Abstract
Purpose
The influenza B virus diverges into two antigenically distinct lineages: B/Yamagata and B/Victoria. Influenza B is the dominant circulating virus during some influenza seasons, and recent data demonstrated that influenza A and B infection similarly cause severe clinical symptoms in hospitalized patients. Nucleoprotein (NP) is a good target for a universal influenza vaccine. This study investigated whether NP epitope variation within two lineages affects the dominant cytotoxic T lymphocyte (CTL) responses induced by vaccination and the resultant protective immunity.
Materials and Methods
The NP of B/Yamagata/16/1988, the representative strain of the Yamagata lineage, includes a dominant CTL epitope, FSPIRITFL, while B/Shangdong/7/1997 from the Victoria lineage has one amino acid difference in this sequence, FSPIRVTFL. Two recombinant replication-deficient adenovirus (rAd)-vectored vaccines expressing either NP were prepared (rAd/B-NP(I) and rAd/B-NP(V), respectively) and administered to BALB/c mice intranasally. To examine the efficacy of vaccination, antibody responses, CTL responses, and morbidity/mortality after challenge were measured.
Results
Both vaccines induce similar antibody and CD8 T-cell responses cross-reacting to both epitopes, and also confer cross-protection against both lineages regardless of amino acid difference.
Conclusion
The rAd-vectored vaccine expressing the NP could be developed as universal influenza B vaccine which provides broader protection.
Keywords: Influenza B virus, Cross protective immunity, Nucleoproteins, Cytotoxic T lymphocytes, Recombinant adenovirus, Epitope
Introduction
The influenza virus is a single-stranded and negative-sense RNA virus classified under the Orthomyxoviridae family. There are three types of influenza viruses: A, B, and C. Both A and B are co-circulating and induce seasonal influenza, a respiratory tract disease that causes high mortality in humans [1,2]. Influenza mutation is mainly caused by antigenic drift and antigenic shift. There are two types of envelope glycoproteins on the surface of the influenza viral particle: hemagglutinin (HA) and neuraminidase (NA), which mediate entry of the viral genome to the target cell and the release of progeny viruses from infected cells [3]. Accumulation of point mutation at HA and NA leads to progressive antigenic changes known as antigenic drift [4]. On the other hand, large antigenic changes can occur when the influenza virus acquires entirely different antigens. This phenomenon is known as antigenic shift and has a risk of triggering a pandemic shift [5]. For these reasons, many researchers have emphasized the need for a vaccine that can protect against a broad range of influenza viruses.
Unlike the influenza A virus, which is comprised of several subtypes, the influenza B virus has two antigenically distinct lineages, Yamagata and Victoria, as determined by phylogenic studies in the 1980s [6]. The influenza B virus only infects humans, seals and ferrets [7,8]. Mutation of the virus is 2–3 times slower than that of influenza A [9], meaning it has little potential for pandemics. Consequently, many studies on cross immunity have been focused on the influenza A virus. However, the two lineages of the influenza B virus have been a menace to public health since the 1980s, when they co-circulated in the human population. Surveillance data suggested that influenza B activity increased in the United States and Europe. In the United States, it was confirmed that 22% to 44% of deaths in the pediatric influenza patient group in each season between 2004 and 2011 were influenza B-related, with the exception of the 2009–2010 pandemic [10]. Due to many factors like variable populations, competition between the two lineages of influenza B, urbanization and travel, the influenza B virus has become more variable and more spreadable [11]. Moreover, it is remarkable that antigenic mismatches between the B strains selected for the seasonal influenza vaccine and actually circulating B strains occurred 5 times over 10 seasons [12]. Nevertheless, there is a lack of studies on the development of a vaccine that can protect against both influenza B virus lineages.
Nucleoprotein (NP) is a highly conserved protein involved in the transcription and duplication of viral genomes and influences the host specificity and virulence of viruses [13]. When a host is infected with the influenza virus, cytotoxic T lymphocytes (CTLs) recognize NP as the main antigen of viral protein. CTLs kill virus-infected cells after recognition of the peptide on infected cells presented by major histocompatibility complex (MHC)-I molecules and subsequently eliminate the viruses. Targeting highly conserved antigens like NP has become one of the major strategies for the development of cross-protective influenza vaccines. Numerous studies have demonstrated the ability of influenza A virus-specific CTLs which can confer protective immunity against antigenically distinct influenza A virus strains, and the CTLs are mostly directed to highly conserved internal proteins [14,15,16]. Furthermore, it has been demonstrated that these CTLs contribute to broad ranges of heterosubtypic protective immunity [17,18,19,20]. Our previous study have identified an immunodominant CTL epitope in NP of B/Yamagata/16/88 [21]. Interestingly, this CTL epitope presents one amino acid difference compared to the sequence of B/Shangdong/7/97, the virus of the Victoria lineage. This study focused on whether the CTLs induced by different epitopes affect cross-protection. To determine the potential for cross-protection, two replication-defective adenovirus (rAd)-vectored vaccines expressing NP (rAd/B-NP) with different epitope sequences were constructed. rAd vectors are known to induce considerable protective immune responses [22]. Immunization with rAd vaccines expressing various antigens of influenza has been reported to confer cross-protective immunity [23] and only a single dose of intranasal vaccination with rAd-encoding NP protected mice from infection by a heterologous influenza A virus including the H1N1 pandemic strain [24]. This study showed that both rAd/B-NP vaccines induce similar CTL responses despite different epitope sequences. Moreover, induced CTLs are cross-protective against viruses of the B/Yamagata and B/Victoria lineages.
Materials and Methods
Animals and virus strains
Six-week-old female BALB/c mice were purchased from Orient Bio (Seoul, Korea) and were housed under specific pathogen free conditions in the experimental facility. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC, Approval No. 18-005) of Ewha Womans University.
Mouse-adapted B/Yamagata/16/1988 (B/Yamagata) and B/Shangdong/7/1997 (B/Shangdong) were provided by Dr. Baek Lin Seong for this study (Yonsei University, Seoul, Korea). Both viruses were grown in Madin-Darby canine kidney (MDCK) cells. After 2 days of infection, the culture supernatant of infected MDCK cells was removed by centrifugation at 600×g for 10 minutes (1580GRM, Gyrozen, Daejeon, Korea). Viral stocks were stored at −70℃.
Cells
MDCK cells and HEK293 cells were cultured in minimum essential medium (Life Technologies, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS).
Construction of rAd expressing NP(I)
From the pShuttle-cytomegalovirus (CMV) vector containing the NP of B/Yamagata, a mutated NP sequence with guanine substituted for adenine at nucleotide position 511 was generated with a site-directed mutagenesis kit (Intron, Seongnam, Korea) using a forward primer (5′-TTCAGCCCTATAAGAGTTACCTTTTTAAAAG-3′) and reverse primer (5′-CTTTTAAAAAGGTAACTCTTATAGGGCTGAA-3′). Consequently, the 171th amino acid became valine instead of isoleucine. As described previously [25], homologous recombination resulted in NP sequence insertion into the adenoviral genome. To produce the rAd/B-NP virus, recombinant adenoviral DNA containing the NP gene was transfected into human embryonic kidney 293 (HEK293) cells. The control adenovirus rAd/mock was generated after transfection of a vacant pShuttle-CMV vector using the same method. Amplification of rAd was performed on HEK293 cells and purification was carried out by CsCl2 density-gradient ultracentrifugation. Infection with 0.002 multiplicity of infection of rAd/B-NP enabled the confirmation of NP expression by western blot using mouse polyclonal B/Yamagata-specific serum and horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (Abcam, Cambridge, UK) as a secondary antibody. Cell lysates from the rAd/B-NP infected HEK293 were prepared after resuspending the cell pellets in a buffer containing 50 mM Tris-HCl, 1% NP-40, 0.5% sodium deoxycholate, 150 mM sodium chloride, and 0.1% sodium dodecyl sulfate. Afterward, the supernatant was separated from the pellet by centrifugation.
Vaccination and challenge
The mice were vaccinated with either rAd/B-NP(I) or rAd/B-NP(V) via the intranasal route. Control mice were inoculated with the control adenovirus, rAd/mock. For intranasal immunization, mice were lightly anesthetized with isoflurane (Ifran, Hana Pharm, Hwaseong, Korea), and 1×108 plaque-forming units (PFUs) of rAd/B-NP(I), rAd/B-NP(V), or rAd/mock were applied to the left nostril at a volume of 50 µL in phosphate-buffered saline (PBS). Lightly anesthetized mice were challenged intranasally with 5 lethal doses (5LD50) of B/Yamagata/16/1988 or B/Shangdong/7/1997 3 weeks after immunization. All animal studies complied with the guidelines of the Ewha Womans University Institutional Animal Care and Use Committee (Approval No. 18-005).
Enzyme-linked immunosorbent assay
Acquisition of blood from the retro-orbital plexus of lightly anesthetized mice by isoflurane (Ifran, Hana Pharm) was performed using a heparinized capillary tube. After centrifugation at 5,800 rpm for 15 minutes, the collected serum was stored at −70℃. Antibody titers against influenza NP were measured using enzyme-linked immunosorbent assay (ELISA). Briefly, the coating antigen was prepared by disrupting each virus, B/Yamagata and B/Shangdong, with 0.5% Triton X-100 (Sigma, St. Louis, MO, USA). Next, 3,600 PFUs of the split influenza virus were added to each well of a polystyrene microtiter plate and incubated overnight at 4℃. After blocking with 1% of non-fat milk and 0.05% Tween 20 for 2 hours at room temperature (RT), serially diluted samples of sera were added and incubated for 2 hours. All wells were washed with PBS containing 0.05% Tween 20. As a secondary antibody, HRP-conjugated rabbit anti-mouse IgG (Abcam) was incubated for 1 hour at RT to measure NP-specific IgG in the sera. For color development, 3,3′,5,5′-tetramethylbenzidine (KPL, Gaithersburg, MD, USA) was added and the reaction was stopped by adding 1 M H3PO4. The color development was analyzed at 450 nm using the Thermo Multiskan EX (Vantaa, Finland).
Tetramer assay
To obtain streptavidin-PE-conjugated tetramer, biotinylated Dd/NP166–174 monomeric protein was purified and tetramerized at an 8:1 molar ratio with streptavidin-PE (Invitrogen, San Diego, CA, USA) according to the manufacturer's protocol. At day 10 post challenge, each group of mice was sacrificed and tracheotomy was performed. The lungs were perfused with 5 mL of PBS with 10 U/mL heparin (Sigma) using a syringe with a 25-gauge needle. The lung tissues were homogenized with 3 mL of Iscove's Modified Dulbecco's Medium (IMDM) through 70-µm cell strainers to obtain single-cell suspensions. Centrifugation was conducted afterward, lymphocytes were resuspended in fresh IMDM and washed with fluorescence-activated cell sorting (FACS) buffer (0.5% FBS and 0.09% NaN_3 in PBS). After blocking with anti-mouse CD16/CD32 (BD Biosciences, Franklin Lakes, NJ, USA), cells were stained with anti-CD8a APC (clone 53-6.7, Biolegend, San Diego, CA, USA), anti-CD44 FITC (clone IM7, Biolegend), and Dd/NP(I) tetramer-PE or Dd/NP(V) tetramer-PE. After staining, the cells were washed and fixed with FACS lysing solution for 15 minutes at RT in the dark. Cells were washed with FACS buffer twice and stored at 4℃ in the dark until FACS analysis.
Intracellular cytokine staining
For cytokine-producing cell analysis, 1×106 lung lymphocytes resuspended in IMDM with 10% FBS were prepared. As a positive control, cells were treated with 50 µg/mL phorbol 12-myristate 13-acetate (1:1,000) and 500 µg/mL ionomycin (1:1,000). NP-specific stimulation was conducted with 10 µM B-NP(I) peptide (FSPIRITFL) or B-NP(V) peptide (FSPIRVTFL). The cells were also treated with 10 ng of recombinant human IL-2 protein (Biolegend) and Brefeldin A (1:1,000, eBioscience, San Diego, CA, USA), and were incubated for 5 hours at 37℃. Stimulated cells were blocked with anti-mouse CD16/32 for 10 minutes at RT and stained with anti-CD8a PE (clone 53-6.7, Biolegend) and anti-CD44 FITC (clone IM7, Biolegend) for 30 minutes at 4℃ in the dark. After permeabilization, intracellular cytokines were stained with anti-interferon-γ (IFN-γ) antibody (clone XMG 1.2, Biolegend) for 30 minutes at RT in the dark. The cells were washed twice and stored at 4℃ in the dark until FACS analysis. All stained cells were analyzed using FACSCalibur (BD Biosciences), and data were analyzed with FlowJo software (TreeStar Inc., Ashland, OR, USA).
Statistical analysis
All experimental data were expressed as mean±standard error of mean (n=3–8) and were compared with one-way ANOVA or two-way ANOVA. Bonferroni's procedure is used for post-hoc analysis and differences were considered statistically significant when p-values were <0.05.
Results
Generation and characterization of rAd vectored vaccine expressing NP of influenza B virus
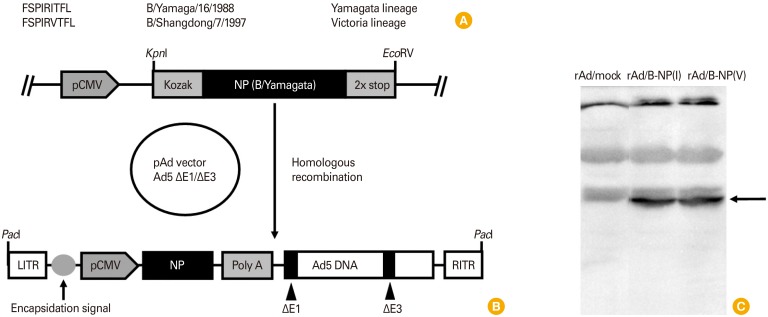
The Yamagata lineage virus used in this study was B/Yamagata/16/1988 (B/Yamagata) and the Victoria/7/97 (B/Victoria) lineage virus was B/Shangdong/7/97. There is one amino acid difference in the 171th sequence of B-NP between B/Yamagata and B/Shangdong, namely isoleucine and valine, respectively (Fig. 1A). Site-directed mutagenesis was performed to change isoleucine into valine from the pShuttle vector expressing the NP of B/Yamagata under the control of CMV promoter. Each of the pShuttle vectors containing B-NP(I) or B-NP(V) was inserted into the early region (E1) of the adenovirus genome by homologous recombination, leading to the generation of replication-defective rAd/B-NP(I) and rAd/B-NP(V), respectively (Fig. 1B). Lysates of HEK293 cells infected with each rAd expressing B-NP were evaluated for protein expression by conducting immunoblot analysis using a polyclonal antibody specific for the B/Yamagata virus. At an approximate molecular weight of 61.6 kDa, a single band was detected, representing B-NP in the rAd/B-NP-infected HEK293 cell lysates. No specific bands were detected in the rAd/mock-infected HEK293 cell lysates used as a negative control (Fig. 1C).
Fig. 1. Construction of replication-defective adenoviruses expressing nucleoprotein (NP) of influenza B virus and expression in HEK293 cells. (A) Cytotoxic T lymphocyte epitope sequences of NP restricted to MHC class I H-2Dd. (B) The recombinant adenovirus encoding NP is generated following homologous recombination with the shuttle vector and adenoviral genome. (C) Expression of NP antigens in HEK293 cells (arrow indicated) infected with each recombinant adenovirus is confirmed by Western blotting as described in the Materials and Methods.
Humoral responses induced by mucosal rAd/B-NP immunization
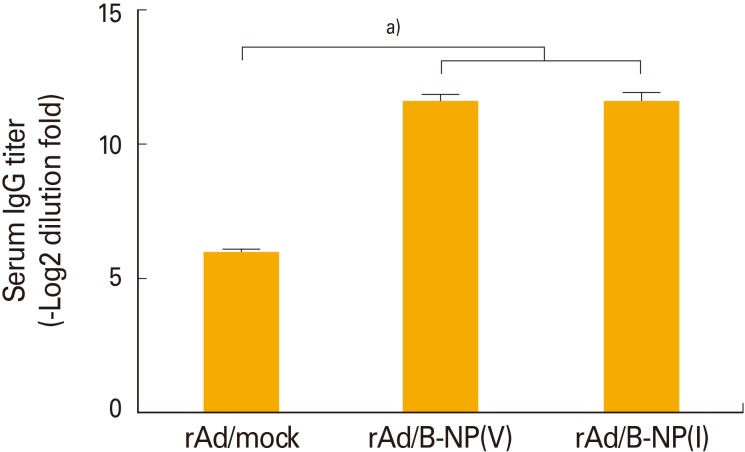
To determine whether rAd/B-NP vaccination elicits sufficient influenza NP-specific immune responses, BALB/c mice were intranasally immunized with 1×108 PFU of rAd/B-NP(I) or rAd/B-NP(V). As a control, rAd/mock was injected with the same route and dose. Two weeks later, serum from all of the immunized mice was harvested and tested for the levels of B/Yamagata-specific IgG through ELISA using detergent-disrupted B/Yamagata virus as the coating antigen. Only vaccine-immunized mice exhibited significantly increased specific IgG titers (Fig. 2). These findings demonstrated that both rAd/B-NP vaccines are equally immunogenic and induce NP-specific IgG responses.
Fig. 2. Characterization of humoral immune responses induced by immunization of the recombinant adenoviruses. BALB/c mice were immunized once with 1×108 PFU rAd/mock, rAd/B-NP(V), or rAd/B-NP(I) via intranasal route. Two weeks later, average anti-nucleoprotein (NP) IgG antibody responses in the sera were measured by enzyme-linked immunosorbent assay. a)p<0.001.
NP-specific CD8+ T-cell responses induced by rAd/B-NP immunization
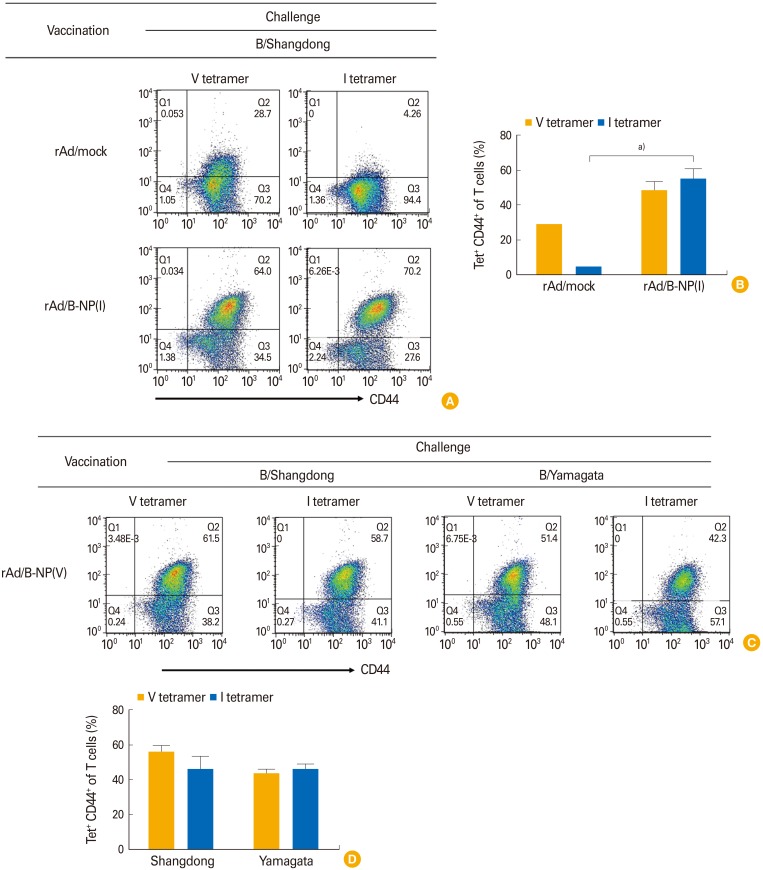
To determine whether the CD8 T-cell responses are induced by vaccination with rAd/B-NP, NP-specific CD8 T cells were analyzed after vaccination and challenge. The mice immunized with rAd/mock or rAd/B-NP(I) were challenged with lethal doses of B/Shangdong 3 weeks after vaccination. Lung lymphocytes were harvested and the frequencies of Dd/NP(I)166–174 or Dd/NP(V)166–174 tetramer-specific CD8 T cells were analyzed using flow cytometry 10 days post infection. Vaccination of the mice with rAd/B-NP(V) led to significant recruitment of Dd/NP(I)166–174 or Dd/NP(V)166–174 tetramer-specific CD8 T cells in the lungs, which was not observed in the control group mice (Fig. 3A, B). The potential of rAd/B-NP(V) vaccination to induce cross-reactive NP-specific CD8 T cells was examined using the same immunization-challenge scheme. Two groups of rAd/B-NP(V) immunized mice were challenged with B/Yamagata or B/Shangdong. After 10 days, lung lymphocytes were harvested and analyzed to detect cells specific to the NP(I) and NP(V) tetramers. Significant and similar numbers of Dd/NP(I)166–174 or Dd/NP(V)166–174 tetramer-specific CD8 T cells were detected in spite of the different viral challenges in identically vaccinated mice (Fig. 3C, D). This indicated that both rAd/B-NP(I) and rAd/B-NP(V) vaccines induce similar NP-specific CD8 T-cell responses.
Fig. 3. Nucleoprotein (NP)-specific CD8 T-cell responses measured by the tetramers. rAd/B-NP(I) (A, B) and rAd/B-NP(V) (C, D) vaccinated mice were challenged with 5LD50 doses of Influenza B/Shangdong or B/Yamagata. Lung cells were harvested 5 days after challenge, and stained with two types of Dd/NP166–174 tetramer (V or I), anti-CD8, and anti-CD44 antibodies. (A, C) Representative dot plots of tetramer staining. The upper right quadrant area shows tetramer+CD44+ cells. (B, D) The percentages of tetramer-positive cells calculated among each group. a)p<0.01.
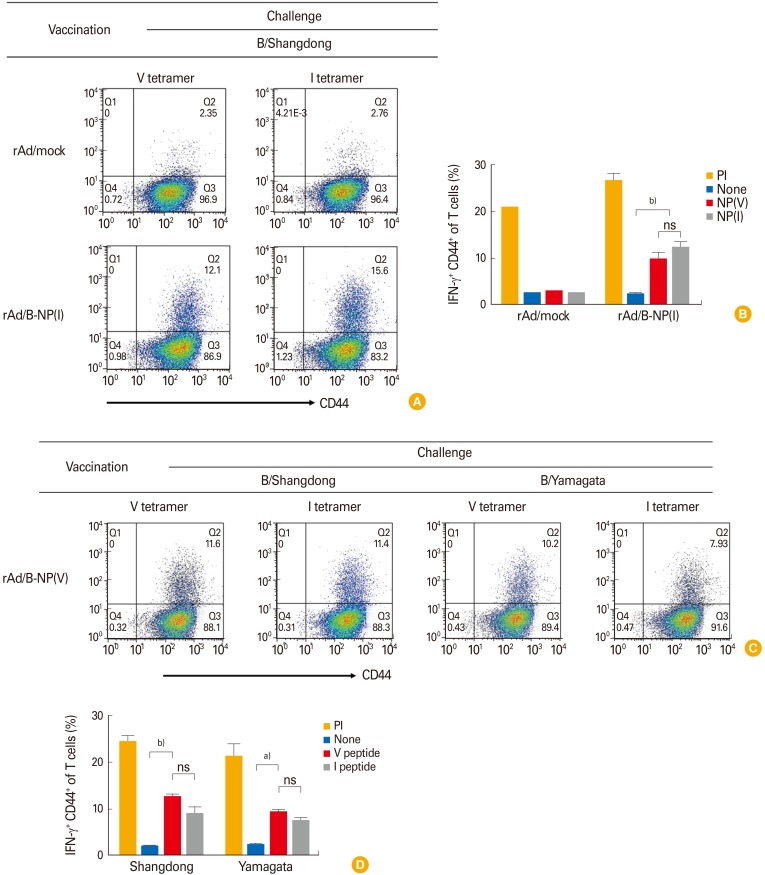
IFN-γ secreting NP-specific CD8 T cells were identified through the same vaccination-challenge regimen. At 10 day post challenge, collected lung lymphocytes were analyzed after 5 hours of stimulation with the I or V peptide. Flow cytometry analyses of the cell population showed that rAd/B-NP(I)-vaccinated mice elicited considerable IFN-γ positive CD8 T cells compared to the rAd/mock vaccinated group (Fig. 4A, B). These results confirmed the cross-reactive functionality of NP-specific CD8 T cells induced by the rAd/B-NP(I) vaccine. Similarly, the ability of the rAd/B-NP(V) vaccine to elicit functional NP-specific CD8 T cells was examined. Mice were immunized with 1×108 PFU of rAd/B-NP(V) and challenged with 5LD50 of B/Shangdong or B/Yamagata. After 10 days, IFN-γ expression was evaluated through 5 hours of stimulation with the I or V peptide. As shown in Fig. 4C and D, the rAd/B-NP(V) vaccine induced IFN-γ-secreting CD8 T cells against both lineages of influenza B, which means that the CD8 T cells induced by rAd/B-NP(V) similarly recognize each epitope, regardless of amino acid difference.
Fig. 4. Nucleoprotein (NP)-specific CD8 T cells in the lungs measured by interferon-γ (IFN-γ) intracellular cytokine staining. Mice were immunized with rAd/B-NP(I) (A, B) or rAd/B-NP(V) (C, D), and lung cells were prepared and stained with anti-CD8, CD44, and IFN-γ after stimulation with two types of peptide (I or V). (A, C) Representative dot plots of CD44+IFN-γ+ cells. (B, D) The percentages of CD44+IFN-γ+ population. ns, not significant. a)p<0.01, b)p<0.001.
Cross-protective immunity against two lineage of influenza B virus by mucosal rAd/B-NP(I) or rAd/B-NP(V) immunization
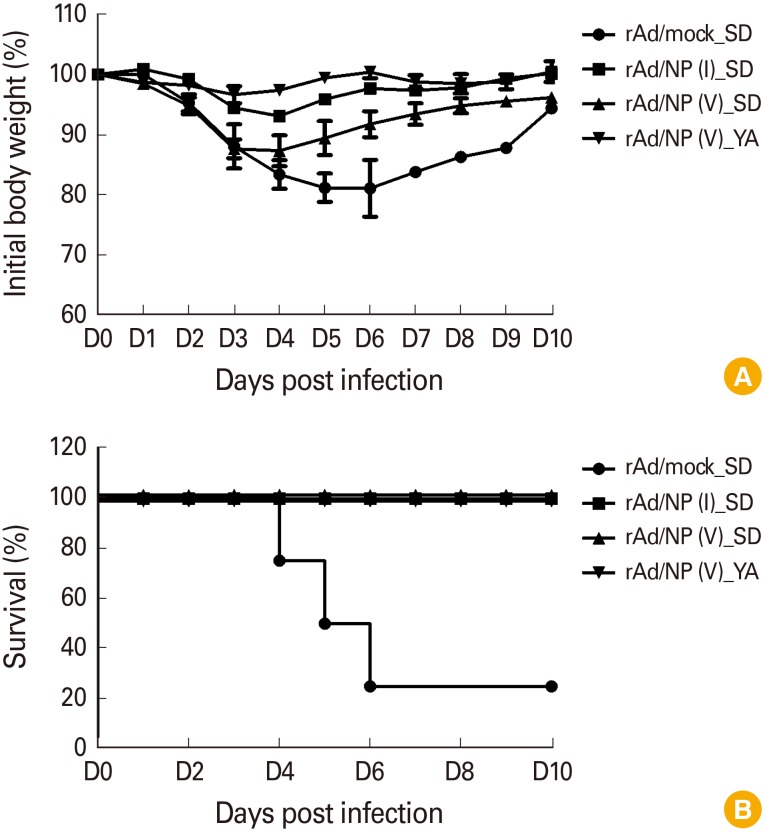
Previous data showed that both rAd/B-NP(I) and rAd/B-NP(V) vaccination similarly elicits NP-specific IgG antibodies and CD8 T cells. Next, the ability of these vaccines to confer cross-protective ability to mice challenged with lethal doses of the influenza B virus was investigated. To this end, mice were challenged with B/Yamagata or B/Shangdong after vaccination, and the body weights and survival rates were measured for 10 days to determine morbidity and mortality, respectively. When the mice were challenged with B/Shangdong, the body weight in all vaccination groups decreased slightly by approximately 10%, while the weight in the rAd/mock control group continually decreased by 20% until day 6 post infection. B/Yamagata-challenged groups maintained their initial body weight following rAd/B-NP(I) (data not shown) or rAd/B-NP(V) vaccination (Fig. 5A). Regardless of the influenza B virus lineage used in challenge experiments, all rAd/B-NP(I) and rAd/B-NP(V) vaccinated groups survived (Fig. 5B). These data suggest that both rAd/B-NP(I) and rAd/B-NP(V) vaccination can provide cross-protective ability against two lineages of the influenza B virus, even though the sequence of the epitope differs for each lineage.
Fig. 5. Protective efficacy of the rAd/B-NP vaccines against influenza B infection. Mice were immunized with 1×108 plaque-forming units of each vaccine intranasally, and were challenged with 5LD50 of B/Shangdong or B/Yamagata. Body weight changes (A) and survival rates (B) were recorded for 10 days.
Discussion
The current strategy for the influenza B vaccine is to induce a strain-specific humoral immune response. This vaccine strategy alone cannot effectively defend against virus mutations such as antigenic drift. Therefore, another vaccine strategy for influenza B is needed. Previous studies have shown that vaccines targeting NPs provide protective immunity against lethal dose infections for a wide range of influenza A strains [26,27,28]. Induction of virus-specific CTLs has been identified as a good vaccine strategy for a broad range of cross-reactive immune responses, and indeed, CTLs have been shown to be an important mediator of cross-protective immunity to the influenza A and B viruses [29,30]. NP is a highly conserved virus protein that induces strong CTL responses, and NP-specific CTLs play an important role in cross-protective immunity to influenza virus infection [31]. In fact, the cross-protective immunity afforded by CTL was confirmed by adoptive transfer and depletion experiments [15,32,33]. For example, transfer of CTLs specific to the influenza virus to naive mice had a protective effect against heterologous influenza virus infection [15]. In addition, depletion of CD8+ T cells prior to challenge infection resulted in poor protection against heterologous viral infections, with high mortality and a high viral titer in the primed animals [34].
In the Yamagata lineage, such as B/Yamagata/16/1988, the immunodominant CTL epitope in the NP sequence appears as “FSPIRITFL,” which is restricted in H-2Dd. This epitope appears as a variant “FSPIRVTFL” with one amino acid difference in B/Shangdong/7/97, the Victoria lineage. Thus, in this study, adenoviruses that express the NP derived from B/Yamagata/16/1988 or B/Shangdong/7/1997 with one amino acid variation in the CTL epitope were prepared as vaccine candidates. Although there is concern that preexisting immunity due to natural infection of adenovirus may be a major factor in reducing the immune response when using the same serotype [35], even hosts infected with adenovirus have been reported to induce sufficient cellular and humoral immune responses when intranasally immunized [24]. Therefore, in this experiment, the nasal route was selected as the immunization route.
To identify whether the mutation in the CTL epitope has a different effect on the NP-specific immune responses and cross-protection ability, vaccine-immunized mice were challenged with the influenza B virus and examined for NP-specific CTL responses in the lungs or spleens using MHC tetramer staining and intracellular cytokine staining (ICS). IgG and CTL responses were similarly elevated in mice immunized with rAd/B-NP regardless of epitope mutation when compared to mice immunized with control rAd/mock. Moreover, mice immunized with the vaccine survived the homologous or heterologous influenza B virus challenge, and showed little or no morbidity. These results demonstrate that I to V mutation in the epitope does not affect the induction of CTLs and there is cross-recognition between the two epitopes, resulting in cross-protective immunity against both lineages of influenza B.
If the peptide does not stably bind to MHC class I, immunogenicity is poor and the peptide is not well recognized by T cells [17,36]. However, the two peptides in this study revealed the same preference for MHC class I binding, according to prediction methods such as BIMAS and IEDB. Cytotoxicity and IFN-γ production are also associated with affinity between peptide-MHC and T-cell receptor (TCR) [18]. Although the affinity between these two peptide-MHC complexes and TCR has not been confirmed, it is thought to be similar based on the levels of IFN-γ production in ICS for both epitopes. Meanwhile, the tetramer population specific for the B-NP(V) in the control group appeared to be higher than the tetramer population specific for the B-NP(I) when the B/Shangdong virus was challenged. However, there was no statistical significance and CD8+ T cells specific for NP(V) did not produce IFN-γ. Thus, it is likely that the corresponding CD8+ T cells in the control group developed a primary response and could not provide protection.
This study demonstrates that single mucosal immunization with replication-defective adenovirus encoding highly conserved NP from both lineages induces strong cross-reactive CTLs, and provides cross-protection regardless of amino acid variations in the CTL epitope. Therefore, our NP vaccines could be further developed as a universal vaccine for the influenza B virus.
Footnotes
No potential conflict of interest relevant to this article was reported.
We thank Dr. Seong and his members of Molecular Medicine Laboratory at Yonsei University for providing Victoria lineage virus.
References
- 1.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 2.McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol. 2004;78:12817–12828. doi: 10.1128/JVI.78.23.12817-12828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- 4.Furuse Y, Suzuki A, Kishi M, et al. Occurrence of mixed populations of influenza A viruses that can be maintained through transmission in a single host and potential for reassortment. J Clin Microbiol. 2010;48:369–374. doi: 10.1128/JCM.01795-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parrish CR, Kawaoka Y. The origins of new pandemic viruses: the acquisition of new host ranges by canine parvovirus and influenza A viruses. Annu Rev Microbiol. 2005;59:553–586. doi: 10.1146/annurev.micro.59.030804.121059. [DOI] [PubMed] [Google Scholar]
- 6.Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175:59–68. doi: 10.1016/0042-6822(90)90186-u. [DOI] [PubMed] [Google Scholar]
- 7.Hay AJ, Gregory V, Douglas AR, Lin YP. The evolution of human influenza viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356:1861–1870. doi: 10.1098/rstb.2001.0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SS, Banner D, Paquette SG, Leon AJ, Kelvin AA, Kelvin DJ. Pathogenic influenza B virus in the ferret model establishes lower respiratory tract infection. J Gen Virol. 2014;95:2127–2139. doi: 10.1099/vir.0.064352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobusawa E, Sato K. Comparison of the mutation rates of human influenza A and B viruses. J Virol. 2006;80:3675–3678. doi: 10.1128/JVI.80.7.3675-3678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103:e43–e51. doi: 10.2105/AJPH.2012.301137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L. Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis. 2011;11:131–141. doi: 10.1016/S1473-3099(10)70223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28:2149–2156. doi: 10.1016/j.vaccine.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 13.Influenza report 2006 [Internet] Paris: Flying Publisher; 2006. [cited 2018 Dec 5]. Available from: http://www.influenzareport.com. [Google Scholar]
- 14.Assarsson E, Bui HH, Sidney J, et al. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J Virol. 2008;82:12241–12251. doi: 10.1128/JVI.01563-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillaire ML, van Trierum SE, Kreijtz JH, et al. Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells. J Gen Virol. 2011;92(Pt 10):2339–2349. doi: 10.1099/vir.0.033076-0. [DOI] [PubMed] [Google Scholar]
- 16.Jameson J, Cruz J, Ennis FA. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J Virol. 1998;72:8682–8689. doi: 10.1128/jvi.72.11.8682-8689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogue RR, Eron J, Frelinger JA, Matsui M. Amino-terminal alteration of the HLA-A*0201-restricted human immunodeficiency virus pol peptide increases complex stability and in vitro immunogenicity. Proc Natl Acad Sci U S A. 1995;92:8166–8170. doi: 10.1073/pnas.92.18.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179:2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 19.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 20.Robbins PA, Rota PA, Shapiro SZ. A broad cytotoxic T lymphocyte response to influenza type B virus presented by multiple HLA molecules. Int Immunol. 1997;9:815–823. doi: 10.1093/intimm/9.6.815. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Kang JO, Kim JY, Jung HE, Lee HK, Chang J. Single mucosal vaccination targeting nucleoprotein provides broad protection against two lineages of influenza B virus. Antiviral Res. 2019;163:19–28. doi: 10.1016/j.antiviral.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Cheng C, Gall JG, Kong WP, et al. Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 2007;3:e25. doi: 10.1371/journal.ppat.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price GE, Soboleski MR, Lo CY, et al. Single-dose mucosal immunization with a candidate universal influenza vaccine provides rapid protection from virulent H5N1, H3N2 and H1N1 viruses. PLoS One. 2010;5:e13162. doi: 10.1371/journal.pone.0013162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SH, Kim JY, Choi Y, Nguyen HH, Song MK, Chang J. Mucosal vaccination with recombinant adenovirus encoding nucleoprotein provides potent protection against influenza virus infection. PLoS One. 2013;8:e75460. doi: 10.1371/journal.pone.0075460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulmer JB, Fu TM, Deck RR, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng M, Liu F, Shen Y, et al. Cross-protection against influenza virus infection by intranasal administration of nucleoprotein-based vaccine with compound 48/80 adjuvant. Hum Vaccin Immunother. 2015;11:397–406. doi: 10.4161/21645515.2014.995056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barefoot BE, Sample CJ, Ramsburg EA. Recombinant vesicular stomatitis virus expressing influenza nucleoprotein induces CD8 T-cell responses that enhance antibody-mediated protection after lethal challenge with influenza virus. Clin Vaccine Immunol. 2009;16:488–498. doi: 10.1128/CVI.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roose K, Fiers W, Saelens X. Pandemic preparedness: toward a universal influenza vaccine. Drug News Perspect. 2009;22:80–92. doi: 10.1358/dnp.2009.22.2.1334451. [DOI] [PubMed] [Google Scholar]
- 30.van de Sandt CE, Dou Y, Vogelzang-van Trierum SE, et al. Influenza B virus-specific CD8+ T-lymphocytes strongly cross-react with viruses of the opposing influenza B lineage. J Gen Virol. 2015;96:2061–2073. doi: 10.1099/vir.0.000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreijtz JH, Bodewes R, van Amerongen G, et al. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007;25:612–620. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Yap KL, Ada GL. The recovery of mice from influenza A virus infection: adoptive transfer of immunity with influenza virus-specific cytotoxic T lymphocytes recognizing a common virion antigen. Scand J Immunol. 1978;8:413–420. doi: 10.1111/j.1365-3083.1978.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 33.Lukacher AE, Braciale VL, Braciale TJ. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J Virol. 2011;85:448–455. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmberg K, Mariathasan S, Ohteki T, Ohashi PS, Gascoigne NR. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. J Immunol. 2003;171:2427–2434. doi: 10.4049/jimmunol.171.5.2427. [DOI] [PubMed] [Google Scholar]