Abstract
Rapid adenosine signaling, on the time frame of seconds, has been discovered in the brain that can modulate neurotransmission or blood flow. Rapid adenosine release can occur spontaneously or be evoked after a mechanical stimulation, but these two modes of adenosine have not been compared. Here, we compared spontaneous and mechanically-stimulated adenosine release in the prefrontal cortex, striatum, and hippocampus of anesthetized mice. For spontaneous adenosine, the number of adenosine events in the prefrontal cortex (40 ± 4 per hour) was significantly lower than in the striatum (54 ± 3) or hippocampus (56 ± 3). Similarly, the concentration per transient was lower in the prefrontal cortex but highest in the striatum. For mechanically-stimulated adenosine, the peak concentration in the prefrontal cortex (8 ± 2 μM) and striatum (8 ± 1 μM) were significantly lower than in the hippocampus (16 ± 2 μM). Comparing the two modes, the hippocampus had high mechanically-stimulated concentration and high spontaneous frequency, while the prefrontal cortex had lower spontaneous frequency and mechanically-stimulated release. However, there is no pattern with the striatum and thus no direct correlations between spontaneous and mechanically-stimulated adenosine. Thus, there may be different pools of adenosine or mechanisms of formation for these two modes. Because of the high frequency of spontaneous events and high concentration of mechanically-stimulated release in the hippocampus, there may be some areas that have stronger adenosine signaling and thus stronger neuromodulatory control by adenosine.
Keywords: Adenosine, in vivo, Mouse brain regions, Fast-scan cyclic voltammetry, Mechanically-stimulated release, Spontaneous release
1. Introduction
Adenosine is an endogenous nucleoside that functions as a neuromodulator and neuroprotector in the central nervous system, particularly during stroke and traumatic brain injury (Lusardi, 2009). While adenosine builds up slowly during pathological events, our lab has also discovered rapid modes of adenosine signaling, lasting only a few seconds (Nguyen et al., 2014, Ross et al, 2014). The function of rapid adenosine is to provide rapid, local neuromodulation; rapid adenosine modulates phasic dopamine release and is correlated with transient oxygen increases (Ross and Venton, 2015; Wang and Venton, 2017). There are different modes of rapid adenosine release and it is important to understand how they vary in order to understand the neuromodulatory effects of adenosine in different regions.
Adenosine signaling varies by brain region, and the mechanism of action varies due to how it is stimulated (Cunha, 2008). In this work, we compare two modes of rapid adenosine signaling for the first time. The first mode is spontaneous adenosine, with no stimulation applied. This mode is important during ischemia-reperfusion injury, where spontaneous adenosine frequency increases (Ganesana and Venton, 2018). The second mode of rapid adenosine is mechanically-stimulated release, where small trauma, such as moving an electrode or probe near the electrode, causes transient adenosine elevation (Ross et al., 2014). This type of adenosine signaling may be important in traumatic brain injury or other physical injuries, which are known to trigger surges in adenosine (Lusardi, 2009). Here, we use mice, because of the future possibilities of studying transgenics, to characterize spontaneous adenosine events using fast-scan cyclic voltammetry. The brain regions chosen were the Cg1 of prefrontal cortex PFC), striatum, and CA1 of hippocampus because these are common brain regions for both traumatic brain injury and memory studies (Kogan et al., 2000). The prefrontal cortex participates in working memory, the striatum is associated with procedural memory, and the hippocampus plays an important role in memory formation. Spontaneous adenosine varied regionally in frequency and concentration, while mechanically-stimulated adenosine varied regionally in concentration. However, there was not clear correlation between spontaneous and mechanically-stimulated adenosine, suggesting that the mechanisms of release may not be the same. The hippocampus did have the highest frequency of spontaneous adenosine and the highest concentration of mechanically-stimulated adenosine, so some regions may have more adenosine neuromodulatory effects.
2. Methods
2.1. Animals and surgery
Male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks old and housed on a 12:12-h light/dark cycle with food and water provided ad libitum. Mice were anesthetized with 4% isoflurane in 100% oxygen for induction and anesthesia maintained with 1.5–3% in 100% oxygen delivered via a facemask (Stoelting, Wood Dale, IL, USA). A heating pad was used to sustained mouse body temperature around 37 °C. The surgical site was shaved and bupivacaine (0.10 mL, APP Pharmaceuticals, Schaumburg, IL, USA) was administered under the skin for local anesthesia. In a stereotaxic frame, the skull was exposed and holes were drilled to allow the placement of the electrode in the prefrontal cortex (AP +1.3 mm, ML + 0.2 mm, and DV −1.5 mm), striatum (AP +1.1 mm, ML + 1.5 mm, and DV −3.0 mm), or hippocampus (AP −2.5 mm, ML + 2.4 mm, and DV −1.8 mm). All experiments were approved by the Institutional Animal Care and Use Committee of the University of Virginia.
2.2. Chemicals
Electrodes were calibrated in phosphate-buffered saline (PBS) solution (3.0 mM KCl, 10.0 mM NaH2PO4, 2.0 mM MgCl2, 131.25 mM NaCl and 1.2 CaCl2, all from Fisher, Fair Lawn, NJ) with pH adjusted to 7.4. A 10.0 mM stock solution of adenosine (Sigma Aldrich, Milwaukee, WI, USA) was prepared in 0.1 mM HClO4 and this was diluted daily in PBS solution to 1 μM for calibration of the electrodes.
2.3. Electrochemistry
Fabrication of carbon-fiber microelectrode with T-650 carbon fiber was previously described (Wang and Venton, 2017). Cylinder electrodes 150–200 μm long and 7 μm in diameter were used. Fast-scan cyclic voltammetry was used to detect adenosine (Wang and Venton, 2017) and the electrode was scanned from −0.4 V to 1. 45 V and back to −0.4 V with a frequency of 10 Hz at 400 V/s.
2.4. Data Analysis and Statistics
Transient adenosine events were identified and characterized using an automated algorithm (Borman et al., 2017) and adenosine events were confirmed by an analyst to exclude any signals that were not adenosine. The primary oxidation peaks of adenosine in Figure 1A were filtered using a Fourier transform 1 Hz filter to reduce noise. All statistics were performed in GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). All data are shown as mean ± SEM. Statistical significance was designated at p < 0.05.
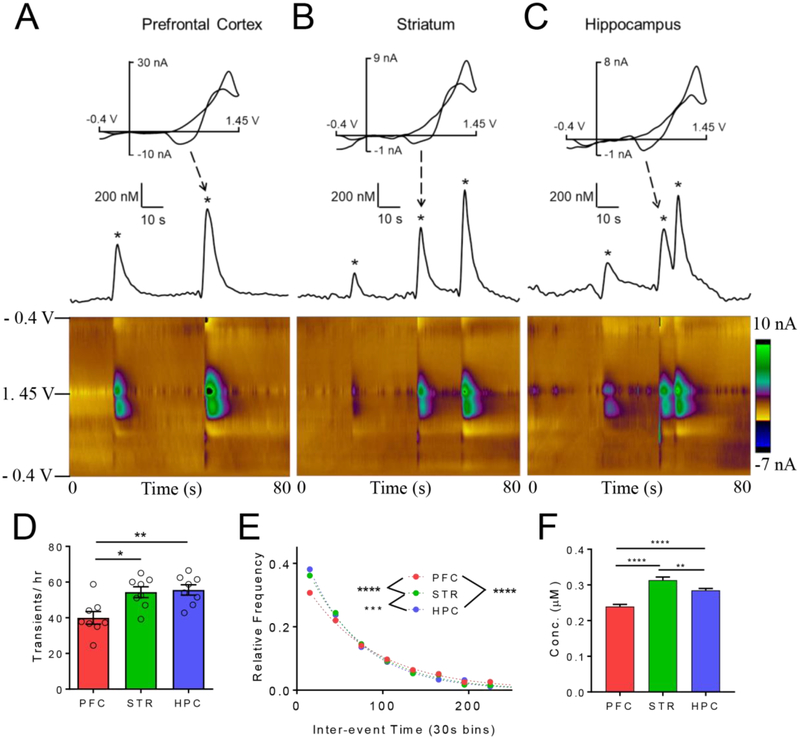
Fig. 1.
Spontaneous, transient adenosine release in various brain regions. Example release in the A) prefrontal cortex (PFC), B) striatum (STR), and C) hippocampus (HPC). Cyclic voltammograms of adenosine have the two characteristic oxidation peaks. Concentration vs. time traces derived from primary oxidation of adenosine (top); adenosine transients marked with stars. Example 3-D color plot show release events in an 80 s time window. Adenosine oxidation is the green/purple area in the middle of the plot. D) Number of adenosine events per hour (main effect, One-way ANOVA, n = 8 animals/group, p = 0.0033). The number of adenosine events was significantly lower in the PFC than the STR and HPC (Tukey’s test, p = 0.011 and p = 0.0056, respectively). E) Inter-event time histogram. The underlying distributions of were significantly different (Kruskal-Wallis test, p < 0.0001) and all regions significantly different from each other. F.) Mean concentrations per event were significantly different (One-way ANOVA, p < 0.0001, n=1279 PFC, 1779 HPC, 1739 STR) and PFC was lower than STR (p < 0.0001) and HPC (p < 0.0001) and the STR was greater than the HPC (p = 0.0072). Error bars are SEM.
3. Results
In this study, regional differences in spontaneous and mechanically-stimulated adenosine were compared in vivo in anesthetized mice. Rapid changes of adenosine were monitored in the prefrontal cortex, striatum (STR), and hippocampus (HPC) using fast-scan cyclic voltammetry. Adenosine is identified by its two characteristic oxidation peaks in the cyclic voltammogram (top) and false color plots (bottom, Fig. 1A–C)(Nguyen et al., 2014).
Spontaneous adenosine events have been reported in vivo and in brain slices in rats (Lee and Venton, 2018; Nguyen et al., 2014; Wang and Venton, 2017) but these are the first measurements in vivo in mice. For spontaneous adenosine (Fig. 1), adenosine transients were measured continuously in anesthetized mice for 4 hours. Multiple, short adenosine events are depicted in the color plot, and marked on the concentration vs. time traces, which show how adenosine varies over time. In the prefrontal cortex, there are only 2 adenosine transient events in this example 80 s window (Fig. 1A) while in the striatum and hippocampus, there are 3 events in an 80 s window (Fig.1B and 1C). Events are short, with an average half width around 2 s in all regions. The number of adenosine events varied by region; on average, there were 40 ± 4 adenosine events per hour in the prefrontal cortex, 54 ± 3 events in the striatum and 56 ± 3 events in the hippocampus, an overall significant effect of brain region (Fig. 1D, one-way ANOVA, n = 8 animals/region, p = 0.0033). The number of adenosine events in the prefrontal cortex was significantly lower than in the striatum or hippocampus (Tukey’s post-test, p = 0.011 and p = 0.0056, respectively). To examine the frequency, the inter-event time, the time between 2 consecutive events, was calculated. The mean inter-event time was 87 ± 3 s in the prefrontal cortex, 66 ± 2 s in the striatum and 64 ± 2 s in the hippocampus. There was a significant effect of brain region on the underlying distributions of inter-event times (Fig. 1E, Kruskal-Wallis test, p < 0.0001) and the prefrontal cortex frequency was significantly lower than the striatum or hippocampus (post-hoc Dunn’s test, p < 0.0001 and p < 0.0001, respectively).
The mean concentration per event was 0.239 ± 0.005 μM in the prefrontal cortex, 0.315 ± 0.009 μM in the striatum, and 0.285 ± 0.005 μM in the hippocampus (Fig. 1F). Because of the large number of transients, there is an overall effect of brain region (One-way ANOVA, p<0.0001, n = 1279 PFC, 1739 STR, 1779 HPC) and each of these groups is significantly different than the other (HPC vs. STR: p = 0.0072, HPC vs. PFC: p < 0.0001, STR vs. PFC: p < 0.0001).
Second, mechanically-stimulated adenosine release was studied (Fig. 2). Other studies have demonstrated ATP release, which could be a precursor to adenosine, due to mechanical perturbation, swelling, shear stress or cell stretching(Xia et al., 2012)(Wan et al., 2008). Here, a carbon-fiber microelectrode was lowered 0.1 mm to mechanically perturb the tissue. Figure 2A shows an example of mechanically-stimulated adenosine in the hippocampus, where adenosine increases immediately after lowering the electrode (at 30 s) with a peak concentration of 21 μM, and a half width of 4.8 s. The extra peaks in the color plot might be background subtraction errors due to ionic changes that affect the double layer charging current.
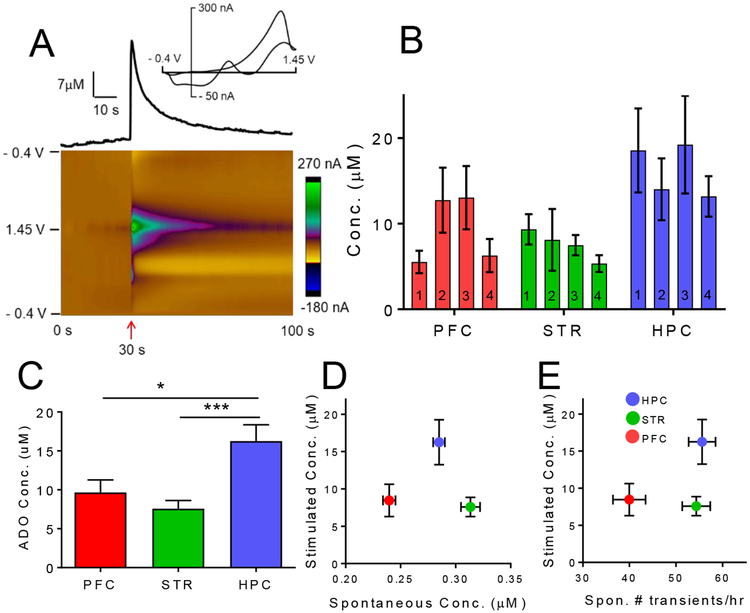
Fig. 2.
Mechanically-stimulated adenosine. A) Example mechanically-evoked adenosine in the hippocampus. Electrode was lowered 0.1 mm at 30 s (arrow). Concentration vs. time trace (top) shows adenosine peak concentration was 21 μM. B) Average data for four consecutive mechanical stimulations in the prefrontal cortex (PFC), striatum (STR), and hippocampus (HPC) in vivo. 4 consecutive stimulations were performed every 15 min. There was a significant effect of brain region on the concentration of mechanically-evoked release (two-way repeated measures ANOVA, p < 0.033). C) Average concentrations significantly vary by region (One-way ANOVA, p = 0.0005) and concentration in the hippocampus was significantly higher than in the prefrontal cortex and striatum (post hoc Tukey’s test, p = 0.012 and p = 0.0004, respectively). D) Mechanically-stimulated release concentration vs. spontaneous release concentration. There appears to be no correlation. E) Mechanically-stimulated concentration vs. number of spontaneous transients per hour. There appears to be no correlation. Error bars are SEM.
In each animal, 4 stimulations performed, every 15 min, and the electrode was continually lowered so new tissue was stimulated each time (although we have shown previously the same tissue can be mechanically-stimulated multiple times)(Ross et al., 2014). Figure 2B shows the concentration of mechanically-evoked adenosine for 4 successive stimulations in each region. Overall, there was a significant effect of brain region on the concentration of mechanically-evoked adenosine but no effect of stimulation number (Fig. 2B, two-way repeated measures ANOVA, n = 7 animals/group, F(2, 12) = 4.567, p < 0.033 and F(3, 18) = 2.070, p = 0.14, respectively). The concentration of mechanically-evoked adenosine was on average 8 ± 2 μM in the prefrontal cortex, 8 ± 1 μM in the striatum, and 16 ± 2 μM in the hippocampus, a significant effect of regions (Fig. 2C, one-way ANOVA, p = 0.0005). The concentration in the hippocampus was significantly higher than in the striatum (Tukey’s multiple comparisons test, p = 0.048).
To examine correlations between spontaneous and mechanically-stimulated adenosine, stimulated adenosine concentration vs. spontaneous concentration or number of spontaneous events were plotted (Fig. 2D–E). The graphs show that there is little correlation between spontaneous and stimulated adenosine throughout the brain. However, the prefrontal cortex did have the least stimulated release and the least number and concentration of transients. There were not strong trends for the other regions. Spontaneous adenosine event concentrations in the striatum are higher while stimulated release are lower, leading to the conclusion that the two modes of adenosine are not correlated.
4. Discussion
The concentrations of spontaneous and mechanically-stimulated adenosine varied by brain region but were not correlated. Each spontaneous event was relatively small, typically only a few hundred nanomolar, which is higher than typical basal levels reported by microdialysis (~40 nM) but similar to kainate-evoked release (Carrozzo et al., 2012). Hundreds of nM levels are sufficient to activate inhibitory A1 receptors and excitatory A2A receptors. Mechanically-stimulated adenosine was larger, in the range of 8–16 μM, so it would activate all adenosine receptors, including lower affinity A2B and A3 receptors. However, there was not a direct correlation between concentration of mechanically-stimulated and spontaneous adenosine. The prefrontal cortex had the lowest concentration of each type of adenosine, but the striatum had opposite trends with lower stimulated adenosine and higher spontaneous adenosine. These first comparisons of spontaneous and stimulated adenosine suggest that there are different release mechanisms or the pools of adenosine may be different. Extracellular formation of adenosine is mainly through metabolism of ATP via CD39 and CD73 enzymes.(Street et al., 2011) Adenosine can also be formed intracellularly and released to extracellular space via vesicular release or equilibrative adenosine transporters. Spontaneous adenosine may be exocytotic, but the larger mechanically-stimulated release may be due to channels opening up or other stress mechanisms (Xia et al., 2012), although past work has suggested that cells are not damaged by mechanical stimulation (Ross et al., 2014). Future studies could investigate these different mechanisms, but the concentrations for the two modes do not appear to be correlated.
Spontaneous and mechanically-stimulated adenosine were measured for the first time in mice, which is useful for future experiments in genetically-altered mice to study the release and formation mechanisms of rapid adenosine. Mice have frequent spontaneous adenosine events in each region, more frequent than previous studies in the rat (Nguyen et al., 2014). Interestingly, the basal concentrations of adenosine in mice are reportedly lower than rats (Delaney and Geiger, 1996), but the concentrations per event in mice were higher than those previously observed in rats (i.e. striatum: 0.3 μM in mice compared to 0.17 μM in rats) (Nguyen et al., 2014). Mechanically-stimulated adenosine was also larger in mice than rats with 8 ± 2 μM observed here in the prefrontal cortex compared to 3.3 ± 0.6 μM in rats. However, the electrode was also slightly larger (150 −200 μm in mice compared to 50 −100 μm in rats), so the amount of perturbation and adenosine detected may be larger.
Spontaneous adenosine is most often regulated not by the concentration of transients but by the frequency of events. Frequent release of spontaneous adenosine may be related to ATP consumption during intense neuronal activity. Regional differences were observed in the number of spontaneous events, with the hippocampus having the highest frequency of events. The hippocampus also has the highest concentration of mechanically-stimulated adenosine. However, the striatum had lower mechanically-stimulated adenosine, but also fairly high numbers of spontaneous events. The prefrontal cortex had the lowest frequency of spontaneous events and the lowest stimulated release (Fig. 2E). In the prefrontal cortex, A1 and A2A receptors modulate cortical acetylcholine (Van Dort et al., 2009), but in the striatum and hippocampus, they also regulate other neurotransmitters, including glutamate and GABA (Sperlágh and Vizi, 2011). In addition, A2B receptors regulate glucose metabolism in the prefrontal cortex and hippocampus but not striatum (Lemos et al., 2015). The hippocampus is a critical area for memory formation and retrieval, so it may be necessary for it to have higher adenosine neuromodulation.
The role of both modes of adenosine would be to act as a neuromodulator, modulating neurotransmission and blood flow. Spontaneous transients are particularly short, with a half width of only 2 s, and thus they would function locally. The concentration of mechanically-stimulated adenosine is 10-fold higher and a half width of more than 5 s, so the neuromodulation would be able to activate more cells and act at a greater region. Thus, mechanically-stimulated release may be able to provide more neuroprotection by activating additional adenosine receptors. Spontaneous adenosine on the other hand is regulated by frequency and not by concentration and would provide lower levels of neuroprotection and neuromodulation, but on a more frequent basis. More comparisons of their mechanism of action as well as mechanism of release are needed, but these studies suggest that there are regional differences in both spontaneous and mechanically-stimulated adenosine but that spontaneous and stimulated release are not generally correlated.
Acknowledgments
This work was supported by a grant from NIH (R01NS076875) to BJV.
Abbreviations
- PFC
Prefrontal cortex
- STR
Striatum
- HPC
Hippocampus
- ADO
Adenosine
Footnotes
Conflicts of interest The authors have no conflict of interest to declare.
References
- Borman RP, Wang Y, Nguyen MD, Ganesana M, Lee ST, Venton BJ, 2017. Automated Algorithm for Detection of Transient Adenosine Release. ACS Chem. Neurosci 8, 386–393. 10.1021/acschemneuro.6b00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrozzo MM, Troisi L, Cannazza G, Cazzato AS, Braghiroli D, Parenti C, Guiducci S, Zoli M, 2012. An improved LC-MS/MS method for the quantitation of adenosine concentration in mice brain microdialysates. J. Pharm. Biomed. Anal 70, 563–566. 10.1016/j.jpba.2012.05.014 [DOI] [PubMed] [Google Scholar]
- Cunha RA, 2008. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem. Int 52, 65–72. 10.1016/j.neuint.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Delaney SM, Geiger JD, 1996. Brain regional levels of adenosine and adenosine nucleotides in rats killed by high-energy focused microwave irradiation. J. Neurosci. Methods 64, 151–156. 10.1016/0165-0270(95)00119-0 [DOI] [PubMed] [Google Scholar]
- Ganesana M, Venton BJ, 2018. Early changes in transient adenosine during cerebral ischemia and reperfusion injury. PLoS One 13, e0196932 10.1371/journal.pone.0196932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Franklandand PW, Silva AJ, 2000. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56. [DOI] [PubMed] [Google Scholar]
- Lee ST, Venton BJ, 2018. Regional Variations of Spontaneous, Transient Adenosine Release in Brain Slices. ACS Chem. Neurosci 9, 505–513. 10.1021/acschemneuro.7b00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos C, Pinheiro BS, Beleza RO, Marques JM, Rodrigues RJ, Cunha RA, Rial D, Köfalvi A, 2015. Adenosine A2B receptor activation stimulates glucose uptake in the mouse forebrain. Purinergic Signal 11, 561–569. 10.1007/s11302-015-9474-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusardi TA, 2009. Adenosine Neuromodulation and Traumatic Brain Injury. Curr. Neuropharmacol 7, 228–237. 10.2174/157015909789152137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ, 2014. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One 9, e87165 10.1371/journal.pone.0087165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AE, Nguyen MD, Privman E, Venton BJ, 2014. Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. J. Neurochem 130, 50–60. 10.1111/jnc.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AE, Venton BJ, 2015. Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors. J. Neurochem 132, 51–60. 10.1111/jnc.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES, 2011. The role of extracellular adenosine in chemical neurotransmission in the hippocampus and Basal Ganglia: pharmacological and clinical aspects. Curr. Top. Med. Chem 11, 1034–46. 10.2174/156802611795347564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street SE, Walsh PL, Sowa NA, Taylor-Blake B, Guillot TS, Vihko P, Wightman RM, Zylka MJ, 2011. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol. Pain 7, 80 10.1186/1744-8069-7-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R, 2009. Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J. Neurosci 29, 871–81. 10.1523/JNEUROSCI.4111-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Ristenpart WD, Stone HA, 2008. Dynamics of shear-induced ATP release from red blood cells. Proc. Natl. Acad. Sci 105, 16432–16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Venton BJ, 2017. Correlation of transient adenosine release and oxygen changes in the caudate-putamen. J. Neurochem 140, 13–23. 10.1111/jnc.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Lim JC, Lu W, Beckel JM, Macarak EJ, Laties AM, Mitchell CH, 2012. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X 7 receptors. J. Physiol 590, 2285–2304. 10.1113/jphysiol.2012.227983 [DOI] [PMC free article] [PubMed] [Google Scholar]