Abstract
Recent reports suggest that in the TKI era, the survival of chronic myeloid leukemia approaches that of general population. The real-world situation may be different. We analyzed patients (≥ 18 years) with chronic phase (CP) CML enrolled over a 7-year period (2002–2008) in an imatinib access program. Event was defined as non-achievement/loss of complete hematological response (CHR), loss of cytogenetic response or progression to accelerated (AP)/blast phase (BC). Progression was defined as development of AP/BC. Any delay of ≥ 1 week in reporting for drug refills was categorized as non-adherence. Of the 443 patients with CP-CML who started imatinib [median age: 36 years (18–70); High risk: 32% (Sokal) and 14% (Hasford/EUTOS)], 162 (37%) had received prior therapy [mostly hydroxyurea (N = 153]. CHR was achieved by 430 (97%). After a median follow up of 109.5 months (3.4–184.3), the EFS, PFS and OS at 10 years was 43%, 75% and 76% respectively. Superior EFS was predicted by low-risk Hasford score and adherence to therapy. Adherence to therapy was the only factor which predicted EFS on multivariate analysis (HR 0.64, 95% CI 0.50–0.83, P = 0.001). Long-term follow up of patients with CP-CML reflects poorer survival than those reported from clinical trials and reflects multiple issues that affect “real-world” patients. The continued drop in EFS, noted during long-term follow up, might take time to impact the PFS and OS due to the chronic nature of the disease. Sustained adherence to therapy is important for optimum long-term outcomes.
Keywords: Chronic myeloid leukemia, Imatinib, Long-term outcomes, 10-Year survival, Adherence
Introduction
Imatinib and other tyrosine kinase inhibitors (TKIs) have changed the paradigm of therapy in chronic myeloid leukemia. Though imatinib was quite expensive when first launched, it was made available to patients in India through various schemes [1, 2]. At our center, we have been using imatinib since 2002 through a company sponsored program where the medicine was supplied free of cost to patients [3]. Imatinib was a landmark in the treatment of CML and many reports suggest that chronic phase CML patients treated with this drug have a similar survival as the general population [4, 5]. The most recent update from the practice changing IRIS (International Randomized Study of Interferon and STI571) trial shows a 10-year overall survival of 83% in those treated with imatinib [6]. Another study showed that only about 44% of the deaths were due to CML among patients on long-term imatinib and more than half the patients who died had other causes [7].
These dramatic survival results are accompanied by certain caveats. Much of these excellent long-term survival data comes from patients treated in clinical trials where the stringency of monitoring, follow up and commitment of patients is very high. Additionally, in the setting of a protocol, the access to study drug is assured at all times. There are concerning reports, even from affluent countries, that the long-term outcomes are dependent on multiple factors, like baseline characteristics [7], drug adherence and quality of life [8] and assured access to medicines through various insurance schemes [9, 10]. Even among high-income countries, outcomes may vary [10, 11]. Very little is known about long-term outcome data from real-world settings, especially from developing countries like India which face unique challenges [1, 2]. We analyzed long-term outcomes of patients with CML treated at a single center where most of the patients were initially supported through a medication access program.
Methods
We had earlier reported our outcomes of CP-CML based on the retrospective review of patients treated at our center between January 2002 and December 2008 with imatinib [12]. Of the 516 patients in the original report, we excluded pediatric patients (N = 29) and patients whose files could not be traced for update (N = 44). The available data from 443 patients was used for the current analysis. All these patients were originally enrolled in the GIPAP (Glivec International Patient Assistance Program) and received free imatinib. Though many of them continued on the scheme, some of them later moved to other support programs for continuing medication and some were switched to generic imatinib. These patients were followed weekly initially, thereafter monthly and then once in 3 months, once the counts stabilized. They were counselled to have cytogenetic or molecular studies at least once a year, but this was not strictly enforced.
The baseline investigations included complete blood counts, chemistries, as well as bone marrow aspiration and biopsy. CML was diagnosed by morphology and demonstration of the Philadelphia chromosome, or by the demonstration of the BCR-ABL transcript. Patients who had received hydroxyurea or busulfan prior to starting imatinib were included. All patients were started with imatinib 400 mg/day. Drug dose adjustment policies and assessment of adherence were according to our earlier report [12]. Patients were considered as non-adherent if they had a documented treatment interruption for more than 1 week at any point of time unless it was as per physician advice as a result of toxicities. This data was derived from the patient record. The cutoff of 1 week was arbitrarily chosen as reported in our earlier paper. Second line TKIs were offered whenever feasible, but only 19 (4%) received 2nd line TKIs (nilotinib or dasatinib) in this cohort. In patients who did not receive second line TKIs attempts were made to give higher doses of imatinib (600–800 mg/day). The impact of subsequent therapies was not separately analyzed.
Data update and statistical analysis For the purpose of this update, we looked into the records for occurrence of events. The event definition was slightly modified to include > 1% BCR ABL transcripts as an event (equivalent to loss of cytogenetic response) as many patients switched to molecular testing from earlier cytogenetic testing. The other definitions for events were as per the original paper (non-achievement of CHR or loss of CHR, loss of CCR, progression to AP/BC, or death due to any cause).
Data was censored as on 1st September 2017. The data for patients who had lost follow-up was censored on the date of last visit and their disease status was updated as on that date. Attempts were made to identify the life status (alive/dead) by making phone calls/through letters/or by contacting the nearest post office/police station through our tumor registry. For patients who were known to be dead, but the exact disease status or cause of death could not be determined, the death date was noted and also coded as an event. Event-free (EFS) and overall survival (OS) from the date of starting imatinib were estimated using the Kaplan–Meier method. Progression free survival (PFS) was calculated from date of start of imatinib till occurrence of accelerated or blast phase. The baseline Hasford [13], EUTOS (European Treatment and Outcome Study) [14] and Sokal [15] scores, prior therapy and adherence to treatment were analyzed to identify predictors of survival.
Results
Out of 443 patients with CP-CML who started imatinib in the period, the median age was 36 years (18–70); 301 (68%) males (Table 1). About one-third (N = 162, 37%) had received prior therapy for CML, most commonly hydroxyurea [N = 153; median duration of use: 3 months (2–80)]. As per risk stratification, 32% were high risk by Sokal and 14% high risk by Hasford/EUTOS scores. All patients started imatinib at 400 mg per day. Complete hematological response (CHR) was achieved in 404 patients (91%) within the first 3 months and 430 (97%) patients achieved CHR at some point. Only 19 patients (4%) of the entire cohort received second line TKI at any point of time.
Table 1.
Patient characteristics at baseline (n = 443)
| Characteristics (n = 443) | N (%) |
|---|---|
| Age, years | |
| Range | 18–70 |
| Median | 36 |
| Age category | |
| ≤ 35 years | 211 |
| > 35 years | 232 |
| Time from diagnosis | |
| ≤ 3 months | 329 (74) |
| > 3–≤ 6 months | 43 (10) |
| > 6 months–≤ 12 months | 26 (6) |
| > 12 months | 45 (10) |
| Male:female | 301:142 |
| Use of non-TKI medicationsa | 162 (37) |
| Sokal risk category | |
| Low | 97 (22) |
| Intermediate | 206 (47) |
| High | 140 (32) |
| Hasford risk category | |
| Low | 192 (43) |
| Intermediate | 191 (43) |
| High | 60 (14) |
| EUTOS risk category | |
| Low | 379 (86) |
| High | 64 (14) |
aPrior therapy given for at > 1 month prior to start of imatinib—153 received hydroxyurea, 11 received busulfan and 5 received interferon
The median follow-up was 109.5 months (range 3.4–184.3) and among surviving patients it was 121 months (range 6.7–184 months). At least 5 years follow-up was available in 78.1% patients and at least 10 years follow up in 41.3% patients. During the last follow up, the following events were documented in 255 out of 443 patients: Non-achievement of CHR (N = 13, 2.9%), loss of CHR after initially achieving CHR (N = 56, 12.6%), loss of complete cytogenetic response (N = 46, 9.9%), progression to accelerated or blast phase (N = 122, 28%), death cause undetermined (N = 18, 4%).
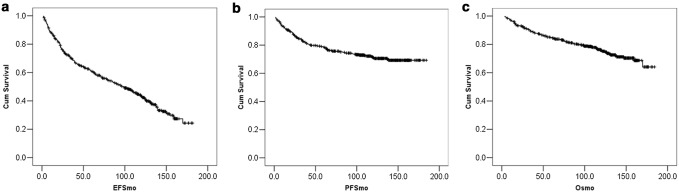
The median EFS (Fig. 1) was 98.5 months (95% CI 81–116) and the actuarial EFS at 5 and 10 years were 60.8% (± 0.024 S. E) and 43.1% (± 0.026 S. E) respectively (Fig. 1a). Superior EFS (Table 2), was predicted by low-risk Hasford score and adherence to therapy (Fig. 2a, b). Adherence to therapy was the only factor which predicted EFS on multivariate analysis (HR 0.64, 95% CI 0.50–0.83, P = 0.001).
Fig. 1.
Survival outcomes. The Kaplan–Meier curves depict the long-term event-free (EFS-1a), progression-free (PFS-1b) and overall survival (OS-1c) of the patients with chronic phase treated with imatinib
Table 2.
Factors affecting survival- univariate analysis
| Parameter | N | 10 yr EFS % | P value | 10-yr PFS % | P value | 10 yr OS % | P value |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| ≤ 35 years | 211 | 46.8 | 0.128 | 76 | 0.57 | 78.5 | 0.1 |
| > 35 years | 232 | 40.3 | 74 | 73.5 | |||
| Sokal risk group | |||||||
| Low | 97 | 51.3 | 0.098 | 80 | 0.17 | 77.5 | 0.42 |
| Intermediate/high | 346 | 40.6 | 74 | 75.5 | |||
| Hasford score | |||||||
| Low | 192 | 50.2 | 0.028 | 81 | 0.018 | 79.3 | 0.06 |
| Intermediate/high | 251 | 37.6 | 71 | 73.1 | |||
| EUTOS score | |||||||
| Low | 379 | 43.6 | 0.62 | 77 | 0.009 | 76.3 | 0.48 |
| High | 64 | 40.0 | 63 | 74.0 | |||
| Adherence | |||||||
| Adherent | 304 | 48.9 | 0.001 | 77 | 0.35 | 77.2 | 0.65 |
| Non-adherent | 139 | 30.7 | 69 | 72.4 | |||
| Prior treatment | |||||||
| Absenta | 284 | 45.2 | 0.086 | 78 | 0.095 | 78.1 | 0.115 |
| Present | 159 | 39.6 | 71 | 72.0 | |||
aUse of hydroxyurea up to 1 month before starting imatinib was considered as no prior therapy
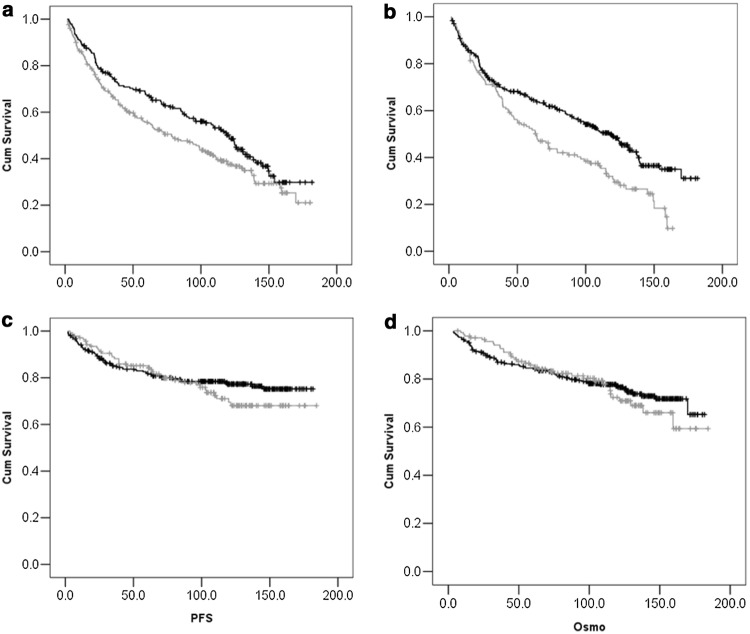
Fig. 2.
Factors affecting EFS. a shows the difference between EFS among those with low risk (black line) and high risk (gray line) Hasford scores at baseline. b shows the difference in EFS between those who were adherent (black line) and non-adherent (gray line) to therapy. c and d shows the difference in PFS(2c) and OS (2d) among those who were adherent and non-adherent to therapy respectively
The median PFS was not reached. The actuarial PFS at 5 and 10 years were 83% (± 0.018) and 75% (± 0.022 S.E) respectively (Fig. 1b). Higher Hasford and EUTOS score predicted for poorer PFS on univariate analysis (Table 2). Though there was poorer PFS in the less adherent group, it was not statistically significant. On analyzing the representative Kaplan–Meier graph looking at PFS and adherence we can see the curve separation starting later (Fig. 2c). CML being a chronic disease, it is likely that the impact of non-adherence on PFS takes time to manifest. Longer follow-up times might better reflect the impact of initial non-adherence on outcomes.
The median overall survival (OS) has not been reached. The actuarial OS at 5 and 10 years was 84.9% (± −0.17 S.E) and 75.9% (± −0.022 S.E) respectively (Fig. 1c). On univariate analysis (Table 2), there was a trend towards better OS in those with lower Hasford scores but no factor was statistically significant. Similar to the PFS curves, the OS curve also shows a late separation favouring patient with treatment adherence again reflecting that the impact on life may take time to manifest in a chronic condition like CML (Fig. 2d). Of the 108, patients who died, 84 (77%) died due to progressive CML, 5 persons died due to other causes (4 due to sepsis, 1 due to ischemic heart disease). In 18 patients, death had occurred but the cause could not be determined.
Discussion
This is one of the first reports demonstrating long-term outcomes of patients with CML treated in a non-trial setting in a developing country. The overall survival was 76% at 10-years which is lower than that reported from other studies [6]. Significantly, there was a continued drop in the event-free survival curves to 43% at 10 years. Hasford score predicted outcome as did poor adherence to therapy which was associated with an inferior EFS (30% at 10 years). This study provides a more sober, “real-world” outcome data on the impact of the “magic bullet” imatinib.
Our data contrasts with the results of the some recent studies, where long-term survival was similar to the general population. Results from the IRIS trial and the CML IV trial showed a 10-year OS of 82–83% [6, 16]. Combined analysis of over 2000 patients from various European trials in CML showed an 8-year OS of 89% [7]. High-volume centers like the MD Anderson Cancer Center where many patients are treated on protocols, have also reported high long term survivals of over 85% [17]. Compared to these, our study shows a survival of 76% at 10 years which is significantly lower. Moreover, the PFS in our study (71%) was also lower than reported from clinical trials (80% in CML IV and 92% in IRIS) [6, 16]. Patients treated in clinical trials are likely to be more carefully selected, more committed, and better monitored and as a result may be expected to have better outcomes. On the other hand, patients treated in routine practice suffer from many challenges. Even within developed countries, treatment in academic centers has been associated with better survival [18]. The 5-year relative survival in patients with CML was lower than the general population and was lower in Germany than in the US (68% in Germany and 72% in the US) [10, 11]. Even in a trial setting, specifically looking at high risk patients, the 6-year EFS was only 44% with no plateau in the curves [4]. What impact this will have on PFS and OS when followed up over a still longer period remains to be seen.
Sustained drug availability is an important consideration in long-term outcomes of chronic diseases. Lack of insurance support leading to poorer outcomes has been described in CML [8]. At our center, though many patients were originally enrolled in the GIPAP program, over a period, this program had been phased out and a number of patients had to be enrolled in other patient support programs where drug availability was not consistent. Another important issue is medication non-adherence, which has been unequivocally associated with poorer outcomes in CML [19, 20]. In our study, adherence was a strong predictor of EFS with patients who were non-adherent, having EFS of only 30% at 10 years. It is possible that patients who were non-adherent and lost remission, and thus had an event, might have regained responses after resuming adherent behavior. This could account for the lack of impact of adherence on OS. We note that the OS and PFS curves flatten over time but the EFS curves continues to fall over long follow up times. However, the fact that 28% of the patients have suffered progression to AP/BC in our study, compared to less than 10% reported in clinical trials; suggest that there exists a persistent significant negative impact of non-adherence to therapy. Though the impact of adherence on OS was not statistically significant at this point, we believe that this will also manifest at some point with longer follow up.
The discontinuation rates of first-line imatinib is around 40%, about half of this is due to intolerance and half due to treatment failure [21]. Hence, the availability and affordability of 2nd line TKI is very important for sustaining long-term outcomes. In our cohort, only 19 patients (4%) went on to receive either nilotinib or dasatinib. Contrast this with the data from the CML IV study where 27% patients switched to 2nd line TKIs [16].
There are multiple shortcomings in this retrospective analysis. The response assessment was not consistently done and the method also changed from FISH and cytogenetics to molecular based methods. Adherence was assessed based on patient reporting for refills—this method can under-diagnose non-adherence. The reasons for non-adherence in a particular patient (financial vs. logistic) could not be captured. Another study from our center has shown that a prospective questionnaire based method shows a non-adherence rate of over 50% compared to the 30% reported here [22]. Quantification of adherence was similarly not possible from this analysis, similarly the exact time point of when the patient became non-adherent could not be captured. We had about a quarter of patients who had received > 3 months of prior treatment before starting imatinib. This could be another reason for poorer outcomes, but this was not borne out in the analyses (Table 2).
Despite these limitations, this analysis reports on very mature survival outcomes of CML in a real-world setting in a developing country. Though the long-term overall survival of CML is still very good, especially when compared to pre-TKI era, it is doubtful whether it will ever approach that of an age-matched general population. Though we have not actually compared to a corresponding general population, we expect the survival to be higher than 76% at 10 years in a population with a median age of only 36 years. Poor adherence is a major cause of failure of therapy, which is multi-factorial and needs to be addressed pro-actively to ensure optimal outcomes. Another important takeaway from this analysis is the need for very long-term follow-up of CML and continued patient motivation to stick with the medications. All care-givers must take efforts to address long-term issues, especially the minor side effects which impact the quality of life. A holistic and intense program of follow up involving multiple dimensions may be needed to achieve optimum outcomes in CML.
Acknowledgements
We acknowledge the support from the Glivec International Patient Assistance Program and the Tamilnadu Government Insurance Scheme for supporting the medication for the patients. We thank Ms Tamilselvarani and Ms Vanitha N for helping patient coordination and data collection.
Author Contribution
Conceptualization, data curation, investigation: all authors. Manuscript writing: PG, NM, JPK, TSG. Supervision and resources: PG, TGS.
Funding
The study was supported by Cancer Institute (WIA) funds. No grant number is applicable.
Compliance with Ethical Standards
Conflict of interest
None of the authors have any relevant conflicts of interests to declare.
References
- 1.Ganesan P, Kumar L. Chronic myeloid leukemia in India. J Glob Oncol. 2017;3(1):64–71. doi: 10.1200/JGO.2015.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman JM. Chronic myeloid leukemia in India. Indian J Med Paediatr Oncol. 2013;34(3):147–148. doi: 10.4103/0971-5851.123700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glivec® International Patient Assistance Program (GIPAP). http://www.themaxfoundation.org/gipap/Default.aspx. Accessed 31 Mar 2018
- 4.Castagnetti F, Gugliotta G, Breccia M, Stagno F, Iurlo A, Albano F, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29(9):1823–1831. doi: 10.1038/leu.2015.152. [DOI] [PubMed] [Google Scholar]
- 5.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. doi: 10.1200/JCO.2015.66.2866. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–927. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfirrmann M, Baccarani M, Saussele S, Guilhot J, Cervantes F, Ossenkoppele G, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48–56. doi: 10.1038/leu.2015.261. [DOI] [PubMed] [Google Scholar]
- 8.Flynn KE, Atallah E. Quality of life and long-term therapy in patients with chronic myeloid leukemia. Curr Hematol Malig Rep. 2016;11(2):80–85. doi: 10.1007/s11899-016-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry AM, Brunner AM, Zou T, McGregor KL, Amrein PC, Hobbs GS, et al. Association between insurance status at diagnosis and overall survival in chronic myeloid leukemia: a population-based study. Cancer. 2017;123(13):2561–2569. doi: 10.1002/cncr.30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulte D, Barnes B, Jansen L, Eisemann N, Emrich K, Gondos A, et al. Population level survival of patients with chronic myelocytic leukemia in Germany compared to the US in the early 21st century. J Hematol Oncol. 2013;6(1):70. doi: 10.1186/1756-8722-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pulte D, Jansen L. Population-level survival for patients with chronic myeloid leukemia: higher survival in Sweden than internationally. J Clin Oncol. 2017;35(6):695–696. doi: 10.1200/JCO.2016.69.6849. [DOI] [PubMed] [Google Scholar]
- 12.Ganesan P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86(6):471–474. doi: 10.1002/ajh.22019. [DOI] [PubMed] [Google Scholar]
- 13.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90(11):850–858. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 14.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–799. [PubMed] [Google Scholar]
- 16.Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017;31(11):2398–2406. doi: 10.1038/leu.2017.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantarjian H, O’Brien S, Jabbour E, Garcia-Manero G, Quintas-Cardama A, Shan J, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–1987. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geelen IGP, Thielen N, Janssen JJWM, Hoogendoorn M, Roosma TJA, Willemsen SP, et al. Impact of hospital experience on the quality of tyrosine kinase inhibitor response monitoring and consequence for chronic myeloid leukemia patient survival. Haematologica. 2017;102(12):e486–e489. doi: 10.3324/haematol.2017.175265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noens L, Hensen M, Kucmin-Bemelmans I, Lofgren C, Gilloteau I, Vrijens B. Measurement of adherence to BCR-ABL inhibitor therapy in chronic myeloid leukemia: current situation and future challenges. Haematologica. 2014;99(3):437–447. doi: 10.3324/haematol.2012.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geelen IGP, Thielen N, Janssen JJWM, Hoogendoorn M, Roosma TJA, Willemsen SP, et al. Treatment outcome in a population-based, ‘real-world’ cohort of patients with chronic myeloid leukemia. Haematologica. 2017;102(11):1842–1849. doi: 10.3324/haematol.2017.174953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unnikrishnan R, Veeraiah S, Mani S, Rajendranath R, Rajaraman S, Vidhubala Elangovan GS, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk. 2016;16:366–371. doi: 10.1016/j.clml.2016.02.040. [DOI] [PubMed] [Google Scholar]