Abstract
Introduction
Sleep disturbances are increasingly recognized as a risk factor for Alzheimer's disease. However, no study has assessed the relationships between objective sleep fragmentation (SF) and brain and cognitive integrity across different cognitive stages, from cognitively unimpaired elderly subjects to patients with subjective cognitive decline and/or mild cognitive impairment.
Methods
30 cognitively unimpaired elderly participants and 36 patients with subjective cognitive decline and/or mild cognitive impairment underwent a neuropsychological evaluation, structural MRI, 18F-fluorodeoxyglucose, and 18F-florbetapir-PET scans, and an actigraphy recording over a minimum of six consecutive nights. Multiple regression and mediation analyses were performed between SF parameters, neuroimaging data, and cognitive scores.
Results
In cognitively unimpaired elderly participants, SF intensity mediated the association between frontohippocampal hypometabolism and lower executive functioning. Moreover, to a lower extent, increased SF variability was related to thalamic atrophy and ventromedial prefrontal amyloid burden. However, in patients with subjective cognitive decline and/or mild cognitive impairment, SF no longer contributed to the expression of cognitive deficits.
Discussion
These findings suggest that SF may directly contribute to lower cognitive performance in cognitively unimpaired elderly subjects. Therefore, treating sleep disturbances before the onset of cognitive deficits may help to cope with brain alterations and maintain cognitive functioning.
Keywords: Sleep, Aging, Subjective cognitive decline, Mild cognitive impairment, MRI, Amyloid, Glucose metabolism, Actigraphy, Sleep fragmentation
1. Introduction
As no curative treatment for Alzheimer's disease (AD) is currently available, it is particularly important to identify modifiable lifestyle factors that might help prevent, delay the onset, or slow down the progression of the disease. In this context, there is a growing interest in better characterizing age-related sleep changes and their associations with AD. Indeed, aging is characterized by a progressive decline in sleep quality, including a decrease in non-rapid eye movement (NREM) sleep, paralleled by greater sleep fragmentation (SF) [1]. Moreover, age-related changes in sleep quality have been related to cognitive decline and increased risk of developing dementia [2], [3], [4]. Sleep disturbances are also a core symptom of AD [1]. They worsen with dementia severity and are related to patients' cognitive deficits, especially memory impairments [5]. Accumulating evidence indicates that the disruption of slow-wave sleep, the deepest NREM sleep stage predominating during the first part of the night, is associated with increased beta-amyloid (Aβ) deposition in the brain [6], [7], [8], a hallmark of AD known to start decades before the apparition of the first cognitive symptoms [9]. Moreover, several self-reported sleep parameters, including longer sleep latency [10], [11] and shorter sleep duration [12], have been associated with increased amyloid deposition in frontal areas and/or in the precuneus. In addition to amyloid deposition, sleep disruption may also be associated with neuronal injury, as measured with gray matter atrophy or hypometabolism, both known to be closely related to cognitive decline [13], [14]. Previous studies reported that, in cognitively unimpaired elderly subjects, poor self-reported sleep quality is related to reduced gray matter volume in frontal regions and the insula [10], [15], and that increased actigraphy-measured sleep fragmentation is associated with atrophy in the orbitofrontal cortex [16].
However, we lack a comprehensive overview of the relationships between objective measures of sleep quality and amyloid deposition, atrophy, and brain glucose metabolism in cognitively unimpaired elderly participants. Furthermore, we have no information about these links in patients with subjective and/or objective cognitive deficits. Clarifying these associations using complementary neuroimaging modalities that reflect different aspects of brain integrity, and across different cognitive stages, would help determining to what extent and at which stage of the disease the treatment of sleep disturbances would be beneficial to prevent cognitive decline.
Thus, the present study aims at investigating and comparing the relationships between objectively measured SF and cognitive, structural, functional, and molecular brain changes in cognitively unimpaired elderly subjects versus in patients with subjective and/or objective cognitive deficits, including patients with subjective cognitive decline (SCD) and mild cognitive impairment (MCI).
2. Materials and methods
2.1. Participants
Sixty-six participants from the IMAP+ study (“Imagerie Multimodale de la Maladie d’Alzheimer à un stade Précoce”, Caen, France) were included in the present study. They were all right-handed, native French speakers, with at least 7 years of education, living at home, without history or clinical evidence of neurological or psychiatric disorders, alcohol use disorder or drug abuse, and with a normal somatic examination. The inclusion and group classification of the participants were based on a clinical interview and a standardized neuropsychological assessment, according to internationally agreed criteria, but did not rely on neuroimaging biomarkers, as they were the main outcome measures in our analyses.
Thirty cognitively unimpaired elderly subjects were recruited from the community. They were aged over 60 years, had no memory complaint and never referred to a memory clinic, and performed in the normal range (i.e., within 1.65 standard deviation [SD] of the mean) for their age and education level in all screening tests. In addition, thirty-six patients aged over 50 years were recruited from local memory clinics, to which they attended for self-reported cognitive concerns. During the clinical interview, the physician ensured i) that the complaint was not related to current medication, psychiatric or neurological diseases (including anxiety or depression), or other medical conditions, ii) that independence in daily life was preserved, and iii) that they did not fulfill NINCDS-ADRDA criteria for probable AD [17]. Among the 36 patients, 22 had objective cognitive deficits and met clinical criteria for single or multiple domain amnestic mild cognitive impairment [18], with predominant episodic memory deficits (1.5 SD from the normal mean for age and education). Fourteen patients did not show significant objective cognitive impairment and met criteria for SCD [19]. Clinical diagnosis for patients was assigned by consensus under the supervision of senior neurologists and neuropsychologists.
Once included in the study, all participants performed a detailed neuropsychological assessment, an actigraphy recording, and three neuroimaging scans within a mean interval of 1.9 ± 3.1 months. The IMAP+ study was approved by the local ethics committee (CPP Nord-Ouest III) and registered at http://clinicaltrials.gov (nb. NCT01638949). All participants gave their written informed consent to the study before the examinations.
2.2. Cognitive assessment
Participants underwent a detailed neuropsychological assessment, encompassing multiple domains of cognition (verbal and visual episodic memory, semantic memory, working memory, executive functioning, processing speed, visuospatial abilities, language skills, and praxis), fully described in previous publications [20], [21]. In the present study, analyses focused on episodic memory and executive functioning, as they are particularly sensitive to aging and AD [22], and closely related to sleep quality [23]. We used composite scores to reflect cognitive abilities with robust proxies and to minimize the issue of multiple statistical testing (see [24] and Supplementary Material for further details). Higher values always indicated better performance. Furthermore, symptoms of depression and anxiety were assessed using the Montgomery-Asberg Depression Rating Scale [25] and the trait version of the State-Trait Anxiety Inventory [26], respectively.
2.3. Actigraphy recording
2.3.1. Actigraphy data collection
The sleep-wake cycle was recorded in all participants using the MotionWatch 8 wrist-worn triaxial actigraph (CamNTech Ltd, Cambridge, UK), for at least six consecutive nights (range: 6-8 nights; mean ± SD: 7.05 ± 0.51 nights). Participants were instructed to wear continuously the device on their nondominant wrist until the end of the recording. They had to press the event marker button at lights off and lights on, and to fill in a sleep diary on awakening each morning [27] to facilitate data analysis.
2.3.2. Actigraphy data analysis
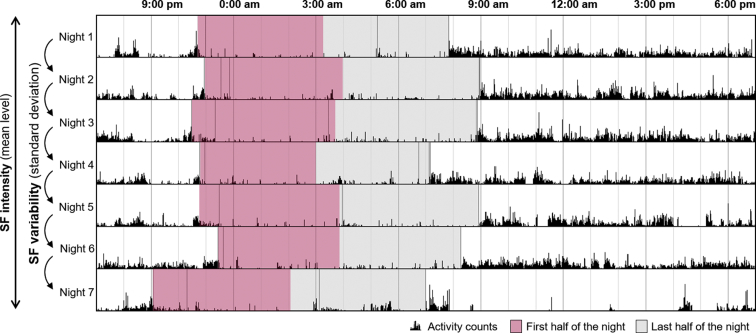
Data were analyzed using the MotionWare software (version 1.1.25, CamNTech Ltd, Cambridge, UK) and sampled using a 5-second epoch. A sensitivity threshold of 20 counts was applied to distinguish activity from rest. For each night, the “Lights Off” and “Got Up” markers were placed by the same experimenter in the appropriate position following the event marker put by the participant and cross-validated with the light sensor data and sleep diary information. Then, “Fell Asleep” and “Woke up” markers were automatically adjusted from the combination of the activity data and the “Lights Off” and “Got Up” markers, respectively, allowing the collection of the assumed sleep time. Then, a fragmentation index was calculated as the sum of the percentages of mobile time and immobile bouts below or equal to 1 minute in the assumed sleep period. Two distinct parameters of SF were considered: SF intensity (corresponding to the mean level of fragmentation over the whole recording) and intra-individual SF variability (computed as SF standard deviation across nights) (Fig. 1). These parameters were computed over the first half of the assumed sleep duration for each subject.
Fig. 1.
Illustration of a representative actigraphy recording with computation of SF parameters. SF was computed over the first half of the night. SF intensity corresponded to the mean level of SF over all recorded nights, and SF variability corresponded to the standard deviation of SF across recorded nights. Abbreviation: SF, sleep fragmentation.
2.4. Neuroimaging procedure
Participants underwent a structural T1 MRI, as well as 18F-fluorodeoxyglucose (FDG)- and florbetapir-PET scans, to measure gray matter volume, brain glucose metabolism, and amyloid burden, respectively. All examinations were performed at the Cyceron Center (Caen, France). Details on MRI and PET images acquisition and preprocessing are available in previous publications [28], [29] and are fully described in the Supplementary Material. Briefly, T1-weighted images were preprocessed in SPM12. FDG-PET and florbetapir-PET images were preprocessed using MRI data for partial volume effect correction and spatial normalization. Partial volume effects-corrected normalized and scaled florbetapir-PET images were also used to extract the individual global cortical amyloid standard uptake value ratio using a predetermined neocortical mask including the entire gray matter, except the cerebellum, occipital and sensory motor cortices, hippocampi, amygdala, and basal nuclei [30].
2.5. Statistical analyses
2.5.1. Group comparisons
Between-group comparisons for demographics, cognitive scores, and sleep parameters were assessed using Student's t-tests for continuous variables and chi-square tests for categorical variables, with statistical significance set to P < .05.
Patients' patterns of atrophy, hypometabolism, and amyloid burden were explored using two-sample t-tests in SPM12, adjusted for age, gender, and body mass index.
2.5.2. Voxelwise regression analyses
Within each group, voxelwise multiple regression analyses were performed between SF parameters and each neuroimaging modality independently in SPM12, controlling for age, gender, body mass index, and the complementary SF parameter (i.e., we controlled for SF variability when SF intensity was the predictive variable, and conversely). Afterward, complementary analyses were performed with the addition of several covariates to the main model. Only results obtained with the initial 4-covariates model are presented, as the main results remained essentially unchanged when adding the Mini-Mental State Examination score, depression score, or sleep medication use as covariates. We report the slight changes observed on these secondary analyses in the Supplementary Material. For all neuroimaging analyses, results were evaluated for significance at P < .001 (uncorrected) combined with a minimum cluster size determined by Monte-Carlo simulation using the AlphaSim program to achieve a corrected statistical significance of P < .05 (Supplementary Table 1). Results obtained at the same statistical threshold but with a lower cluster size were considered as trends.
2.5.3. Multiple regression analyses
Multiple regression analyses were performed between SF parameters and cognitive scores (i.e., composite scores for executive functioning and episodic memory) within each group. Age and gender were included as covariates in the regression models, and results were considered significant after applying a Bonferroni correction for multiple comparisons: the statistical threshold for significance was set to P = (.05/number of comparisons).
2.5.4. Mediation analyses
When the same SF parameter was significantly associated to both a brain and a cognitive variable, then causal mediation analyses were performed to assess the directionality of the relationships. For this purpose, brain data (i.e., gray matter volume, glucose metabolism, or amyloid values) were extracted in the clusters significantly associated with SF parameters in the voxelwise analyses described previously. Then, two different models were tested to determine whether the brain variable mediated the relationships between sleep and cognition or whether the sleep variable mediated the relationships between the brain variable and cognition. These analyses were performed using the R package “mediation” [31], and we report the average direct effects and average causal mediation effect estimated using nonparametric bootstrapping (5000 bootstrap resamples, P < .05) for both models.
3. Results
3.1. Between-group differences
Demographic data, neuropsychological performance, and sleep parameters for each subgroup, as well as between-group differences, are reported in Table 1. The two groups did not differ for age, gender, years of education, body mass index, state-trait anxiety, and sleep parameters. As expected, patients with SCD/MCI presented lower global cognitive functioning (Mini-Mental State Examination score: t = 2.03, P = .046; Mattis Dementia Rating Scale: t = 2.88, P = .005), episodic memory (t = 2.92, P = .005), and executive performance (t = 2.39, P = .020). They also presented higher depressive symptoms (Montgomery and Asberg depression rating scale: t = −3.27, P = .002). Moreover, patients with SCD/MCI presented significant brain changes compared with cognitively unimpaired elderly subjects including (i) atrophy within the parahippocampal and hippocampal region, (ii) hypometabolism in the precuneus and posterior cingulate cortex, and (iii) widespread amyloid deposition mainly in frontal regions (Supplementary Fig. 1). Consistently, the global cortical amyloid standard uptake value ratio was significantly higher in patients with SCD/MCI compared with cognitively unimpaired elderly subjects (t = −2.61, P = .011).
Table 1.
Participants characteristics
| Variables | Cognitively unimpaired elderly subjects (n = 30) | SCD/MCI patients (n = 36)∗ | Group comparison† |
|---|---|---|---|
| Demographics | |||
| Age (years ± SD) | 73.3 ± 7 | 71.5 ± 8.2 | NS |
| Gender (% women) | 56.7% | 38.9% | NS |
| Education (years ± SD) | 12.1 ± 3.5 | 11.8 ± 3.2 | NS |
| Body mass index (mean ± SD) | 24.5 ± 3 | 24.6 ± 4.5 | NS |
| Florbetapir SUVr (% positive)‡ | 1 ± 0.2 (30.8 %) | 1.2 ± 0.3 (50 %) | P = .011 |
| MADRS (mean ± SD) | 1 ± 1.9 | 3.4 ± 3.7 | P = .002 |
| STAI-B (mean ± SD) Participants on sleep medication (number, (%))§ |
37.4 ± 10 1 (3.3%) |
38.8 ± 8.2 3 (8.3%) |
NS NS |
| Cognition | |||
| MMSE (mean ± SD) | 28.6 ± 1.2 | 27.6 ± 2.4 | P = .046 |
| Mattis total score (mean ± SD) | 140.2 ± 3.3 | 136.2 ± 6.9 | P = .005 |
| Episodic memory composite score (mean ± SD) | 0.0 ± 0.7 | −0.8 ± 1.3 | P = .005 |
| Executive functioning composite score (mean ± SD) | 0.0 ± 0.7 | −0.8 ± 1.7 | P = .020 |
| Sleep parameters | |||
| SF intensity (mean ± SD) | 30.3 ± 14.7 | 28 ± 11.1 | NS |
| SF variability (mean ± SD) | 10.9 ± 4.7 | 11.5 ± 5.2 | NS |
Abbreviations: MADRS, Montgomery and Asberg Depression Rating Scale; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NS, nonsignificant; SCD, subjective cognitive decline; SD, standard deviation; SF, sleep fragmentation; STAI-B, State-Trait Anxiety Inventory form B; SUVr, standard uptake value ratio.
Owing to missing data in some patients, n = 30 for episodic memory, n = 33 for the MADRS, n = 34 for executive functioning.
Between-groups differences were assessed using Student's t-tests for all variables, except for gender for which chi-square statistics were used. Statistical significance was set to P < .05.
n = 26 healthy elderly and 34 patients with valid florbetapir-PET scan. Amyloid positivity was defined as >0.985, based on mean SUVr + 2 SDs in a group of 41 healthy young individuals (aged <40 years).
Use of sleep medication on a regular basis (>1/week), excluding phytotherapy and homeopathy.
3.2. Relationships between SF and neuroimaging
Results of significant voxelwise regression analyses are presented in Fig. 2, and detailed peak statistics and coordinates of significant clusters are reported in Supplementary Table 2.
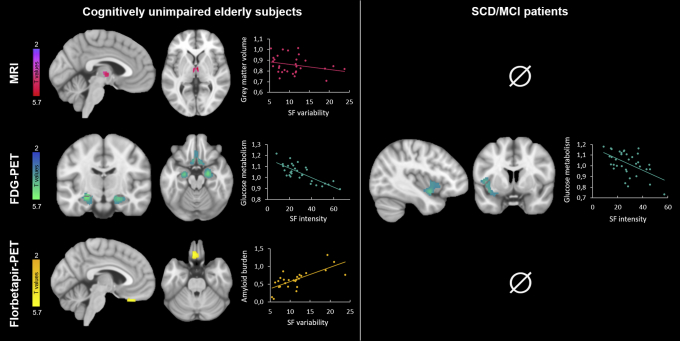
Fig. 2.
Neuroimaging correlates of SF. Results of the voxelwise regression analyses between SF parameters and neuroimaging data in cognitively unimpaired elderly participants (left part) and patients with SCD/MCI (right part). Results are presented at P < .001 (uncorrected) and adjusted for age, gender, BMI, and the complementary SF parameter (i.e., SF variability when SF intensity was considered, and conversely). Abbreviations: BMI, body mass index; FDG: 18F-fluorodeoxyglucose; MCI, mild cognitive impairment; SCD, subjective cognitive decline; SF, sleep fragmentation.
3.2.1. Cognitively unimpaired elderly subjects
SF intensity negatively correlated to brain glucose metabolism in the ventromedial prefrontal cortex, hippocampus and parahippocampus, bilaterally. No significant association was found with gray matter volume or amyloid burden.
SF variability negatively correlated to gray matter volume within the thalamus. Moreover, we observed a trend toward a positive correlation with amyloid burden in the left rectus gyrus. When sleep medication was added as a covariate in the model, both associations became trends (Supplementary Table 3). No association was found between SF variability and brain glucose metabolism.
3.2.2. Patients with SCD/MCI
SF intensity negatively correlated with brain glucose metabolism in the left insula, whereas no significant association was found with gray matter volume or amyloid burden. SF variability was not associated with any neuroimaging modality.
3.3. Relationships between SF and cognition
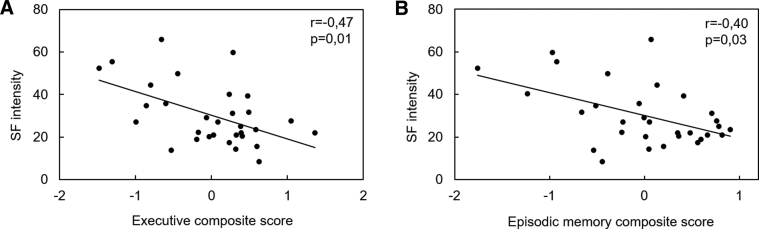
In cognitively unimpaired elderly subjects, SF intensity was significantly associated to worse performance in executive functioning (Fig. 3A; r = −0.47, P = .01) and episodic memory (Fig. 3B; r = −0.40, P = .03), although this latter did not survive the Bonferroni correction for multiple comparisons. By contrast, SF variability was not related to any cognitive score in this group.
Fig. 3.
Significant associations between SF intensity and cognitive performance in cognitively unimpaired elderly subjects. Scatterplots illustrating the associations between SF intensity and (A) executive performance, and (B) episodic memory performance. Partial correlation coefficients and P-values adjusted for age and gender are indicated on corresponding graphs. Results were considered significant after applying a Bonferroni correction for multiple testing (alpha = .05/4 = .01). Abbreviation: SF, sleep fragmentation.
We did not find any significant association between SF parameters and cognitive performance in patients with SCD/MCI (see Supplementary Table 4 for detailed results).
3.4. Mediation analyses in cognitively unimpaired elderly subjects
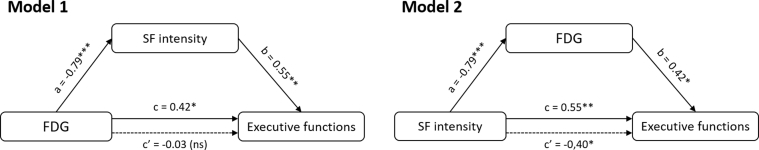
As SF intensity was significantly associated to both executive performance and hippocampal and prefrontal glucose metabolism, these three variables were entered in causal mediation analyses. The results of the two models tested in cognitively unimpaired elderly subjects are summarized in Fig. 4. They showed that SF significantly mediated the relationship between total brain glucose metabolism and executive performance (P = .02; Table 2). No other mediation analysis was conducted, as no other sleep variable was significantly associated to both a brain and a cognitive variable.
Fig. 4.
Causal mediation analyses performed in cognitively unimpaired elderly subjects. Direct effects in filled arrows (simple regressions between variables) are expressed as standardized regression coefficients, and indirect effects in dotted arrows (multiple regressions in which the predictor and the mediator are both added in the model) as partial correlation coefficients. *P < .05, **P < .01, ***P < .001. Abbreviations: FDG, 18F-fluorodeoxyglucose; NS, nonsignificant; SF, sleep fragmentation.
Table 2.
Detailed statistics of causal mediation analyses in cognitively unimpaired elderly subjects
| Model | ADE |
ACME |
||||
|---|---|---|---|---|---|---|
| Estimate | CI95% | P-value | Estimate | CI95% | P-value | |
| Model 1 | −0.344 | [−5.396; 3.526] | .85 | 4.051 | [0.657; 8.723] | .02 |
| Model 2 | −0.028 | [−0.059; −0.005] | .02 | 0.002 | [−0.016; 0.024] | .87 |
Abbreviations: ADE, average direct effect; ACME, average causal mediation effect; CI, confidence interval.
In both models, executive functions were entered as the dependent variable. In model 1, brain glucose metabolism was the independent variable and SF intensity the mediator, whereas in model 2, SF intensity was the independent variable and brain glucose metabolism the mediator.
4. Discussion
The present study aimed at investigating and comparing the brain and cognitive correlates of SF in healthy aging versus in a group of patients with SCD/MCI. In cognitively unimpaired elderly participants, SF intensity was related to frontal and medial temporal metabolism, and to cognitive performance, especially executive functioning. Moreover, SF variability was related to thalamic atrophy and, to a lesser extent, to frontal amyloid deposition. By contrast, in patients with SCD/MCI, we only report a significant association between SF intensity and glucose metabolism in the left insula, whereas no relationship between sleep and cognition was found.
The brain regions found to be associated with the fragmentation of the first hours of sleep include frontal and medial temporal areas, as well as the thalamus in cognitively unimpaired elderly participants, and the insula in patients. These regions are known to be closely involved in sleep physiology. They are largely involved in the generation of NREM-sleep oscillations, such as sleep spindles [32] and slow waves [33], [34], that are notably critical for sleep-dependent memory processes [35], [36]. Besides, these regions are also known to be particularly sensitive to aging and affected in the early stages of AD. Indeed, on the one hand, the medial prefrontal cortex is one of the first brain regions exhibiting amyloid deposition [37], and is also sensitive to glucose metabolism changes in aging and AD [38], [39], [40]. On the other hand, the medial temporal lobe is early affected by tau pathology [41], with atrophy already detectable in the preclinical and prodromal stages [42], [43]. The involvement of this common set of brain regions in both sleep physiology and AD pathophysiology might at least partly underlie the (possibly bidirectional) links between sleep problems and AD.
As a first step toward characterizing the directionality of these associations, we performed causal mediation analyses. We showed that the intensity of the fragmentation of the first hours of sleep mediated the relationship between frontohippocampal hypometabolism and lower executive performance. This suggests that sleep disruption resulting from frontohippocampal hypometabolism may directly contribute to cognitive deficits in cognitively unimpaired elderly subjects. Therefore, treating sleep disturbances in cognitively unimpaired elderly subjects exhibiting a high level of SF could improve their ability to cope with brain changes and potentially reduce their risk of cognitive decline. Nevertheless, owing to a limited statistical power, mediation analyses were restricted to a single set of variables (i.e., sleep fragmentation, brain glucose metabolism in frontohippocampal areas, and executive functioning), as they were the only ones for which the same sleep parameter was related to both a brain and a cognitive variable. Thus, these analyses are likely to picture only part of the potential associations existing between sleep, brain, and cognitive integrity. They do not preclude that some sleep changes could induce brain alterations that may, in turn, underlie cognitive deficits [44]. Moreover, it is also likely that some brain changes are directly underlying cognitive deficits, independently from sleep disturbances.
Besides the associations between metabolic changes and SF intensity, our results reveal that a higher night-to-night variability of SF was rather associated to structural and molecular brain alterations, namely gray matter atrophy and amyloid deposition. Of note, these results should be taken cautiously as they were only trends when taking into account regular use of sleep medication. This suggests that experiencing inconsistent sleep quality over time might be associated with brain changes that might be less reversible, that is, more durable and that might conduct to long-term cognitive deficits and dementia. This interpretation is in line with existing data showing that a greater variability in sleep quality is related to a higher risk of cognitive impairment and dementia [4], [45], as well as other physical and mental health conditions [46], [47]. Further investigations need to be conducted to unravel the determinants of this aspect of sleep, such as the use of sleep medication, lifestyle, or psychoaffective factors that might vary from one day to another and induce changes in sleep quality. Addressing this question is essential to be able to take over sleep problems in their entirety in the elderly population.
By contrast to our results in cognitively unimpaired individuals, the relationships between sleep and brain or cognitive variables were almost nonexistent in patients with SCD/MCI—only a link between SF intensity and insula glucose metabolism was found. We hypothesize that while cognitive performances are directly impacted by sleep quality (as probably other lifestyle factors) in asymptomatic individuals, they may be mainly and more directly driven by underlying neuropathological processes (such as amyloid deposition) at a cognitively impaired (SCD/MCI) stage, so that the direct influence of sleep disruption would no longer be detectable. This observation is supported by previous works showing no association between slow-wave sleep fragmentation nor the amount of REM sleep and cognitive performance in patients with MCI [48], [49]. Taken together, these results suggest that preserving sleep quality could help to cope with brain alterations and maintain cognitive performance in the normal range, but such interventions may be less efficient once patients experience measurable cognitive deficits.
The present study has some strengths, including an objective sleep assessment over several consecutive nights combined to multimodal neuroimaging data, allowing us to assess for the first time the relationships between sleep fragmentation intensity versus variability on structural, metabolic, and molecular brain alterations in the same sample. Moreover, these associations were evaluated both in cognitively impaired and unimpaired elderly subjects, thus helping to give a more comprehensive picture of their role at different cognitive stages. However, some limitations must be mentioned. First, our study is limited by the small sample size and the cross-sectional nature of the analyses. Further analyses should be conducted using a longitudinal design, together with larger sample sizes to complement our mediation analyses and assess the nature and directionality of the relationships between sleep and brain and cognitive integrity more exhaustively. Second, we were not able to characterize the origin of sleep fragmentation (e.g., obstructive sleep apnea, periodic limb movements, or restless legs syndrome), as participants did not undergo polysomnography recordings. Further studies should aim at determining the relative contribution of the multiple factors that can cause sleep fragmentation, such as sleep disorders or lifestyle risk factors. In addition, polysomnography will also be necessary to specifically assess changes in NREM-sleep oscillations, and to test the potential impact of other sleep alterations such as REM-sleep disruption [50].
Our interpretation of the data is that the fragmentation of the first hours of sleep is associated with an alteration of brain regions typically affected in aging and AD, and may directly contribute to lower cognitive performance. However, in patients with SCD/MCI, sleep disruption may no longer contribute to the expression of cognitive deficits. Therefore, preserving sleep quality in cognitively unimpaired elderly subjects may help to cope with brain changes and maintain cognitive functioning.
Research in Context.
-
1.
Systematic Review: In the existing literature, sleep disturbances are increasingly recognized as a risk factor for Alzheimer's disease. However, no previous study has assessed the associations between sleep disruption, brain and cognitive integrity across different cognitive stages.
-
2.
Interpretation: We showed that the disruption of the first hours of sleep is related to the alteration of brain regions typically affected in aging and Alzheimer's disease, and may directly contribute to lower cognitive performance in cognitively unimpaired elderly participants. However, this is no longer the case in patients with subjective cognitive decline and/or mild cognitive impairment.
-
3.
Future Direction: These findings have important clinical implications, as they suggest that treating sleep disturbances before the onset of cognitive deficits may help to cope with brain changes and maintain cognitive performance. Further studies need to be conducted on larger samples and using polysomnography to assess changes in sleep architecture and microstructure more specifically.
Acknowledgments
The authors are grateful to E. Arenaza-Urquijo, A. Bejanin, A. Chocat, S. Egret, M. Fouquet, J. Gonneaud, R. La Joie, A. Laniepce, M. Leblond, C. Malle, K. Mevel, J. Mutlu, V. Ourry, A. Pélerin, A. Perrotin, G. Poisnel, A. Quillard, S. Segobin, N. Villain, A. Manrique, the Cyceron MRI-PET staff members, and to the participants of the study.
This work was supported by the Programme Hospitalier de Recherche Clinique (PHRC National 2011 and 2012), the Agence Nationale de la Recherche (ANR LONGVIE 2007), Fondation Plan Alzheimer (Alzheimer Plan 2008-2012), Fondation LECMA-Vaincre Alzheimer (grant no. 13732), Fondation Thérèse et René Planiol, the Fonds Européen de Développement Régional (FEDER), the Région Basse-Normandie, and the Institut National de la Santé et de la Recherche Médicale (INSERM). Funding sources were not involved in the study design, data acquisition, analysis, and manuscript writing.
Footnotes
Declarations of interest: The authors have no conflict of interest to disclose.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dadm.2018.12.009.
Supplementary data
References
- 1.Petit D., Gagnon J.F., Fantini M.L., Ferini-Strambi L., Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–496. doi: 10.1016/j.jpsychores.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Oosterman J.M., Van Someren E.J.W., Vogels R.L.C., Van Harten B., Scherder E.J.A. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–135. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Lim A.S., Kowgier M., Yu L., Buchman A.S., Bennett D.A. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diem S.J., Blackwell T.L., Stone K.L., Yaffe K., Tranah G., Cauley J.A. Measures of Sleep–Wake Patterns and Risk of Mild Cognitive Impairment or Dementia in Older Women. Am J Geriatr Psychiatry. 2016;24:248–258. doi: 10.1016/j.jagp.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauchs G., Schabus M., Parapatics S., Bertran F., Clochon P., Hot P. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. 2008;19:1159–1162. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mander B.A., Marks S.M., Vogel J.W., Rao V., Lu B., Saletin J.M. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga A.W., Wohlleber M.E., Giménez S., Romero S., Alonso J.F., Ducca E.L. Reduced Slow-Wave Sleep Is Associated with High Cerebrospinal Fluid Aβ42 Levels in Cognitively Normal Elderly. Sleep. 2016;39:2041–2048. doi: 10.5665/sleep.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju Y.S., Ooms S.J., Sutphen C., Macauley S.L., Zangrilli M.A., Jerome G. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branger P., Arenaza-Urquijo E.M., Tomadesso C., Mézenge F., André C., de Flores R. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–114. doi: 10.1016/j.neurobiolaging.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Brown B.M., Rainey-Smith S.R., Villemagne V.L., Weinborn M., Bucks R.S., Sohrabi H.R. The Relationship between Sleep Quality and Brain Amyloid Burden. Sleep. 2016;39:1063–1068. doi: 10.5665/sleep.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spira A.P., Gamaldo A.A., An Y., Wu M.N., Simonsick E.M., Bilgel M. Self-reported Sleep and β-Amyloid Deposition in Community-Dwelling Older Adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fjell A.M., Walhovd K.B., Amlien I., Bjørnerud A., Reinvang I., Gjerstad L. Morphometric Changes in the Episodic Memory Network and Tau Pathologic Features Correlate with Memory Performance in Patients with Mild Cognitive Impairment. AJNR Am J Neuroradiol. 2008;29:1183–1189. doi: 10.3174/ajnr.A1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouquet M., Desgranges B., Landeau B., Duchesnay E., Mézenge F., De La Sayette V. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimers disease. Brain. 2009;132:2058–2067. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sexton C.E., Storsve A.B., Walhovd K.B., Johansen-Berg H., Fjell A.M. Poor sleep quality is associated with increased cortical atrophy in community-dwelling adults. Neurology. 2014;83:967–973. doi: 10.1212/WNL.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim A.S.P., Fleischman D.A., Dawe R.J., Yu L., Arfanakis K., Buchman A.S. Regional Neocortical Gray Matter Structure and Sleep Fragmentation in Older Adults. Sleep. 2016;39:227–235. doi: 10.5665/sleep.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Petersen R.C., Morris J.C. Mild Cognitive Impairment as a Clinical Entity and Treatment Target. Arch Neurol. 2005;62:1160–1164. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 19.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mevel K., Landeau B., Fouquet M., La Joie R., Villain N., Mézenge F. Age effect on the default mode network, inner thoughts, and cognitive abilities. Neurobiol Aging. 2013;34:1292–1301. doi: 10.1016/j.neurobiolaging.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Tomadesso C., Perrotin A., Mutlu J., Mézenge F., Landeau B., Egret S. Brain structural, functional, and cognitive correlates of recent versus remote autobiographical memories in amnestic Mild Cognitive Impairment. Neuroimage Clin. 2015;8:473–482. doi: 10.1016/j.nicl.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hooren S.A.H., Valentijn A.M., Bosma H., Ponds R.W.H.M., van Boxtel M.P.J., Jolles J. Cognitive Functioning in Healthy Older Adults Aged 64–81: A Cohort Study into the Effects of Age, Sex, and Education. Aging Neuropsychol Cogn. 2007;14:40–54. doi: 10.1080/138255890969483. [DOI] [PubMed] [Google Scholar]
- 23.Yaffe K., Falvey C.M., Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 24.Perrotin A., La Joie R., de La Sayette V., Barré L., Mézenge F., Mutlu J. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimer’s Dement. 2017;13:550–560. doi: 10.1016/j.jalz.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery S.A., Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C.D., Sydeman S.J. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In: Maruish M.E., editor. Use Psychol. Test. Treat. Plan. Outcome Assessment. Lawrence Erlbaum Associates; Hillsdale, NJ: 1994. pp. 292–321. [Google Scholar]
- 27.Ellis B.W., Johns M.W., Lancaster R., Raptopoulos P., Angelopoulos N., Priest R.G. The St. Mary’s Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4:93–97. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Mutlu J., Landeau B., Gaubert M., de La Sayette V., Desgranges B., Chételat G. Distinct influence of specific versus global connectivity on the different Alzheimer’s disease biomarkers. Brain. 2017;140:3317–3328. doi: 10.1093/brain/awx279. [DOI] [PubMed] [Google Scholar]
- 29.Tomadesso C., de La Sayette V., de Flores R., Bourgeat P., Villemagne V.L., Egret S. Neuropsychology and neuroimaging profiles of amyloid-positive versus amyloid-negative amnestic mild cognitive impairment patients. Alzheimer’s Dement (Amst) 2018;10:269–277. doi: 10.1016/j.dadm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.La Joie R., Perrotin A., de La Sayette V., Egret S., Doeuvre L., Belliard S. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. Neuroimage Clin. 2013;3:155–162. doi: 10.1016/j.nicl.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tingley D., Yamamoto H.T., Hirose K., Keele L., Princeton K.I. mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59:1–38. [Google Scholar]
- 32.Schabus M., Dang-Vu T.T., Albouy G., Balteau E., Boly M., Carrier J. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci. 2007;104:13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G. The Sleep Slow Oscillation as a Traveling Wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy M., Riedner B, Huber R., Massimini M., Ferrarelli F., Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzsáki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 36.Maingret N., Girardeau G., Todorova R., Goutierre M., Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016;19:959–964. doi: 10.1038/nn.4304. [DOI] [PubMed] [Google Scholar]
- 37.Thal D.R., Rüb U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 38.Herholz K., Carter S.F., Jones M. Positron emission tomography imaging in dementia. Br J Radiol. 2007;80:S160–S167. doi: 10.1259/bjr/97295129. [DOI] [PubMed] [Google Scholar]
- 39.Kalpouzos G., Chételat G., Baron J.C., Landeau B., Mevel K., Godeau C. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh T.C., Lin W.Y., Ding H.J., Sun S.S., Wu Y.C., Yen K.Y. Sex- and age-related differences in brain FDG metabolism of healthy adults: an SPM analysis. J Neuroimaging. 2012;22:21–27. doi: 10.1111/j.1552-6569.2010.00543.x. [DOI] [PubMed] [Google Scholar]
- 41.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 42.Chételat G., Landeau B., Eustache F., Mézenge F., Viader F., de la Sayette V. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Apostolova L.G., Green A.E., Babakchanian S., Hwang K.S., Chou Y.-Y., Toga A.W. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment (MCI), and Alzheimer Disease. Alzheimer Dis Assoc Disord. 2012;26:17–27. doi: 10.1097/WAD.0b013e3182163b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ju Y.-E.S., Lucey B.P., Holtzman D.M. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho-Bos S.S., Riemersma-van der Lek R.F., Waterhouse J., Reilly T., Van Someren E.J.W. Strong association of the rest-activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. 2007;15:92–100. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- 46.Lemola S., Ledermann T., Friedman E.M. Variability of Sleep Duration Is Related to Subjective Sleep Quality and Subjective Well-Being: An Actigraphy Study. PLoS One. 2013;8:e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bei B., Wiley J.F., Trinder J., Manber R. Beyond the mean: A systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:104–120. doi: 10.1016/j.smrv.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Hita-Yanez E., Atienza M., Gil-Neciga E., Cantero J.L. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE epsilon4 genotype. Curr Alzheimer Res. 2012;9:290–297. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- 49.Hita-Yañez E., Atienza M., Cantero J.L. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep. 2013;36:1327–1334. doi: 10.5665/sleep.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pase M.P., Himali J.J., Grima N.A., Beiser A.S., Satizabal C.L., Aparicio H.J. Sleep architecture and the risk of incident dementia in the community. Neurology. 2017;89:1244–1250. doi: 10.1212/WNL.0000000000004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.