Abstract
Introduction
This study evaluates rates of all-cause emergency department visits, all-cause hospitalizations, potentially avoidable hospitalizations, and falls in 3 years preceding Alzheimer's disease (AD) diagnosis.
Methods
Patients with AD and controls with no cognitive impairment were identified from the Medicare claims data. Patients were required to be aged ≥ 65 years and have continuous Medicare enrollment for ≥4 years before the index date (AD cohort: first AD diagnosis in 2012–2014; controls: randomly selected medical claim). Outcomes for each preindex year were compared among propensity score-matched cohorts.
Results
Each year, before index, patients with AD were more likely to have all-cause emergency department visits, all-cause hospitalizations, potentially avoidable hospitalizations, and falls (P < .05 for all comparisons) than matched controls (N = 19,679 pairs). Increasing absolute and relative risks over time were observed for all outcomes.
Discussion
The study findings highlight the growing burden of illness before AD diagnosis and emphasize the need for timely recognition and management of patients with AD.
Keywords: PAH, Hospitalizations, Costs, Alzheimer's disease, Falls
1. Introduction
Potentially avoidable hospitalizations (PAHs) are admissions to a hospital for certain chronic and acute illnesses that could be prevented with successful management of these conditions in outpatient settings [1], [2], [3]. PAHs have been considered as indicators of quality of care provided in ambulatory care settings. In 2006, the Medicare Payment Advisory Commission revised a set of indicators called the Medicare Ambulatory Care Indicators for the Elderly (MACIEs) to assess the quality of care provided in ambulatory settings using the Medicare claims data [1]. Similarly, the Agency for Healthcare Research and Quality developed the Prevention Quality Indicators (PQIs) to identify PAHs using criteria that are fairly similar to those developed by the Medicare Payment Advisory Commission [4]. Both the MACIE and PQI criteria outline high-level paradigms of care that, if properly delivered, should help patients avoid hospitalization for conditions such as hypertension, chronic obstructive pulmonary disease/asthma, heart failure, and urinary tract infection. Because hospitalizations tend to be costlier than ambulatory care, minimizing the incidence of preventable hospital visits can also help reduce the overall cost of care.
Patients with Alzheimer's disease (AD) and related dementia (ADRD) are at a particularly increased risk of experiencing hospitalizations given the substantially higher rates of comorbidities among these patients than among those with normal cognition [5], [6]. Indeed, several studies have shown that the presence of dementia complicates the management of comorbid conditions and that hospitalization for these comorbidities involves a substantial share of expenditures for patients with AD [7], [8]. In addition, it is estimated that in 2013, nearly one in ten patients with ADRD experienced at least one PAH, which resulted in approximately $2.6 billion in Medicare expenditures [9]. Using data from an integrated care system, Phelan et al. [10] found that the incidence of dementia significantly increased the risk of all-cause hospitalizations and PAHs compared to those without dementia. In a different study using Medicare data, Lin et al. [11] found similar results with regard to PAHs for short-term and long-term diabetes complications and hypertension. The study also found that among patients with PAH, those with ADRD had significantly higher costs than control patients with no ADRD. In another study, using data from the Health and Retirement Study linked with Medicare claims, Feng et al. [12] found that after controlling for patient differences, 7.3% of Medicare fee-for-service beneficiaries with ADRD had a PAH annually compared with 4.2% among those with no cognitive impairment. Using the same data source, Davydow et al. [13] also found that compared with respondents without dementia, those with dementia had a 32% greater likelihood of experiencing a PAH. The same study also reported that respondents with cognitive impairment but no dementia—a cognitive status that can develop several years before clinical AD diagnosis [14] and affects as many as 20% of Americans aged 65 years and older [15], [16], [17]—were 25% more likely to experience a PAH than respondents with normal cognition. In addition, even though falls are not formally considered a quality metric by the Centers for Medicare and Medicaid Services/Agency for Healthcare Research and Quality, these could also potentially be avoided by adequate patient management and supervision [2]. Indeed, several studies have documented the high rates of falls among patients with AD [18], [19].
To the best of our knowledge, however, no study to date has evaluated the likelihood of PAHs or falls and associated costs among patients with AD in the extended period leading up to their AD diagnosis using real-world data. This prediagnosis period is of particular importance as the perceived onset of cognitive impairment among patients eventually diagnosed with AD could precede an initial diagnosis by over 4 years [14], [20], [21], [22], [23]. For example, recent studies detected episodic memory decline 7 to 10 years before the diagnosis of mild cognitive impairment or AD dementia [21], [22]. In another study, using longitudinal clinical assessment data from the National Alzheimer's Coordinating Center, we found that the subjective report of cognitive decline occurred, on average, 4.5 years before the diagnosis of AD [20]. A lack of diagnosis among patients already experiencing cognitive impairment could in turn increase complexities in the management of other acute and chronic conditions commonly present among this population and increase the overall medical resource use. Indeed, in a recent study by Rosenbloom et al. [24], compared with patients who screened negative for dementia, those who screened positive for dementia had experienced 32% higher rates of emergency department (ED) visits and 39% higher rates of hospitalization in the 18 months before the screening.
The objective of this study was to examine the rates of and costs associated with PAHs—for both chronic and acute conditions—and falls in the 3 years preceding an AD diagnosis. In addition, to characterize the relationship between PAHs and the overall resource use, the study also evaluated the burden associated with all-cause hospitalizations and ED visits before AD diagnosis.
2. Methods
2.1. Data
This study was conducted using deidentified administrative claims data from the Standard Analytical Files for a 5% random sample of Medicare beneficiaries. The data span the years 1999–2014 and include information on Medicare enrollment history, patient demographics, and detailed medical claims including diagnosis codes, procedure codes, place of service (e.g., hospital, physician office), dates of service (year and quarter; precise dates available from 2009 onwards), provider type, provider specialty, and associated payments made by Medicare to providers. However, the data do not include Medicare Part D (i.e., prescription drug) claims.
2.2. Study sample and time periods
Two mutually exclusive cohorts of patients were identified: patients with AD (“AD patients”) and without ADRD (“controls”). Patients with AD were identified as Medicare beneficiaries with at least two distinct medical claims with a diagnosis code for AD (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 331.0x), with the first such claim—defined as the study index date—occurring between 2012 and 2014. In addition, to minimize the likelihood of including patients with comorbid or misdiagnosed dementia, patients with AD were required to have no claims for other dementia etiologies (i.e., vascular dementia, Parkinson's dementia, dementia with Lewy bodies, frontotemporal dementia, or normal pressure hydrocephalus) between or after the two most recent AD diagnoses.
The control cohort was defined as patients with no claims for AD, related dementia etiologies, or other dementia conditions (i.e., dementia - unspecified, senile dementia, presenile dementia, and dementia in conditions specified otherwise) at any point in their medical history. The index date for controls was a randomly selected medical claim between 2012 and 2014 to ensure comparability with the AD cohort who, by definition, had a medical interaction on their index date. In addition, control patients were required to have no indication of cognitive impairment (i.e., amnestic disorders, drug- or alcohol-induced dementia, mild cognitive impairment, other cerebral degeneration, other persistent mental disorders, or other senile psychotic conditions) any time before the index date. To increase computational efficiency, a 25% sample of the control cohort was selected using a probability-based simple random sampling approach [25], [26]. The sampling approach consisted of two steps. First, all patients were assigned a random number between 0 and 1 using the SAS software. After this, patients with values less than 0.25 (representing 25% of the overall sample) were included in the final sample. A random sampling approach was used to ensure that the selected subset of patients was representative of the overall cohort meeting the selection criteria.
Continuous, non-HMO Medicare enrollment was required during the period 4 years before the index date for all patients. Patients were also required to be aged ≥ 65 years and have no hospice use during this period. The year beginning 4 years before the index date constituted the baseline period, whereas the period 3 years before the index date constituted the study period for both cohorts (Supplementary Appendix Fig. 1).
2.3. Baseline patient characteristics
Patient characteristics—including demographics (age, gender, race, US census region), year of index date, Charlson Comorbidity Index and its components [27], [28], select additional comorbidities (hyperlipidemia, depression, psychosis, and bipolar disorders), proportions of patients with ≥1 hospitalization, ED visit, outpatient/physician office visit, skilled nursing facility (SNF) visit, or home health-care visit and total medical costs during the baseline period—were compared between the AD and control cohorts. Costs are reported in 2015 US dollars [29] and reflect amounts paid by Medicare to providers on a fee-for-service basis. Statistical significance of differences was assessed using chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous measures.
2.4. Outcomes
Measures of all-cause ED visits, hospitalizations, PAHs for chronic and acute conditions, and falls were compared between cohorts in each year of the study period. Specifically, for each outcome, we quantified the proportions of patients experiencing at least one event within the year. In addition, we estimated the average per-patient costs associated with all-cause hospitalizations and all-cause ED visits for the two cohorts. Furthermore, for all outcomes, we also calculated the average number of events experienced by the patients with at least one event and average medical costs associated with the outcome to examine potential differences in the frequency and complexity of events across the two cohorts.
All-cause hospitalizations and ED visits were identified based on the definitions suggested by the Centers for Medicare and Medicaid Services [30], [31]. Falls were identified as hospitalizations or ED visits with a diagnosis code for fall (ICD-9-CM: E880-E888). PAHs were identified based on criteria defined by MACIE and PQI [2], [4]. In this study, we analyzed hospitalizations associated with serious short-term complications of diabetes, serious long-term complications of diabetes, chronic obstructive pulmonary disease or asthma, hypertension, heart failure, and ED visits with unstable angina to identify PAHs associated with these chronic conditions. For acute conditions, hospitalizations associated with dehydration, urinary tract infection, and bacterial pneumonia as the primary reasons were considered. These conditions were selected as they represent common acute and chronic conditions among the Medicare population, which are considered sensitive to ambulatory care in the most recent MACIE guidance and are readily identifiable in the Medicare claims data [2], [4].
2.5. Statistical analyses
Our analytical approach consisted of two steps. First, to identify potential differences in outcomes resulting from an AD diagnosis, patients with AD were matched one-to-one to controls using propensity score-based greedy matching algorithm [32], [33]. Propensity scores (i.e., the predicted probability of being a patient with AD) were estimated using a logistic regression model with cohort assignment (AD vs. control) as the dependent variable and baseline patient characteristics including demographics, Charlson Comorbidity Index components with ≥3% prevalence in the AD cohort, rates of select additional comorbidities, rates of medical resource use, and medical costs as independent variables. After estimating the propensity scores, the two cohorts were matched one-to-one using a greedy matching algorithm with a caliper of 0.25 standard deviations of the propensity score (on the log odds scale).
Then, the statistical significance of differences in baseline characteristics and outcomes was compared between the matched cohorts using McNemar's test for categorical variables and Wilcoxon sign-rank test for continuous measures. In addition, relative risks of experiencing an all-cause ED visit, hospitalization, PAH, or fall among the patients in the AD cohort relative to matched controls were estimated during each year of the study period.
Because propensity score matching can result in exclusion of certain patients for whom a match is not obtained, potentially affecting the generalizability of findings, we also conducted a regression-based sensitivity analysis. Logistic regression models were used to estimate the risk of all-cause hospitalizations, ED visits, and ≥1 PAH (overall) for patients with AD relative to controls. The models were estimated for the unmatched cohorts with cohort assignment as the key predictor variable and baseline characteristics (similar to those used to estimate propensity scores) as covariates.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). A P value < .05 was considered statistically significant.
3. Results
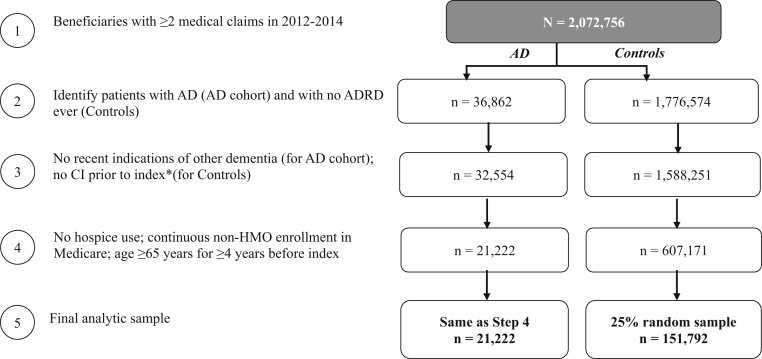
The selection criteria before matching resulted in a sample of 21,222 patients with AD and 151,792 control patients (Fig. 1).
Fig. 1.
Sample selection and resulting patient counts. Abbreviations: AD, Alzheimer's disease; ADRD, Alzheimer's disease and related dementia; CI, cognitive impairment; HMO, health maintenance organization. *Index for AD cohort: first AD diagnosis; for controls: date of a randomly selected medical claim.
3.1. Baseline characteristics
Before matching, patients with AD were older (83.6 vs. 77.3 years) than controls on index and had significantly higher rates of comorbidities such as cerebrovascular disease (16.8% vs. 7.5%), congestive heart failure (14.2% vs. 8.4%), and depression (14.3% vs. 5.9%) during the baseline period (Table 1). Furthermore, patients with AD were significantly more likely to use medical services and incurred higher medical costs during baseline than control patients ($9597 vs. $6051). P values were < .05 for all comparisons.
Table 1.
Baseline patient characteristics before and after matching
| Patient characteristics | Unmatched |
Matched |
||||
|---|---|---|---|---|---|---|
| AD |
Controls |
P | AD |
Controls |
P | |
| (N = 21,222) | (N = 151,792) | (N = 19,679) | (N = 19,679) | |||
| Age, mean (SD) | 83.59 (6.78) | 77.28 (6.19) | ** | 83.34 (6.67) | 83.47 (6.97) | |
| Male (%) | 33.5% | 43.2% | ** | 34.0% | 33.9% | |
| Race (%) | - | - | ** | - | - | |
| White | 87.9% | 88.9% | 87.9% | 87.8% | ||
| Black | 7.3% | 6.3% | 7.2% | 7.1% | ||
| Asian | 1.5% | 1.6% | 1.5% | 1.6% | ||
| Hispanic | 2.1% | 1.2% | 2.1% | 2.1% | ||
| North American Native | 0.3% | 0.4% | 0.3% | 0.3% | ||
| US Census region (%) | - | - | ** | - | - | |
| West | 15.0% | 17.8% | 15.2% | 15.3% | ||
| Midwest | 23.7% | 24.1% | 23.7% | 23.8% | ||
| Northeast | 18.2% | 18.3% | 17.9% | 17.8% | ||
| South | 42.7% | 39.5% | 42.7% | 42.8% | ||
| Year of index date (%) | - | - | ** | - | - | |
| 2012 | 38.9% | 33.7% | 38.8% | 38.7% | ||
| 2013 | 35.2% | 32.4% | 35.3% | 35.3% | ||
| 2014 | 25.9% | 33.9% | 26.0% | 26.0% | ||
| Patients with ≥1 CCI components | ||||||
| Chronic pulmonary disease | 18.3% | 15.3% | ** | 18.0% | 18.1% | |
| Peripheral vascular disease | 17.9% | 10.0% | ** | 16.7% | 16.5% | |
| Cerebrovascular disease | 16.8% | 7.5% | ** | 15.2% | 15.1% | |
| Mild to moderate diabetes | 16.4% | 16.6% | 16.4% | 16.8% | ||
| Congestive heart failure | 14.2% | 8.4% | ** | 13.5% | 13.5% | |
| Any malignancy including lymphoma and leukemia | 11.5% | 11.9% | 11.7% | 12.0% | ||
| Diabetes with chronic complications | 10.4% | 7.4% | ** | 10.0% | 10.0% | |
| Renal disease | 9.6% | 6.3% | ** | 9.2% | 9.3% | |
| Myocardial infarction | 4.9% | 3.4% | ** | 4.6% | 4.5% | |
| Rheumatologic disease | 3.5% | 2.9% | ** | 3.4% | 3.4% | |
| Patients with ≥1 additional comorbidities | ||||||
| Hyperlipidemia | 57.1% | 58.3% | ** | 57.1% | 57.8% | |
| Depression | 14.3% | 5.9% | ** | 12.7% | 12.2% | |
| Psychosis | 3.3% | 0.4% | ** | 2.1% | 1.7% | ** |
| Bipolar disorder | 1.1% | 0.4% | ** | 0.9% | 0.8% | |
| Baseline resource use (patients with ≥1 visit [%]) | ||||||
| Emergency department visits | 35.8% | 21.3% | ** | 33.8% | 33.1% | |
| Inpatient visits | 19.6% | 11.9% | ** | 17.9% | 17.2% | |
| Outpatient/physician office visits | 97.0% | 96.4% | ** | 96.8% | 96.9% | |
| Skilled nursing facility visits | 6.1% | 1.6% | ** | 4.6% | 4.1% | * |
| Home health-care visits | 13.2% | 4.6% | ** | 11.4% | 10.6% | ** |
| Baseline costs, mean (SD) | $9597 ($18,379) | $6051 ($13,395) | ** | $8711 ($17,074) | $8457 ($17,254) | ** |
Abbreviations: AD, Alzheimer's disease; CCI, Charlson Comorbidity Index; SD, standard deviation.
**P < .01 and *P < .05, evaluated before matching using chi-square tests for proportions and Wilcoxon rank-sum tests for continuous measures and evaluated after matching using McNemar's tests for proportions and Wilcoxon sign-rank test for continuous measures.
The matching process resulted in 19,679 matched pairs of patients with AD and controls with similar demographic characteristics and comorbidities (Table 1).
3.2. Relative risks of experiencing the outcomes over time
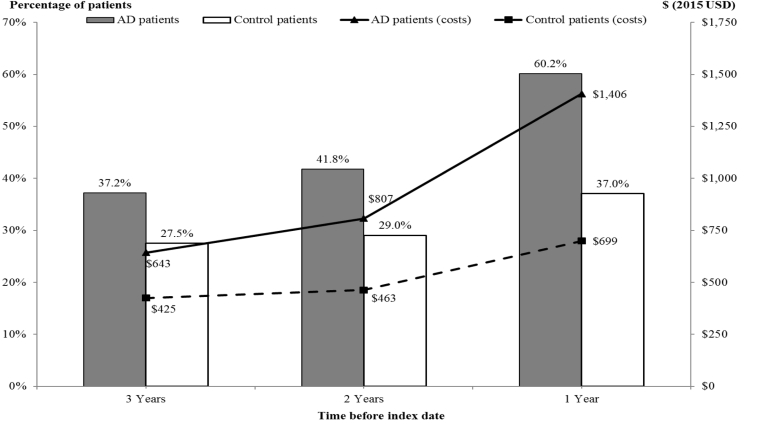
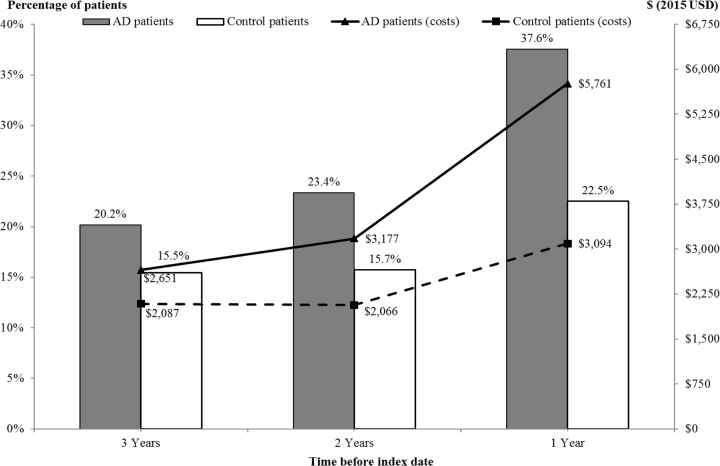
During each year before index, patients with AD were significantly (P < .01) more likely than matched controls to have ≥1 all-cause ED visit and all-cause hospitalization, with the likelihoods and relative risks increasing over time (Figs. 2 and 3, Table 2). In addition, patients with AD were significantly more likely to have ≥1 PAH (overall) during each year before index: (1) 6.5% vs. 4.8% three years before; (2) 8.3% vs 5.5% two years before; and (3) 14.6% vs. 8.6% one year before index (P < .01 for all comparisons). As with all-cause ED visits and hospitalizations, the relative risk of experiencing a PAH in the AD cohort increased from 1.35 three years before index to 1.70 during the year leading up to the index date compared with matched controls (Table 2). However, for both cohorts, the proportion of patients with ≥1 all-cause hospitalization and ≥1 PAH was similar: (1) approximately 33% in the second and third years before index and (2) approximately 40% in the year before index.
Fig. 2.
Rates of and costs associated with all-cause ED visits during each year before index (N = 19,679 matched pairs). P < .01 for all comparisons, evaluated using McNemar's tests for proportions and Wilcoxon sign-rank test for continuous measures. Average per-patient costs (from Medicare perspective) were estimated among all patients within a cohort, including those with no ED visit during the relevant year. Abbreviations: AD, Alzheimer's disease; ED, emergency department.
Fig. 3.
Rates of and costs associated with all-cause hospitalizations during each year before index (N = 19,679 matched pairs). P < .01 for all comparisons, evaluated using McNemar's tests for proportions and Wilcoxon sign-rank test for continuous measures. Average per-patient costs (from Medicare perspective) were estimated among all patients within a cohort, including those with no hospitalization during the relevant year. Abbreviation: AD, Alzheimer's disease.
Table 2.
Relative risks of health service use, PAHs, and falls in each year before index date (N = 19,679 matched pairs)
| Condition | Time before index date |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year 3 |
Year 2 |
Year 1 |
||||||||||
| AD |
Controls |
RR |
P | AD |
Controls |
RR |
P | AD |
Controls |
RR |
P | |
| % | % | AD/controls | % | % | AD/controls | % | % | AD/controls | ||||
| All-cause ED visits | 37.2 | 27.5 | 1.35 | ** | 41.8 | 29.0 | 1.44 | ** | 60.2 | 37.0 | 1.62 | ** |
| All-cause hospitalizations | 20.2 | 15.5 | 1.30 | ** | 23.4 | 15.7 | 1.49 | ** | 37.6 | 22.5 | 1.67 | ** |
| Any PAH | 6.5 | 4.8 | 1.35 | ** | 8.3 | 5.5 | 1.51 | ** | 14.6 | 8.6 | 1.70 | ** |
| PAH for chronic conditions | ||||||||||||
| Short-term complications of diabetes | 0.05 | 0.02 | 2.25 | 0.07 | 0.03 | 2.17 | 0.15 | 0.04 | 3.75 | ** | ||
| Long-term complications of diabetes | 1.2 | 0.7 | 1.61 | ** | 1.4 | 0.8 | 1.89 | ** | 2.4 | 1.2 | 1.95 | ** |
| COPD/asthma | 2.6 | 2.2 | 1.17 | * | 3.3 | 2.7 | 1.23 | ** | 5.5 | 4.2 | 1.32 | ** |
| Hypertension | 0.1 | 0.1 | 1.16 | 0.1 | 0.1 | 1.25 | 0.2 | 0.2 | 1.00 | |||
| Heart failure | 1.9 | 1.6 | 1.17 | * | 2.5 | 1.9 | 1.31 | ** | 4.9 | 3.4 | 1.45 | ** |
| PAH for acute conditions | ||||||||||||
| Dehydration | 0.4 | 0.2 | 1.93 | ** | 0.4 | 0.1 | 2.88 | ** | 0.7 | 0.2 | 3.72 | ** |
| Bacterial pneumonia | 1.1 | 0.8 | 1.36 | ** | 1.5 | 1.0 | 1.60 | ** | 2.5 | 1.6 | 1.59 | ** |
| Urinary tract infection | 1.0 | 0.4 | 2.44 | ** | 1.5 | 0.6 | 2.60 | ** | 3.5 | 0.6 | 5.35 | ** |
| Any fall | 7.0 | 3.4 | 2.04 | ** | 6.9 | 3.0 | 2.27 | ** | 10.6 | 3.8 | 2.80 | ** |
Abbreviations: AD, Alzheimer's disease; COPD, chronic obstructive pulmonary disease; ED, emergency department; PAH, potentially avoidable hospitalization; RR, risk ratio.
**P < .01 and *P < .05, evaluated using McNemar's tests for proportions and Wilcoxon sign-rank test for continuous measures.
When considering individual conditions comprising the overall rates of PAH, for both cohorts, PAHs for chronic obstructive pulmonary disease/asthma were most frequent (2%–6% in years 3-1 before index), and PAHs for short-term complications of diabetes were the least common (≤0.2% in each year before index). As with the overall rate of PAHs, the relative risks of PAHs for the AD cohort increased over time for all conditions other than short-term complications for diabetes, hypertension, and bacterial pneumonia (Table 2).
Results were similar with regard to relative risks for falls. Specifically, patients in the AD cohort were significantly (P < .01) more likely to have a hospitalization or ED visit related to falls in every year before the index than matched controls; the likelihoods increased over time (7.0% vs. 3.4%—risk ratio: 2.04 three years before index to 10.6% vs. 3.8%—risk ratio: 2.80 one year before index; Table 2).
Results of regression-adjusted sensitivity analyses were similar to the core study results (Supplementary Appendix Table 1).
3.3. Relative frequencies and costs associated with outcomes over time
Among patients with ≥1 hospitalization, on average, patients with AD experienced more hospitalizations per year than matched controls: (1) 1.54 vs. 1.43 three years before index; (2) 1.60 vs. 1.47 two years before index; and (3) 1.78 vs. 1.56 the year before index (Table 3). Results were similar among patients with ≥1 ED visit. Similarly, among patients experiencing at least one PAH, the average number of PAH per patient increased from 1.46 three years before index to 1.67 during the year before index for AD cohort compared with 1.41 and 1.57, respectively, for controls; the differences, however, were not statistically significant. Results were similar when evaluating each condition separately (other than hypertension and bacterial pneumonia).
Table 3.
Number of and costs associated with health service use, PAHs, and falls in each year before the index date among patients with at least one event (N = 19,679 matched pairs)
| Condition | Time before index date |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Year 3 |
Year 2 |
Year 1 |
|||||||
| AD | Controls | P | AD | Controls | P | AD | Controls | P | |
| Average number of, mean (SD) | |||||||||
| All-cause ED visits | 5.00 (4.53) | 4.25 (3.40) | ** | 5.56 (4.94) | 4.34 (3.40) | ** | 6.71 (6.08) | 4.84 (4.01) | ** |
| All-cause hospitalizations | 1.54 (0.97) | 1.43 (0.84) | ** | 1.60 (1.08) | 1.47 (0.90) | ** | 1.78 (1.27) | 1.56 (1.04) | ** |
| Any PAH | 1.46 (0.94) | 1.41 (0.84) | 1.53 (1.03) | 1.48 (0.96) | 1.67 (1.25) | 1.57 (1.10) | |||
| PAH for chronic conditions | |||||||||
| Short-term complications of diabetes | 1.11 (0.33) | 1.00 (0.00) | 1.23 (0.60) | 1.00 (0.00) | 1.03 (0.18) | 1.00 (0.00) | |||
| Long-term complications of diabetes | 1.27 (0.63) | 1.24 (0.66) | 1.35 (0.84) | 1.28 (0.66) | 1.42 (0.92) | 1.28 (0.74) | |||
| COPD/asthma | 1.64 (1.07) | 1.46 (0.83) | 1.71 (1.18) | 1.53 (0.97) | 1.89 (1.43) | 1.65 (1.19) | |||
| Hypertension | 1.00 (0.00) | 1.16 (0.69) | 1.16 (0.55) | 1.10 (0.31) | 1.02 (0.16) | 1.07 (0.26) | |||
| Heart failure | 1.52 (0.99) | 1.49 (0.92) | 1.55 (0.98) | 1.57 (0.99) | 1.81 (1.38) | 1.67 (1.15) | |||
| PAH for acute conditions | |||||||||
| Dehydration | 1.01 (0.11) | 1.00 (0.00) | 1.00 (0.00) | 1.00 (0.00) | 1.03 (0.16) | 1.00 (0.00) | |||
| Bacterial pneumonia | 1.10 (0.30) | 1.11 (0.33) | 1.07 (0.31) | 1.08 (0.33) | 1.09 (0.33) | 1.06 (0.26) | |||
| Urinary tract infection | 1.11 (0.48) | 1.05 (0.27) | 1.07 (0.29) | 1.07 (0.34) | 1.11 (0.37) | 1.06 (0.24) | |||
| Any fall | 1.27 (0.63) | 1.18 (0.46) | 1.22 (0.59) | 1.12 (0.35) | 1.28 (0.67) | 1.08 (0.35) | ** | ||
| Associated costs, mean (SD) | |||||||||
| All-cause ED visits | $1729 ($1955) | $1546 ($1892) | ** | $1930 ($2181) | $1595 ($1871) | ** | $2337 ($2492) | $1886 ($2209) | ** |
| All-cause hospitalizations | $13,144 ($16,394) | $13,504 ($15,924) | $13,595 ($17,976) | $13,155 ($15,914) | $15,342 ($20,363) | $13,749 ($17,233) | ** | ||
| Any PAH | $11,433 ($13,948) | $11,975 ($15,141) | $12,124 ($15,465) | $12,073 ($14,216) | $14,015 ($18,978) | $14,009 ($17,176) | |||
| PAH for chronic conditions | |||||||||
| Short-term complications of diabetes | $17,363 ($16,350) | $5229 ($3103) | $12,433 ($8694) | $4079 ($2065) | $9733 ($9215) | $4571 ($2204) | |||
| Long-term complications of diabetes | $11,715 ($11,881) | $12,063 ($12,384) | $12,608 ($16,124) | $12,186 ($13,412) | $14,140 ($18,399) | $13,950 ($16,390) | |||
| COPD/asthma | $13,249 ($14,098) | $12,569 ($14,174) | $13,982 ($16,398) | $13,033 ($15,296) | $17,324 ($21,066) | $15,015 ($17,746) | |||
| Hypertension | $4533 ($4769) | $4490 ($3776) | $4884 ($6729) | $5933 ($5697) | $3742 ($2550) | $3966 ($2470) | |||
| Heart failure | $13,907 ($16,384) | $15,090 ($19,785) | $14,941 ($16,900) | $12,946 ($12,505) | $17,361 ($20,618) | $16,485 ($18,761) | |||
| PAH for acute conditions | |||||||||
| Dehydration | $3654 ($1961) | $3438 ($1712) | $3839 ($2685) | $3788 ($2870) | $4545 ($3647) | $4672 ($3628) | |||
| Bacterial pneumonia | $6254 ($4776) | $7107 ($6620) | $6378 ($5522) | $6960 ($5533) | $6872 ($6893) | $6334 ($5784) | * | ||
| Urinary tract infection | $4803 ($3728) | $4112 ($1946) | $4818 ($3284) | $4670 ($2859) | $5337 ($4304) | $5077 ($3798) | |||
| Any fall | $2798 ($5874) | $2474 ($5937) | $1568 ($4676) | $1487 ($4544) | $845 ($3401) | $734 ($2942) | |||
Abbreviations: AD, Alzheimer's disease; COPD, chronic obstructive pulmonary disease; ED, emergency department; PAH, potentially avoidable hospitalization; SD, standard deviation.
**P < .01 and *P < .05, evaluated using McNemar's tests for proportions and Wilcoxon sign-rank test for continuous measures.
With regard to costs, in each year of the study period, the average per-patient medical costs associated with hospitalizations and ED visits were significantly higher for patients with AD than those for matched controls, with the costs increasing over time (Figs. 2 and 3). Results were similar among patients with ≥1 ED visit in a given year (Table 3). Among those with ≥1 hospitalization, the average per-patient cost associated with hospitalizations was higher for the AD cohort in the year before index ($15,342 vs. $13,749, P < .01); however, the two cohorts had similar costs three years and two years before index. The average per-patient costs associated with PAH, among those experiencing ≥1 PAH, increased similarly over time for both cohorts from ∼$11,000 three years before index to ∼$14,000 during the year before index.
Regarding patients experiencing a fall, in each year of the study period, the AD cohort appeared to have slightly higher number of falls and associated costs than matched controls, although the differences were not statistically significant (Table 3). In contrast to the results seen for all-cause costs and PAH, both cohorts experienced a decline in fall-related costs over time.
4. Discussion
Previous studies evaluating the prevalence of and costs associated with PAH among elderly patients with dementia have found that the presence of ADRD is associated with an increased risk of resource use, including PAHs, and costs [9], [10], [11], [12], [13]. These studies, however, have largely focused on the postdiagnosis period. Our study results provide evidence that even before an AD diagnosis, Medicare fee-for-service beneficiaries have substantially higher rates of all-cause ED visits, all-cause hospitalizations, PAHs, and hospitalizations or ED visits for falls than matched control patients with no cognitive impairment. Over time, the likelihoods of these events increased for both cohorts; however, the relative risks were higher in the AD cohort relative to matched controls. For example, three years before index, patients with AD were 1.4 times more likely to experience all-cause hospitalizations and PAHs than matched controls; the relative risk of experiencing the same events in the AD cohort increased to 1.7 in the year before index. However, for both cohorts, the proportion of patients with ≥1 all-cause hospitalization and ≥1 PAH in a given year was similar: (1) approximately 33% in the second and third years before index and (2) approximately 40% in the year before index. These findings suggest that the increased likelihood of experiencing a PAH may, in part, be driven by the fact that patients with AD are generally more likely to have a hospitalization than patients without AD—a finding that is consistent with prior research [10], [12], [24]. In addition, among those with at least one hospitalization, on average, the number of hospitalization for the AD cohort was higher than that for matched controls in each year of the study period, suggesting a greater intensity of resource use among patients with AD. Results were similar when evaluating subsets with PAHs, although the differences were not statistically significant.
Taken together, these results suggest that patients eventually diagnosed with AD are not only more likely to have a hospitalization or ED visit, and consequently PAHs or falls in the years leading up to the diagnosis, but are also likely to have more of these events than matched controls. For example, in the year before the diagnosis, 7390 patients with AD experienced 13,154 hospitalizations for any reason, and 4806 (37%) of these were for conditions that, if managed appropriately in ambulatory settings, could have been potentially prevented. By comparison, 4429 matched controls experienced 6909 all-cause hospitalizations, and 2664 (39%) of these were potentially avoidable. Similarly, for falls, in the year before diagnosis, the proportion of patients with at least one event was nearly three times higher in the AD cohort than that of the matched controls (10.6% vs. 3.8%), resulting in 1860 more fall-related hospitalizations or ED visits than matched controls.
With regard to costs, we find that on average, patients with AD have higher average costs related to hospitalizations and ED visits during the three years before the formal diagnosis, with the estimates increasing up to the point of diagnosis. An increased economic burden before AD diagnosis has also been demonstrated by other studies such as that by Geldmacher et al. [34] who found that compared with matched controls, patients with AD had $5549 in excess total medical costs during the year before diagnosis and by Lin et al. [35] who found that during the year before diagnosis, the average medical costs for patients newly diagnosed with ADRD or mild cognitive impairment were ∼40% higher than those of the matched controls. However, we find that conditional on having a hospitalization (or PAHs or falls, for that matter) in a given year, the average per-patient costs associated with the event did not differ across the two cohorts. Although the precise mechanism behind this finding remains unknown, results suggest that despite patients with AD experiencing more events before the index date, the severity of the events may be similar. Nevertheless, the fact that more patients among the AD cohort experience these events suggests that the incremental cost burden associated with these outcomes in the prediagnosis period is greater among patients eventually diagnosed with AD than among those with no cognitive impairment.
The findings of this study could be attributed to the fact that patients eventually diagnosed with AD experience symptoms of cognitive impairment several years earlier, which could result in complexities in the management of their comorbidities and general health. Although our study findings underscore the need for timely diagnosis and optimal management of patients with cognitive impairment, additional research is needed to understand the extent to which interventions targeted to improve dementia evaluation and care management might reduce medical resource use in the real world [24], [36].
Recently, initiatives such as the Medicare Annual Wellness Visit [37] and the National Alzheimer's Project Act [38] have been introduced to improve the detection and management of patients with AD. Similarly, the Alzheimer's Association has proposed several strategies to improve dementia care and reduce the rates of PAHs and falls, including (but not limited to) following best practices for care management, educating providers about dementia and its impact on comorbidities, and targeting cognitively impaired patients who live alone in an effort to prevent falls [5]. However, dementia-related quality measures are not yet an integral part of the current health-care system [39]. Future real-world studies, particularly those that characterize the trajectory of health-care resource utilization across the early continuum of AD, could help inform strategies for optimal care management of patients at the earliest stages of the disease.
4.1. Strengths and limitations
To the best of our knowledge, this is the first study to provide comprehensive estimates of the incremental resource use, costs, and PAHs in the period leading up to the AD diagnosis using data from a nationally representative sample of Medicare beneficiaries. In addition, the study used robust analytical techniques to minimize the effects of confounding and selection bias. Nevertheless, the study is subject to limitations associated with an observational study design, including any inaccuracies or incompleteness of the ICD-9-CM codes used to identify diagnoses and any lack of clinical information (e.g., to assess dementia etiology/severity), and therefore, the study findings should be interpreted with caution. In particular, previous studies have reported that a substantial proportion of individuals with dementia were not informed about their diagnosis [40]. To the extent that a similar pattern of underreporting of AD diagnoses exists in administrative claims data, this analysis may not characterize the full cohort of patients with AD in Medicare. Relatedly, although the study used algorithms to minimize the inclusion of patients with cognitive impairment in the control cohort, an unknown proportion of these patients may, in fact, have symptoms of cognitive impairment, and the true differences in outcomes may be larger than those reported in the present study. Similarly, although we excluded patients with other known etiologies of dementia, the proportion of patients with mixed dementia etiology is not known, and the findings may not be applicable to patients with other forms of dementia. In addition, while the propensity score matching analysis and the regression sensitivity analyses controlled for observable differences across patient cohorts, it cannot account for unobserved heterogeneity such as factors related to care provision in the ambulatory settings. Furthermore, the cost estimates reported in the study do not reflect prescription drug use or medical services covered by other payers (e.g., Medicare/Medicaid dual eligibility) and over-the-counter medications or informal care. Finally, findings are limited to beneficiaries enrolled in fee-for-service Medicare and may not be generalized to other populations (e.g., covered by managed Medicare).
5. Conclusions
Our study results suggest that even before AD diagnosis, patients have a considerable burden in terms of increased hospitalizations and ED visits, a third to 40% of which could be considered potentially avoidable according to the MACIE/PQI quality metrics. These findings further highlight the growing burden in the pre-AD diagnosis period and emphasize the need for timely recognition and management of patients with AD.
Research in Context.
-
1.
Systematic review: Recent literature suggests that people with Alzheimer's disease (AD) are at an increased risk of experiencing hospitalizations, many of which could be potentially preventable with better ambulatory care. Little is known, however, about the trends in health services provided during the extended period leading up to the AD diagnosis, during which time many people experience cognitive impairment symptoms.
-
2.
Interpretation: We find that even before AD diagnosis, people with AD have substantially higher and increasing likelihoods of hospital and emergency department visits than similar people with no cognitive impairment. A third to 40% of these visits could be potentially avoidable according to the existing quality metrics.
-
3.
Future directions: Future research should explore the specific mechanisms, particularly related to comorbidity management, associated with medical services used during the earliest stages of cognitive impairment, and assess the implications of timely diagnosis and treatment among people with AD.
Acknowledgments
The authors thank Yanying Sheng, who was an employee of Analysis Group, Inc., at the time of the study, for her support in conducting the data analysis. Parts of the findings from this analysis were presented at the 2017 Alzheimer's Association International Conference in London UK. Research funding provided by Eli Lilly and Company, Indianapolis, IN.
Footnotes
Conflict of interest: J.S.A. and W.Y. are employees of Eli Lilly and Company, Indianapolis, Indiana. U.D., N.Y.K., N.R.M., and J.W. are employees of Analysis Group, Inc., a company that received funding from Eli Lilly and Company to conduct this study.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.dadm.2018.12.005.
Supplementary data
References
- 1.MEDPAC 2006 Report. http://www.medpac.gov/documents/reports/Jun06_Ch01.pdf?sfvrsn=0 Available at:
- 2.Westrick E., Kogut S. Medicare Ambulatory Care Indicators for the Elderly: Refinement of the Access to Care for the Elderly Project Indicators (final report to MedPac, January 2006) http://permanent.access.gpo.gov/lps78740/MACIEFeb1206Final.pdf Available at:
- 3.Moy E., Barrett M., Ho K. Potentially preventable hospitalizations – United States, 2004-2007, CDC MMWR. January 2011. https://www.cdc.gov/mmwr/preview/mmwrhtml/su6001a17.htm Available at: [PubMed]
- 4.Agency for Healthcare Research and Quality (AHRQ) Guide to Prevention Quality Indicators. http://www.ahrq.gov/downloads/pub/ahrqqi/pqiguide.pdf Available at:
- 5.Alzheimer’s Association Policy Brief Reducing potentially preventable hospitalizations for people living with Alzheimer’s and other dementias. http://www.alz.org/publichealth/downloads/policy-brief-preventable.pdf 2017. Available at:
- 6.Bunn F., Burn A.M., Goodman C., Rait G., Norton S., Robinson L. Comorbidity and dementia: A scoping review of the literature. BMC Med. 2014;12:192. doi: 10.1186/s12916-014-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloan F.A., Taylor D.H., Jr. Effect of Alzheimer disease on the cost of treating other diseases. Alzheimer Dis Assoc Disord. 2002;16:137–143. doi: 10.1097/00002093-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hill J.W., Futterman R., Duttagupta S., Mastey V., Lloyd J.R., Fillit H. Alzheimer's disease and related dementias increase costs of comorbidities in managed Medicare. Neurology. 2002;58:62–70. doi: 10.1212/wnl.58.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Lin P.J., Rane P.B., Fillit H.M., Cohen J.T., Neumann P.J. National estimates of potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer’s disease and related dementia. Alzheimers Dement. 2016;12:253–254. doi: 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Phelan E.A., Borson S., Grothaus L., Balch S., Larson E.B. Association of incident dementia with hospitalizations. JAMA. 2012;307:165–172. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin P.J., Fillit H.M., Cohen J.T., Neumann P.J. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer's disease and related disorders. Alzheimers Dement. 2013;9:30–38. doi: 10.1016/j.jalz.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Feng Z., Coots L., Kaganova Y., Wiener J. 2013. Hospital and emergency department use by people with Alzheimer’s disease and related disorders: final report.https://aspe.hhs.gov/basic-report/hospital-and-emergency-department-use-people-alzheimer%E2%80%99s-disease-and-related-disorders-final-report#execsum Available at: [Google Scholar]
- 13.Davydow D.S., Zivin K., Katon W.J., Pontone G.M., Chwastiak L., Langa K.M. Neuropsychiatric disorders and potentially avoidable hospitalizations in a prospective cohort study of older Americans. J Gen Intern Med. 2014;29:1362–1371. doi: 10.1007/s11606-014-2916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan K.B., Wilson R.S., Weuve J., Barnes L.L., Evans D.A. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology. 2015;8:898–904. doi: 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langa K.M., Larson E.B., Karlawish J.H., Cutler D.M., Kabeto M.U., Kim S.Y. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez O.L., Jagust W.J., DeKosky S.T., Becker J.T., Fitzpatrick A., Dulberg C. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 17.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of Cognitive Impairment without Dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan L.M., Ballard C.G., Rowan E.N., Kenny R.A. Incidence and predictors of falls in dementia: a prospective study in older people. PLoS One. 2009;4:e5521. doi: 10.1371/journal.pone.0005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph J.L., Zanin N.M., Jones R.N., Marcantonio E.R., Fong T.G., Yang F.M. Hospitalization in Community-Dwelling Persons with Alzheimer's Disease: Frequency and Causes. J Am Geriatr Soc. 2010;58:1542–1548. doi: 10.1111/j.1532-5415.2010.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirson N.Y., Andrews J.S., Desai U., King S.B., Schonfeld S., Birnbaum H.G. Patient Characteristics and Outcomes Associated with Receiving an Earlier Versus Later Diagnosis of Probable Alzheimer’s Disease. J Alzheimers Dis. 2018;61:295–307. doi: 10.3233/JAD-170078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grober E., Hall C.B., Lipton R.B., Zonderman A.B., Resnick S.M., Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias M.F., Beiser A., Wolf P.A., Au R., White R.F., D'Agostino R.B. The preclinical phase of Alzheimer's disease – A 22-year prospective study of the Framingham cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 23.Albert M., Soldan A., Gottesman R., McKhann G., Sacktor N., Farrington L. Cognitive changes preceding clinical symptom onset of mild cognitive impairment and relationship to ApoE genotype. Curr Alzheimers Res. 2014;11:773–784. doi: 10.2174/156720501108140910121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbloom M., Barclay T.R., Borson S., Werner A.M., Erickson L.O., Crow J.M. Screening positive for cognitive impairment: impact on healthcare resource utilization and provider action in primary and specialty care practices. J Gen Intern Med. 2018;33:1746–1751. doi: 10.1007/s11606-018-4606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinbaum D.G., Kupper L.L., Mogenstern H. John Wiley & Sons, Inc.; New York, NY: 1982. Epidemiologic Research: Principles and Quantitative Methods. [Google Scholar]
- 26.Bryman A. Oxford University Press; New York, NY: 2012. Social Research Methods. [Google Scholar]
- 27.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Romano P.S., Roos L.L., Jollis J. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 29.US Bureau of Labor Statistics Consumer Price Index, Medical Care Component. https://www.bls.gov/cpi/ Available at:
- 30.Merriman K., Caldwell D. Research Data Assistance Center for CMS, University of Minnesota; Minneapolis, MN: 2015. How to Identify Emergency Room Services in the Medicare Claims Data.http://www.resdac.org/resconnect/articles/144 Available at: [Google Scholar]
- 31.OPPS Visit codes frequently asked questions. Centers for Medicare and Medicaid Services; 2008. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Downloads/OPPS_QandA.pdf Available at: [Google Scholar]
- 32.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Austin P.C. A comparison of 12 algorithms for matching on the propensity score. Statist Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geldmacher D.S., Kirson N.Y., Birnbaum H.G., Eapen S., Kantor E., Cummings A.K. Pre-diagnosis excess acute care costs in Alzheimer's patients among a US Medicaid population. Appl Health Econ Health Policy. 2013;11:407–413. doi: 10.1007/s40258-013-0038-9. [DOI] [PubMed] [Google Scholar]
- 35.Lin P.J., Zhong Y., Fillit H.M., Chen E., Neumann P.J. Medicare expenditures of individuals with Alzheimer's disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64:1549–1557. doi: 10.1111/jgs.14227. [DOI] [PubMed] [Google Scholar]
- 36.Phelan E.A., Debnam K.J., Anderson L.A., Owens S.B. A systematic review of intervention studies to prevent hospitalizations of community-dwelling older adults with dementia. Med Care. 2015;53:207–213. doi: 10.1097/MLR.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Medicare and Medicaid Services Annual Wellness Visit. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/awv_chart_icn905706.pdf Available at:
- 38.US Department of Health and Human Services National Alzheimer’s Project Act. http://napa.alz.org/national-alzheimers-project-act-backgroun Available at:
- 39.US Department of Health and Human Services . 2014. Priority Setting for Healthcare Performance Measurement: Addressing Performance Measure Gaps for Dementia, Including Alzheimer's Disease. Final report.http://www.qualityforum.org/priority_setting_for_healthcare_performance_measurement_alzheimers_disease.aspx Available at: [Google Scholar]
- 40.Alzheimer's Association . 2018. Alzheimer's Disease Facts and Figures.https://www.alz.org/media/HomeOffice/Facts%20and%20Figures/facts-and-figures.pdf Available at: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.