Abstract
Obesity increases the risk of heart failure and atrial fibrillation. Left atrial (LA) dysfunction is increasingly recognized as a mediator of cardiovascular disease. Early effects of obesity on LA function have not been examined in large population samples. We quantified LA strain and strain rate (SR) via speckle tracking echocardiography among 1,531 middle-aged community-based participants enrolled in the Asklepios study. We compared LA function between individuals with body mass index (BMI) <25 kg/m2 (n=779), 25–29.9 kg/m2 (n=618) and ≥30 kg/m2 (n=134). Significant differences in reservoir longitudinal LA strain (BMI<25 kg/m2 =35.3%, BMI 25–29.9 kg/m2=33.1%, BMI ≥30 kg/m2=30.9%; P<0.00001) strain rate ([SR] BMI<25 kg/m2=151; BMI 25–29.9 kg/m2=141; BMI ≥30 kg/m2=135 %/s; P<0.00001) and expansion index (BMI<25 kg/m2=1.6, BMI 25–29.9 kg/m2=1.4; BMI ≥30 kg/m2=1.4; P<0.00001) were seen, indicating reduced reservoir function with increasing BMI. Obesity was also associated with impaired LA conduit function, including conduit longitudinal LA strain (BMI<25 kg/m2=21.6%, BMI 25–29.9 kg/m2=18.9%, BMI ≥30 kg/m2=16.7%; P<0.00001), SR (BMI<25 kg/m2=− 189, BMI 25–29.9 kg/m2=166 and BMI ≥30 kg/m2=150 %/sec; P<0.0001) and passive LA emptying fraction (BMI<25 kg/m2=40.5, BMI 25–29.9 kg/m2=36.5, BMI ≥30 kg/m2=36%, P<0.00001). These differences persisted after adjustment for age, sex and other potential confounders. In contrast to reservoir and conduit function, obesity was associated with increased booster pump function (active LA emptying fraction: BMI<25 kg/m2=19.4%, BMI 25–29.9 kg/m2=20.5% and BMI ≥30 kg/m2=21.5%; P<0.00001). Among middle-aged adults, obesity is associated with impaired reservoir and conduit LA function and higher booster function, which may be compensatory. Loss of booster LA function, either because of more advanced LA dysfunction or atrial fibrillation, may play an important role in precipitating heart failure in obese individuals.
Keywords: Obesity, left atrial function, atrial remodeling, heart failure, speckle-tracking echocardiography, left atrial strain
Obesity is a strong modifiable risk factor for the development of atrial fibrillation and heart failure.1 An increased LA size has previously been studied as a marker of obesity-associated LA remodeling.2–5 Using LA enlargement as a measure of atrial remodeling might not differentiate compensatory LA remodeling (due to increased cardiac output in obesity6) from the pathologic remodeling (due to increased LA afterload7,8 and/or potential myopathic effects of adipokines 9,10 and other neurohormonal pathways). In contrast to LA volume, LA reservoir function increases in states of increased cardiac output, but decreases with increase in LA afterload and stiffness.11 Therefore, phasic LA dysfunction might better identify adverse atrial remodeling of obesity. 12–14 In this study, we aimed to determine whether overweight and obesity are independently associated with measures of LA dysfunction assessed via volumetric methods and LA longitudinal strain measurements among middle-aged adults from the community enrolled in the Asklepios study.
Methods
The methods and design for the Asklepios study have previously been described. Briefly, the study recruited a cohort of 2524 community-dwelling apparently healthy volunteers aged 35 to 55 years.15,16 A complete echocardiographic examination was performed on 2368 participants using a standardized protocol. None of the participants had apparent cardiovascular diseases (including valvular heart disease) at the time of enrolment (and echocardiography). The Ghent University Hospital Ethical Committee approved the study protocol and participants provided written informed consent.
Doppler-echocardiographic examinations were performed using a Vivid-7 ultrasound platform (Vingmed Ultrasound; Horten, Norway) as previously described in detail.15. Left ventricular (LV) end-diastolic volume and mass were indexed linearly for body surface area. This analysis is based on data from 1,531 participants who had optimal images for LA speckle-tracking (visualizing the entire atrium throughout the cardiac cycle). LA analyses were performed using speckle tracking analyses on EchoPAC (GE Healthcare; Chalfont St. Giles, UK). LA endocardial borders were manually traced in apical 2- and 4-chamber views using atrial diastasis as the point of reference. An automated tracking algorithm was applied, with manual adjustments performed as needed to optimize wall tracking (Figure 1). Time-resolved numerical values derived from speckle tracking were exported from the echoPAC software for further calculations in custom-designed software written in Python (Python Software Foundation, Wilmington, Delaware, USA). We computed longitudinal atrial strain, defined as the change of atrial myocardial length throughout the atrial cycle (L1) compared to its resting (or reference) length (L0) in a relaxed state at diastasis (end of atrial diastole), as (L1-L0)/L0. Strain rate (SR) was calculated as the rate of change in longitudinal strain over time (units: %/sec). Strain and SR were calculated for reservoir, conduit, and booster phases. Additionally, the maximum (LAmax), minimum (LAmin), and diastatic (LAdias) LA volumes were measured. LA expansion index, passive LA emptying fraction (LAEmF), and active LAEmF were calculated as volumetric measures of reservoir, conduit, and booster phases, respectively. LA expansion index was calculated as (LAmax–LAmin)/LAmin, passive LAEmF as (LAmax–LAdias)/LAmax, and active LAEmF as (LAdias–LAmin)/LAmax. Interobserver coefficients of variation were 3.2% for maximum LA volume, 6.7% for minimum LA volume, 2.5% for diastatic LA volume, 4.9% for conduit strain, 4.4% for conduit strain rate, 6.7% for booster strain, 6% for booster strain rate, 4.5% for reservoir strain and 6.5% for reservoir strain rate. Intra-observer coefficients of variation for all volume and strain measures were <6%.
Figure 1: Representative example of LA strain measurements.
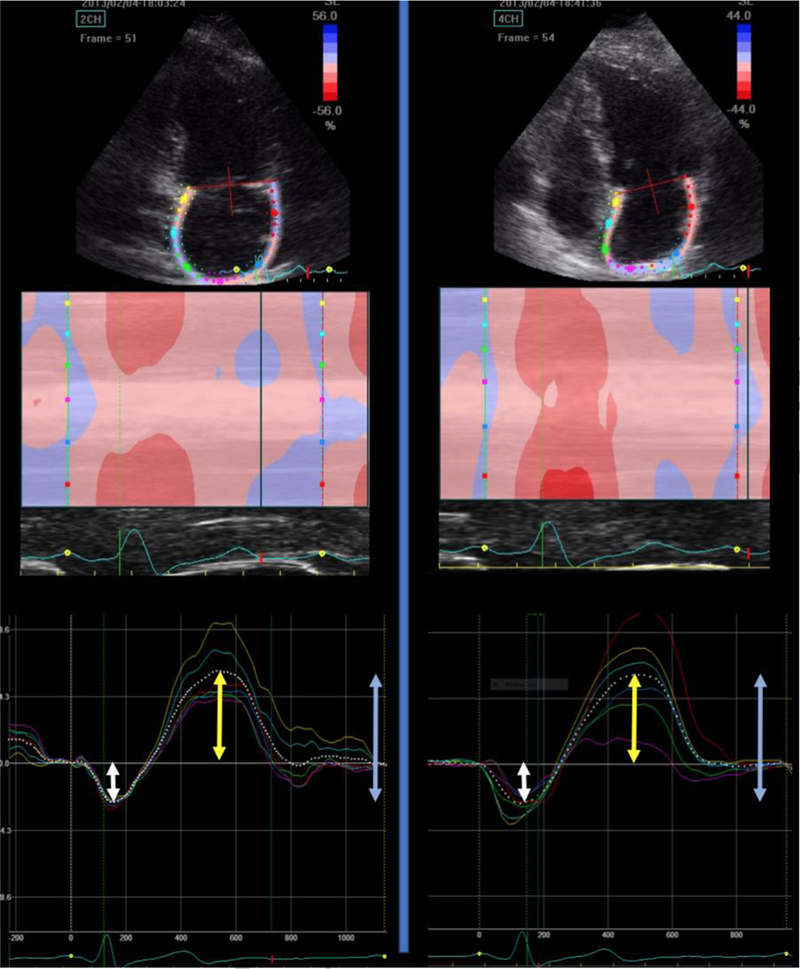
The upper panels demonstrate the region of interest, corresponding to the left atrial wall in apical 4-chamber and 2-chamber views. The middle panels demonstrate a color-coded map of atrial wall deformation over time. The lower panels demonstrate the LA strain curve. Notice that the diastatic phase (end of atrial diastole) is used as the reference phase for LA strain computations. The white arrows demonstrate the amplitude of booster pump strain (atrial kick). The yellow arrows demonstrate the amplitude of conduit strain. The blue arrows demonstrate the amplitude of reservoir strain.
Continuous variables were presented as means and 95% confidence intervals. Categorical variables were shown as total counts with percentages and were compared using the χ2 test or the Fisher’s exact test. We stratified participants into three groups based on the body mass index (BMI): <25 kg/m2 (n=779), 25–29.9 kg/m2 (n=618) and >30 kg/m2 (n=134). Continuous variables were compared between the groups using analysis of variance. Adjusted comparisons between the groups were made with analysis of covariance (ANCOVA). Models were adjusted for age and gender. Additional adjustments were performed for history of hypertension, diabetes mellitus, total cholesterol, high density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, and antihypertensive medication use. A two-sided P value of 0.05 was used to define statistical significance. Post-hoc pairwise comparisons were made with Bonferroni correction for alpha error. Statistical significance was defined as a 2-tailed P<0.05. Analyses were performed using SPSS v24 for Mac (IBM, Chicago IL) and the Statistics and Machine Learning Toolbox in Matlab v2016b (The Mathworks; Natick MA).
Results
A comparison of general characteristics of Asklepios study participants who were included in the analyses (n=1531) vs. those not included (n=993) is shown in Supplemental Table 1. Included participants had a greater proportion of men and a slightly lower average BMI (24.9 vs. 26.3 kg/m2) and burden of cardiovascular risk factors. Table 1 shows demographic, clinical and echocardiographic characteristics of participants included in this analysis, stratified by BMI. Nearly half of the included participants had BMI>25 kg/m2 (n=752, 49%). Overall, the BMI groups 25–29.9 kg/m2 and ≥30 kg/m2 included slightly older participants, with a higher proportion of men. The BMI groups 25–29.9 kg/m2 and ≥30 kg/m2 also included a higher proportion of participants with hypertension, diabetes, and dyslipidemia.
Table 1.
General demographic, clinical and echocardiographic characteristics of normal weight, overweight and obese participants
| Parameter | Normal Weight (n=779) |
Overweight (n=610) |
Obese (n=142) |
P value |
|---|---|---|---|---|
| Mean (95%CI) or count (%) |
Mean (95%CI) or count (%) |
Mean (95%CI) or count (%) |
||
| Age | 44.7 (44.3 to 45.1) | 46.3 (45.8 to 46.7) | 47.2 (46.2 to 48.3) | <0.0001 * # |
| Male sex | 323 (41.46%) | 438 (70.87%) | 89 (66.42%) | <0.0001 |
| Body mass index | 22.3 (22.2 to 22.4) | 27 (26.8 to 27.1) | 32.7 (32.3 to 33) | <0.0001 * # $ |
| Systolic blood pressure | 123 (122 to 123) | 130 (129 to 131) | 134 (131 to 136) | <0.0001 * # $ |
| Diastolic blood pressure | 76 (76 to 77) | 82 (81 to 82) | 87 (86 to 89) | <0.0001 * # $ |
| Pulse pressure | 46 (45 to 47) | 48 (47 to 49) | 46 (44 to 47) | <0.0001 * $ |
| Heart rate | 69 (68 to 69) | 68 (68 to 69) | 74 (72 to 76) | <0.0001 # $ |
| Current smoker | 188 (24.13%) | 134 (21.68%) | 33 (24.63%) | 0.51 |
| Hypertension | 127 (16.30%) | 215 (34.79%) | 78 (58.21%) | <0.0001 |
| Antihypertensive therapy | 33 (4.24%) | 70 (11.33%) | 35 (26.12%) | <0.0001 |
| Diabetes | 1 (0.13%) | 9 (1.46%) | 8 (5.97%) | <0.0001 |
| Medications | ||||
| Aspirin | 5 (0.64%) | 12 (1.94%) | 3 (2.24%) | 0.0637 |
| Lipid lowering therapy | 28 (3.59%) | 36 (5.83%) | 14 (10.45%) | 0.0022 |
| Beta-blocker | 19 (2.44%) | 36 (5.83%) | 12 (8.96%) | 0.0002 |
| ACE inhibitor | 6 (0.77%) | 14 (2.27%) | 9 (6.72%) | <0.0001 |
| ARB | 8 (1.03%) | 11 (1.78%) | 4 (2.99%) | 0.1736 |
| Calcium channel blocker | 3 (0.39%) | 7 (1.13%) | 0 (0.00%) | 0.1399 |
| Laboratory variables | ||||
| Total cholesterol | 208 (205 to 210) | 219 (216 to 221) | 217 (210 to 223) | <0.0001 * # |
| HDL | 66.6 (65.4 to 67.8) | 57.2 (56 to 58.4) | 51.3 (49 to 53.6) | <0.0001 * # $ |
| Triglycerides | 78 (75 to 81) | 107 (103 to 112) | 135 (123 to 147) | <0.0001 * # $ |
| Non-HDL cholesterol | 137 (135 to 140) | 158 (154 to 161) | 162 (155 to 169) | <0.0001 * # |
| Estimated GFR (ml/min) | 91.7 (90.6 to 92.8) | 91.8 (90.6 to 93) | 91 (88.4 to 93.7) | 0.8705 |
| Glucose | 88.8 (88.2 to 89.5) | 91.7 (90.9 to 92.4) | 95.7 (94 to 97.4) | <0.0001 * # $ |
| Echocardiographic variables | ||||
| LV end-diastolic volume index | 57.3 (56.6 to 57.9) | 60.9 (60.1 to 61.7) | 59.5 (57.8 to 61.3) | <0.0001 * # |
| LV ejection fraction | 64.4 (63.9 to 65) | 65.6 (64.9 to 66.2) | 65.5 (64.2 to 66.9) | 0.0270 * |
| LV mass index (g/m2) | 74.7 (73.5 to 75.8) | 86.4 (84.9 to 87.9) | 84.6 (81.4 to 87.8) | <0.0001 * # |
| LV mass index (g/1.7) | ||||
| Relative wall thickness | 0.359 (0.355 to 0.363) | 0.387 (0.382 to 0.392) | 0.409 (0.398 to 0.421) | <0.0001 * # $ |
| Cardiac output, ml/min | 4224 (4161 to 4287) | 4852 (4770 to 4933) | 5322 (5128 to 5516) | <0.0001 * # $ |
| Cardiac index, ml min−1 BSA−1 | 2428 (2394 to 2462) | 2477 (2438 to 2516) | 2503 (2418 to 2588) | 0.0813 |
Significant pairwise comparisons:
Normal weight vs. Overweight
Normal weight vs. Obese
Overweight vs Obese
ACE=angiotensin convertase enzyme; ARB=angiotensin receptor blocker; HDL=high density lipoprotein; GFR=glomerular filtration rate; LV=left ventricle; CI=confidence intervals
Figure 2A and 2B depict the distribution and correlation various measures of phasic LA function and LA size in relation to each other. Measures of reservoir function demonstrated strong correlations with conduit function and moderate correlations with booster function, however, conduit and booster function demonstrated very weak correlations. Interestingly, volumetric and longitudinal strain-based measures correlated weakly, even within domains of LA function, suggesting that transverse contraction accounts for an important proportion of the variability in LA volumetric phasic measures. Finally, LA volume index (LA maximum volume linearly indexed to body surface area) correlated weakly with all measures of phasic LA function.
Figure 2. Panel A shows a matrix of density plots depicting correlation of various measures of LA size and function for the sample. Panel B shows a color correlation map between these measures.
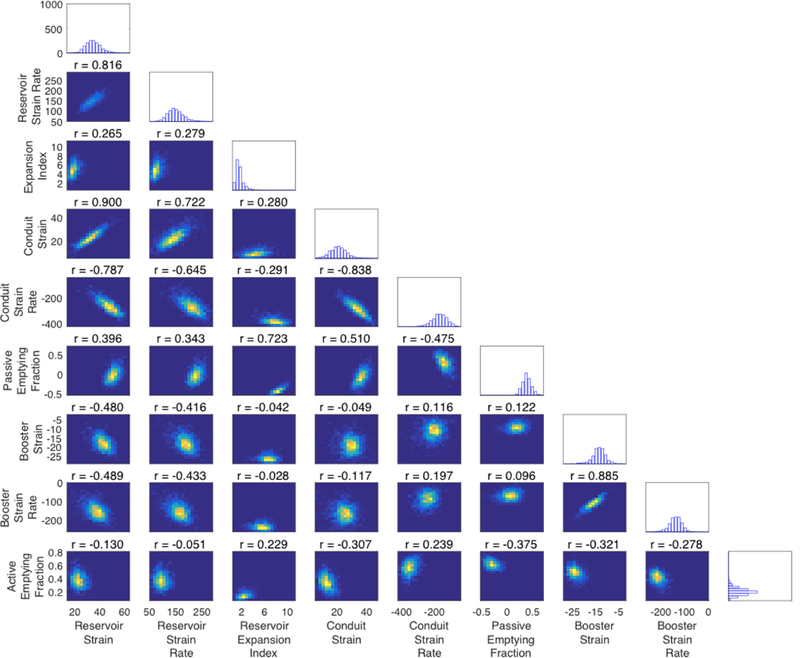

Correlation coefficients are shown in each cell of the color map.
Table 2 and Figure 3 show unadjusted analyses comparing measures of LA size and phasic LA function among various BMI groups. Reservoir function was lower for participants in the BMI 25–29.9 kg/m2 and ≥30 kg/m2 groups as compared to BMI <25 kg/m2 group, as indicated by lower average reservoir strain (35.3% vs. 33.1% vs. 30.9% in BMI <25 kg/m2, 25–29.9 kg/m2 and ≥30 kg/m2 groups respectively, P<0.00001), SR (151%/sec vs. 141%/sec vs. 135%/sec, P<0.00001) and expansion index (1.6 vs. 1.4 vs. 1.4, P<0.00001). In these analyses, participants with BMI≥30 kg/m2 demonstrated lower reservoir longitudinal strain compared to those with BMI 25–29.9 kg/m2, whereas no significant differences were found in reservoir SR and expansion index between BMI 25–29.9 kg/m2 and ≥30 kg/m2 participants.
Table 2.
Unadjusted comparison of LA measures between the BMI groups.
| LA Parameter | Normal Weight | Overweight | Obese | P value |
|---|---|---|---|---|
| Mean (95%CI) | Mean (95%CI) | Mean (95%CI) | ||
| Reservoir Strain | 35.3 (34.8 to 35.7) | 33.1 (32.6 to 33.6) | 30.9 (29.9 to 31.8) | <0.00001 * # $ |
| Reservoir Strain Rate | 151 (149 to 153) | 141 (139 to 143) | 135 (130 to 139) | <0.00001 * # $ |
| Reservoir Expansion Index | 1.6 (1.5 to 1.6) | 1.4 (1.4 to 1.5) | 1.4 (1.3 to 1.5) | <0.00001 * # |
| Conduit Strain | 21.6 (21.1 to 22) | 18.9 (18.5 to 19.3) | 16.7 (15.9 to 17.5) | <0.00001 * # $ |
| Conduit Strain Rate | −189 (−193 to −185) | −166 (−171 to −161) | −150 (−161 to −139) | <0.00001 * # $ |
| Conduit Passive emptying fraction | 40.5% (39.8% to 41.1%) | 36.5% (35.8% to 37.2%) | 36% (34.5% to 37.6%) | <0.00001 * # |
| Booster Strain | −13.9 (−14.1 to −13.6) | −14.3 (−14.6 to −14.1) | −14.1 (−14.7 to −13.5) | 0.03060 * |
| Booster Strain Rate | −144 (−147 to −141) | −148 (−151 to −145) | −145 (−151 to −138) | 0.22551 |
| Booster Active Emptying Fraction | 19.4% (19.1% to 19.8%) | 20.5% (0.20048 to 0.20938) | 21.5% (20.5% to 22.5%) | 0.00001 * # |
| Left Atrial Volume Index | 21.3 (20.9 to 21.8) | 23.4 (22.8 to 23.9) | 22.6 (21.5 to 23.9) | <0.00001 * |
Significant pairwise comparisons:
Normal weight vs. Overweight
Normal weight vs. Obese
Overweight vs Obese
LA=left atrium; BMI=body mass index; CI=confidence intervals
Figure 3: Unadjusted comparisons of reservoir, conduit, and booster function between participant with BMI <25kg/m2, 25–29.9 kg/m2 and >30kg/m2.
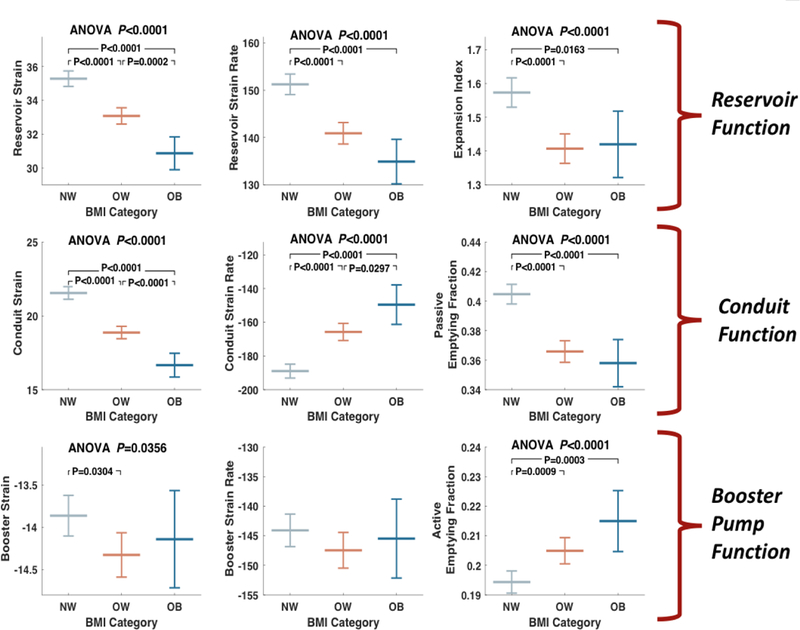
BMI=body mass index; ANOVA=analysis of variance.
Conduit function was lower in the BMI 25–29.9 kg/m2 and ≥30 kg/m2 groups, as indicated by lower conduit strain (21.6% vs. 18.9% vs. 16.7%, P<0.00001), SR (−189%/sec vs. −166%/sec vs. −150%/sec, P<0.00001), and passive LAEmF (40.5% vs. 36.5% vs. 36%, P<0.00001). In these analyses, longitudinal conduit strain and SR, but not the passive LAEmF, were significantly different between the BMI 25–29.9 kg/m2 and ≥30 kg/m2 groups.
In contrast to conduit and reservoir LA function, the active LAEmF was significantly greater in the BMI 25–29.9 kg/m2 and ≥30 kg/m2 groups compared to the BMI <25 kg/m2 group (19.4% vs. 20.5% vs. 21.5%, P<0.00001). Longitudinal booster strain was significantly greater (i.e., more negative) among participants with BMI 25–29.9 kg/m2 (−13.9%, −14.3% and −14.1% in the BMI <25 kg/m2, 25–29.9 kg/m2 and ≥30 kg/m2 groups respectively, P=0.03). In pairwise comparisons, the difference between the BMI <25 kg/m2 and 25– 29.9 kg/m2 groups was significant.
Supplemental Table 2 shows age- and sex-adjusted comparisons of measures of phasic LA function and LA volume index across BMI groups. Overall, the results were similar to those found in unadjusted models, i.e. reservoir and conduit functions were decreased, whereas the active LAEmF was increased in the BMI 25–29.9 kg/m2 and ≥30 kg/m2 groups when compared to the <25 kg/m2 group. Supplemental Table 3 shows the results from models that were further adjusted for history of hypertension, diabetes, antihypertensive medication use, total cholesterol, HDL-cholesterol, triglycerides, and glomerular filtration rate. In these comparisons, participants with a BMI ≥30 kg/m2 demonstrated significantly lower reservoir strain and conduit strain compared to participants in with BMI <25 kg/m2 and 25–29.9 kg/m2. Between-group differences in booster function and LA volume index became non-significant in these models.
Discussion
In this study, which included a large community-based sample of middle-aged adults, we assessed the impact of obesity on volumetric and longitudinal strain-based measures of phasic LA function. We demonstrate that obesity is characterized by reduced reservoir and conduit LA function, with an increase in booster LA function. Our findings suggest that LA booster function may compensate for reduced LA conduit function in uncomplicated obesity in middle-age. These findings contrast with more advanced stages of cardiac dysfunction, which are typically characterized by loss of conduit and booster pump LA function, suggesting that loss of compensatory booster pump function accompanies the progression from uncomplicated obesity to heart failure.
LA enlargement is seen early in the course of obesity. 2–5 Various mechanisms for obesity-related LA enlargement have been postulated, including increased stroke volume/total blood volume in response to the increase in metabolic demands,6 increased LA afterload due to impaired LV lusitropy and/or abnormal LA-LV-aortic coupling,7,8 atrial remodeling related to systemic inflammation and adipokines,10 and paracrine effects from epicardial adipose tissue.9 LA enlargement associated with an increased stroke volume in athletes is considered a compensatory response, and is not associated with adverse coutcomes.17 In the setting of uncomplicated obesity, using LA enlargement as a measure of atrial remodeling might not differentiate expected compensatory responses from the adverse LA remodeling and dysfunction. Recent studies suggest that phasic LA function is prognostic of adverse outcomes independent of LA size;12–14 however, the ability of measures of phasic LA function to characterize obesity-related atrial remodeling and dysfunction has not been previously assessed.
In age- and sex-adjusted analyses, we observed that measures of LA conduit and reservoir function were reduced, whereas booster active emptying fraction was increased in participants in a BMI 25–29.9 kg/m2 and ≥30 kg/m2. Among 386 middle-aged to older participants without structural heart disease, Abou et al. observed that reservoir LA strain was inversely associated with BMI.18 However, they did not assess conduit and booster function. Similar to our findings, in a small sample of 70 community-based healthy participants, Erdem et al. observed significantly lower passive LAEmF and higher active LAEmF in participants with BMI≥30 kg/m2 compared to participants with BMI<30 kg/m2.19 Similar findings were reported in sample samples of participants with hypertension and diabetes with concomitant obesity.20,21 On the contrary, Tugcu et al. did not identify an association between BMI and volumetric measures of reservoir function in community-based elderly adults, although reservoir function was associated with abdominal obesity.22 In comparison to prior studies, we studied phasic LA function using both volumetric and longitudinal LA strain-based measures in a large sample of community-dwelling middle-aged participants. Our findings indicate that obesity is associated with a functional LA phenotype characterized by reduced conduit and reservoir function, with an increase in booster function, which may be compensatory. Interestingly, the progressive reduction in reservoir function in individuals with BMI 25–29.9 kg/m2 and BMI≥30 kg/m2 was more apparent from longitudinal strain-derived measures, whereas the increase in booster pump function was more apparent from volume-based measures of LA function. These findings suggest that transverse contraction of the LA may be important in maintaining LA phasic function with obesity, whereas LA longitudinal reservoir strain may be a more sensitive indicator of LA dysfunction and abnormal LA-LV coupling in early stages of obesity-related myocardial disease.
Cardiac output is a major determinant of LA reservoir function, with increased cardiac output being associated with increased reservoir strain.11 In our analyses, participants with BMIs 25–29.9 kg/m2 and ≥30 kg/m2 demonstrated lower reservoir LA strain despite higher cardiac output compared to participants with a BMI <25 kg/m2. This suggests that LA longitudinal strain can identify obesity-associated LA dysfunction. A similar phenotype of LA remodeling (i.e. lower reservoir/conduit function with higher booster function) has been observed in adults with mild hypertension.23 With the progression of hypertensive heart disease, booster function also declines and is associated with heart failure symptoms.24 It is plausible that a similar reduction of booster function in asymptomatic participants with obesity precedes the onset heart failure symptoms or other complications of obesity (such as atrial fibrillation), although this needs to be assessed in future studies.
Our study is the largest study regarding the effect of obesity of LA phasic function, and is derived from a large community-based sample of middle-aged adults, thus preventing confounding by referral bias. Our study has various limitations. Our study sample was primarily composed of Caucasian individuals of European ancestry, and further studies in racially diverse populations are warranted. Second, ours was a cross-sectional analysis and causality cannot be determined, nor can residual confounding be excluded based on our findings. Not all of our subjects had analyzable LA images, which introduced modest selection bias within our general Asklepios study sample.
In conclusion, in this cross-sectional analysis of a large middle-aged community-based sample, we identified an association between higher BMI and reduced reservoir and conduit LA function, with a compensatory increase in booster function. Our findings suggest that longitudinal LA strain measured using speckle-tracking echocardiography is a sensitive indicator of adverse functional LA remodeling in obesity. Future studies should investigate the role of aggressive risk factor modification in middle-aged adults with obesity and LA dysfunction to reduce the risk of obesity-related cardiovascular comorbidities, specifically heart failure and atrial fibrillation.
Supplementary Material
Acknowledgments
Funding
This research was funded by Fonds voor Wetenschappelijk Onderzoek Vlaanderen research grant G.0427.03 (for the Asklepios Study) and NIH grant R01 HL 121510-01A1 (J.A.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: J.A.C. has received consulting honoraria from BMS, OPKO Healthcare, Fukuda-Denshi, Microsoft, Ironwood, Sanifit, Pfizer, Vital Labs, Bayer, Merck and Akros Pharma. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda-Denshi, BMS and Microsoft. Other authors have no disclosures.
REFERENCES
- 1.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol 2006;26:968–976. [DOI] [PubMed] [Google Scholar]
- 2.Stritzke J, Markus MR, Duderstadt S, Lieb W, Luchner A, Doring A, Keil U, Hense HW, Schunkert H, Investigators MK. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol 2009;54:1982–1989. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension 1995; 25:1155–1160 [DOI] [PubMed] [Google Scholar]
- 4.Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA. Factors Associated With Left Atrial Remodeling in the General Population. Circ Cardiovasc Imaging 2017;10:e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayer JG, Sholler GF, Celermajer DS. Left atrial size increases with body mass index in children. Int J Cardiol 2010;141:61–67. [DOI] [PubMed] [Google Scholar]
- 6.de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation 1981;64:477–482. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers P, Asklepios i. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension 2009;54:558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvaraj S, Martinez EE, Aguilar FG, Kim KY, Peng J, Sha J, Irvin MR, Lewis CE, Hunt SC, Arnett DK, Shah SJ. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circ Cardiovasc Imaging 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahabadi AA, Lehmann N, Kalsch H, Bauer M, Dykun I, Kara K, Moebus S, Jockel KH, Erbel R, Mohlenkamp S. Association of epicardial adipose tissue and left atrial size on non-contrast CT with atrial fibrillation: the Heinz Nixdorf Recall Study. Eur Heart J Cardiovasc Imaging 2014;15:863–869. [DOI] [PubMed] [Google Scholar]
- 10.Ybarra J, Resmini E, Planas F, Navarro-Lopez F, Webb S, Pou JM, Santos A, Ballesta-Lopez C. Relationship between adiponectin and left atrium size in uncomplicated obese patients: adiponectin, a link between fat and heart. Obes Surg 2009;19:1324–1332. [DOI] [PubMed] [Google Scholar]
- 11.Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999;100:427–436. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, Drazner MH. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J 2013;34:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle-Valle TM, Zareian M, Almeida AL, Choi EY, Wu C, Alonso A, Heckbert SR, Bluemke DA, Lima JA. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JA. Association of CMR-measured LA function with heart failure development: results from the MESA study. JACC Cardiovasc Imaging 2014;7:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rietzschel ER, De Buyzere ML, Bekaert S, Segers P, De Bacquer D, Cooman L, Van Damme P, Cassiman P, Langlois M, van Oostveldt P, Verdonck P, De Backer G, Gillebert TC, Asklepios I. Rationale, design, methods and baseline characteristics of the Asklepios Study. Eur J Cardiovasc Prev Rehabil 2007;14:179–191. [DOI] [PubMed] [Google Scholar]
- 16.Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR, Asklepios i Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women [DOI] [PubMed]
- 17.Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, Roselli A, Caselli S, Culasso F. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol 2005;46:690–696. [DOI] [PubMed] [Google Scholar]
- 18.Abou R, Leung M, Tonsbeek AM, Podlesnikar T, Maan AC, Schalij MJ, Ajmone Marsan N, Delgado V, Bax JJ. Effect of Aging on Left Atrial Compliance and Electromechanical Properties in Subjects Without Structural Heart Disease. Am J Cardiol 2017;120:140–147. [DOI] [PubMed] [Google Scholar]
- 19.Erdem FH, Ozturk S, Baltaci D, Donmez I, Alcelik A, Ayhan S, Yaz M. Detection of atrial electromechanical dysfunction in obesity [DOI] [PubMed]
- 20.Tadic M, Cuspidi C, Ilic I, Suzic-Lazic J, Zivanovic V, Jozika L, Celic V. The relationship between blood pressure variability, obesity and left atrial phasic function in hypertensive population. Int J Cardiovasc Imaging 2016;32:603–612. [DOI] [PubMed] [Google Scholar]
- 21.Evin M, Broadhouse KM, Callaghan FM, McGrath RT, Glastras S, Kozor R, Hocking SL, Lamy J, Redheuil A, Kachenoura N, Fulcher GR, Figtree GA, Grieve SM. Impact of obesity and epicardial fat on early left atrial dysfunction assessed by cardiac MRI strain analysis. Cardiovasc Diabetol 2016;15:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tugcu A, Russo C, Jin Z, Homma S, Nakanishi K, Elkind MSV, Rundek T, Sacco RL, Di Tullio MR. Association of body size metrics with left atrial phasic volumes and reservoir function in the elderly. Eur Heart J Cardiovasc Imaging 2017. [DOI] [PubMed]
- 23.Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging 2009;2:93–99. [DOI] [PubMed] [Google Scholar]
- 24.Soullier C, Niamkey JT, Ricci JE, Messner-Pellenc P, Brunet X, Schuster I. Hypertensive patients with left ventricular hypertrophy have global left atrial dysfunction and impaired atrioventricular coupling. J Hypertens 2016;34:1615–1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


