neurosci Lett. 1992;145(2):201–4. [DOI] [PubMed] [Google Scholar]
- Gould J, Reeve HL, Vaughan PF, Peers C. Nicotinic acetylcholine receptors in human neuroblastoma (SH-SY5Y) cells. Neurosci Lett. 1992;145(2):201–4. [DOI] [PubMed] [Google Scholar]
- Han AR, Yang JW, Na JM, Choi SY, Cho SW. Protective effects of N,4,5-trimethylthiazol-2amine hydrochloride on hypoxia-induced β-amyloid production in SH-SY5Y cells. BMB Rep. 2018. October 24 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013;23(2):131–44. J Trace Elem Med Biol. 2015;31:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inden M, Takata K, Yanagisawa D, Ashihara E, Tooyama I, Shimohama S, Kitamura Y. α4 nicotinic acetylcholine receptor modulated by galantamine on nigrostriatal terminals regulates dopamine receptor-mediated rotational behavior. Neurochem Int. 2016;94:74–81. [DOI] [PubMed] [Google Scholar]
- Jones C α7 Nicotinic Acetylcholine Receptor: A Potential Target in Treating Cognitive Decline in Schizophrenia. J Clin Psychopharmacol. 2018;38(3):247–249. [DOI] [PubMed] [Google Scholar]
- Jurado-Coronel JC, Avila-Rodriguez M, Capani F, Gonzalez J, Moran VE, Barreto GE. Targeting the nicotinic acetylcholine receptors (nAChRs) in astrocytes as a potential therapeutic target in Parkinson’s disease. Curr Pharm Des. 2016;22(10):1305–11. [DOI] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Urushitani M, Sawada H, Kimura J, Kume T, Maeda T, Akaike A. Stimulation of alpha4beta2 nicotinic acetylcholine receptors inhibits beta-amyloid toxicity. Brain Res. 1998;792(2):331–4. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Kim YM, Park SK, Lee MK. Effects of tributyltin chloride on L-DOPA-induced cytotoxicity in PC12 cells. Arch Pharm Res. 2006;29(8):645–50. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Picciotto MR. High-affinity nicotinic acetylcholine receptor expression and trafficking abnormalities in psychiatric illness. Psychopharmacology (Berl). 2013;229(3):477–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZZ, Guo ZZ, Zhang Z, Cao QA, Zhu YJ, Yao HL, Wu LL, Dai QY. Nicotine-induced upregulation of VCAM-1, MMP-2, and MMP-9 through the α7-nAChR-JNK pathway in RAW264.7 and MOVAS cells. Mol Cell Biochem. 2015;399(1–2):49–58. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hao S, Yang B, Fan Y, Qin X, Chen Y, Hu J. Wnt/β-catenin signaling plays an essential role in α7 nicotinic receptor-mediated neuroprotection of dopaminergic neurons in a mouse Parkinson’s disease model. Biochem Pharmacol. 2017;140:115–123. [DOI] [PubMed] [Google Scholar]
- Liu W, Li MD. Insights into nicotinic receptor signaling in nicotine addiction: implications for prevention and treatment. Curr Neuropharmacol. 2018;16(4):350–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JYD, Su P, Barber JEM, Nash JE, Le AD, Liu F, Wong AHC. The neuroprotective effect of nicotine in Parkinson’s disease models is associated with inhibiting PARP-1 and caspase-3 cleavage. PeerJ. 2017;5:e3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Norman SA, Lucero L. Characterization of nicotinic acetylcholine receptors expressed by cells of the SH-SY5Y human neuroblastoma clonal line. Mol Cell Neurosci. 1993;4(1):1–12. [DOI] [PubMed] [Google Scholar]
- Manavalan S, Getachew B, Manaye KF, Khundmiri SJ, Csoka AB, McKinley R, Tamas A, Reglodi D, Tizabi Y. PACAP protects against ethanol and nicotine toxicity in SH-SY5Y cells: implications for drinking-smoking co-morbidity. Neurotox Res. 2017;32(1):8–13. [DOI] [PubMed] [Google Scholar]
- McMillan DE. A brief history of the neurobehavioral toxicity of manganese: some unanswered questions. NeuroToxicology. 1999;20(2–3):499–508. [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol Clin Exp Res. 2006;30(2):253–64. [DOI] [PubMed] [Google Scholar]
- Miller MB, Wilson RS, Lam TT, Nairn AC, Picciotto MR. Evaluation of the Phosphoproteome of Mouse Alpha 4/Beta 2-Containing Nicotinic Acetylcholine Receptors In Vitro and In Vivo. Proteomes. 2018. October 15;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Nagy GM. Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology. 2004;25(1–2):193–204. [DOI] [PubMed] [Google Scholar]
- O’Neal SL, Zheng W. Manganese Toxicity Upon Overexposure: a Decade in Review. Current environmental health reports. 2015;2(3):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, Matsuda H, Nomura L, Imai S, Denda M, Nakagawa S, Yonezawa A, Nakagawa T, Yano I, Matsubara K. Ubiquitin ligase HMG-CoA reductase degradation 1 (HRD1) prevents cell death in a cellular model of Parkinson’s disease. Biochem Biophys Res Commun. 2018. October 22 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M. “Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies”. BMC Pharmacol Toxicol. 2016;17(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Quik M. α6ß2* and α4ß2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson’s disease. Mol Pharmacol. 2010;78(5):971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Calabresi P. Switching on the lights of dyskinesia: Perspectives and limits of the optogenetic approaches. Mov Disord. 2017;32(4):485–486. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Mineur YS. Molecules and circuits involved in nicotine addiction: The many faces of smoking. Neuropharmacology. 2014;76 Pt B:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx E, Piva M, Tian MK, Bailey CD, Lambe EK. Nicotinic acetylcholine receptors in attention circuitry: the role of layer VI neurons of prefrontal cortex. Cell Mol Life Sci. 2014;71(7):1225–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualls Z, Brown D, Ramlochansingh C, Hurley LL, Tizabi Y. Protective effects of curcumin against rotenone and salsolinol-induced toxicity: implications for Parkinson’s disease. Neurotox Res. 2014;25(1):81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Zhang D, McGregor M, Bordia T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson’s disease. Biochem Pharmacol. 2015;97(4):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Rane A, Chinta SJ, Andersen JK. Regulation of ATP13A2 via PHD2-HIF1α signaling is critical for cellular iron homeostasis: implications for Parkinson’s disease. J Neurosci. 2016;36(4):1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlochansingh C, Taylor RE, Tizabi Y. Toxic effects of low alcohol and nicotine combinations in SH-SY5Y cells are apoptotically mediated. Neurotox Res. 2011;20(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989; 52, 515–520 [DOI] [PubMed] [Google Scholar]
- Roth JA, Garrick MD. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochemical Pharmacology. 2003;66(1):1–13. [DOI] [PubMed] [Google Scholar]
- Saksonová S, Brodňanová M, Dibdiaková K, Pilchová I, Klačanová K, Hatok J, Račay P. Cobalt chloride affects the death of SH-SY5Y cells induced by inhibition of ubiquitin proteasome system. Role of heat shock protein 70 and caspase 3. Gen Physiol Biophys. 2018. October 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Salvador GA, Uranga Romina M, and Giusto Norma M., “Iron and Mechanisms of Neurotoxicity,” Intern J Alzheimer’s Disease, vol. 2011, Article ID 720658, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology. 2012;292(2–3):90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Aguilar J, Kostrzewa RM. Neurotoxins and neurotoxic species implicated in neurodegeneration. Neurotox Res. 2004;6(7–8):615–30. [DOI] [PubMed] [Google Scholar]
- Serres F, Carney SL. Nicotine regulates SH-SY5Y neuroblastoma cell proliferation through the release of brain-derived neurotrophic factor. Brain Res. 2006;1101(1):36–42. [DOI] [PubMed] [Google Scholar]
- Sorenson EM, Shiroyama T, Kitai ST. Postsynaptic nicotinic receptors on dopaminergic neurons in the substantia nigra pars compacta of the rat. Neuroscience. 1998;87(3):659–73. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S The clinical symptoms of Parkinson’s disease. J Neurochem. 2016; 139 Suppl 1:318–324. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environmental Health Perspectives. 2011;119(6):866–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42(5):413–416. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26(3):394–9. [PubMed] [Google Scholar]
- Tizabi Y, Getachew B. Nicotinic receptor intervention in Parkinson’s disease: future directions. Clin Pharmacol Transl Med. 2017;1(1):14–19. [PMC free article] [PubMed] [Google Scholar]
- Nicotine Tizabi Y. and nicotinic system in hypoglutamatergic models of schizophrenia. Neurotox Res. 2007;12(4):233–46. [DOI] [PubMed] [Google Scholar]
- Tizabi Y Duality of antidepressants and neuroprotectants. Neurotox Res. 2016;30(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari C, El Dib R, de Camargo JLV. Paraquat and Parkinson’s disease: a systematic review protocol according to the OHAT approach for hazard identification. Systematic Reviews. 2017;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg MG, Manetti D, Romanelli MN, Arias HR. Significance of the nicotinic alpha7 receptor in cognition and antipsychotic-like behavior in the rat. Behav Brain Res. 2017;333:129134. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Bertrand D. Neuronal α7 Nicotinic Receptors as a Target for the Treatment of Schizophrenia. Int Rev Neurobiol. 2015;124:79–111. [DOI] [PubMed] [Google Scholar]
- Wang JY, Zhuang QQ, Zhu LB, et al. Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Scientific Reports. 2016;6:36669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wang J, Dwyer JB, Mangold J, Cao J, Leslie FM, Li MD. Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol. 2011;14(1):91–106. [DOI] [PubMed] [Google Scholar]
- Xue MQ, Liu XX, Zhang YL, Gao FG. Nicotine exerts neuroprotective effects against βamyloid-induced neurotoxicity in SH-SY5Y cells through the Erk1/2-p38-JNK-dependent signaling pathway. Int J Mol Med. 2014;33(4):925–33. [DOI] [PubMed] [Google Scholar]
- Yousefi Babadi V, Sadeghi L, Shirani K, Malekirad AA, & Rezaei M The Toxic Effect of Manganese on the Acetylcholinesterase Activity in Rat Brains. Journal of Toxicology, 2014, 946372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Mechawar N, Krantic S, Quirion R. α7 Nicotinic receptor activation reduces β-amyloidinduced apoptosis by inhibiting caspase-independent death through phosphatidylinositol 3-kinase signaling. J Neurochem. 2011;119(4):848–58. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu Q, Chen Q, Liu NQ, Li FL, Lu ZB, Qin C, Zhu H, Huang YY, He W, Zhao BL. Nicotine attenuates beta-amyloid-induced neurotoxicity by regulating metal homeostasis. FASEB J. 2006;20(8):1212–4. [DOI] [PubMed] [Google Scholar]