Abstract
Chlamydia are a genus of successful obligate intracellular pathogens spread across humans, wildlife, and domesticated animals. The most common species reported in livestock in this genus are Chlamydia abortus, Chlamydia psittaci, Chlamydia suis, and Chlamydia pecorum. Chlamydial infections trigger a series of inflammatory disease-related sequelae including arthritis, conjunctivitis, pneumonia, and abortion. Other bacteria in the phylum Chlamydiae have also been reported in livestock and wildlife but their impact on animal health is less clear. Control of chlamydial infections relies on the use of macrolides, fluoroquinolones, and tetracyclines. Tetracycline resistance (TETR) reported for porcine C. suis strains in association with the use of tetracycline feed is a potentially significant concern given experimental evidence highlighting that the genetic elements inferring TETR may be horizontally transferred to other chlamydial species. As documented in human Chlamydia trachomatis infections, relapse of infections, bacterial shedding post-antibiotic treatment, and disease progression despite chlamydial clearance in animals have also been reported. The identification of novel chlamydiae as well as new animal hosts for previously described chlamydial pathogens should place a renewed emphasis on basic in vivo studies to demonstrate the efficacy of existing and new antimicrobial treatment regimes. Building on recent reviews of antimicrobials limited to C. trachomatis and C. suis, this review will explore the use of antimicrobials, the evidence and factors that influence the treatment failure of chlamydial infections in animals and the future directions in the control of these important veterinary pathogens.
Keywords: Chlamydia, treatment failure, tetracycline resistance, antichlamydials, veterinary medicine, veterinary chlamydiae, antimicrobial treatment
Introduction
Bacteria within the phylum Chlamydiae are globally significant human and animal pathogens causing asymptomatic infections, as well as acute and chronic diseases in the host. The most well described family in this phylum is the Chlamydiaceae, consisting of 13 taxonomically classified chlamydial species (Sachse et al., 2014) and three Candidatus species (Vorimore et al., 2013; Taylor-Brown et al., 2016, 2017; Staub et al., 2018): C. trachomatis, C. muridarum, C. suis, C. psittaci, C. abortus, C. caviae, C. felis, C. pneumoniae, C. pecorum, C. avium, C. gallinacea, C. serpentis, C. poikilothermis, Candidatus C. ibidis, Ca. C. corallus, and Ca. C. sanzinia. Outside of the family Chlamydiaceae within the phylum Chlamydiae, significant taxonomic diversity awaits to be discovered with novel families and new species regularly described.
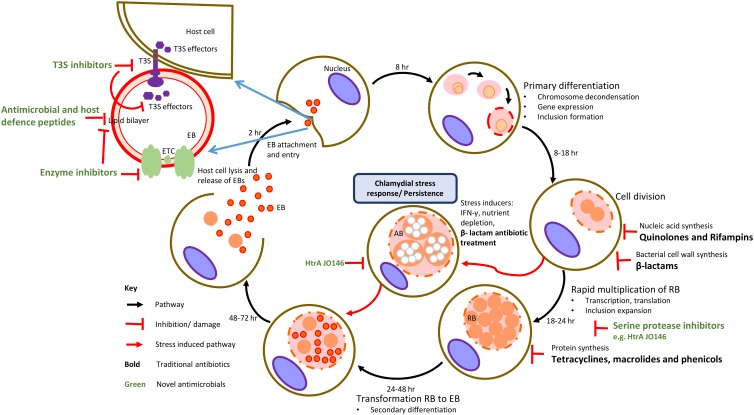
A common feature of these bacteria is a unique and complex intracellular biphasic developmental cycle (Figure 1). The cycle begins when a chlamydial EB attaches to the host cell, is internalized and forms a membrane bound cytoplasmic inclusion. In the inclusion, the EB develops into a non-infectious RB whereby the cell now actively replicates, parasitizing the host cell for metabolites that it acquires through its inclusion. Subsequent populations of RBs mature into infectious EBs that are then released upon host cell lysis to then infect neighboring cells (Abdelrahman and Belland, 2005). During sub-optimal growth conditions, antibiotic treatment or viral co-infection, chlamydial RBs may enter into a non-replicative, non-infective state, yet remaining viable until optimal growth conditions are restored (Bavoil, 2014; Figure 1). Evidence of this chlamydial stress response in vivo is rarer with some in vitro and in vivo evidence of β-lactam-induced persistence reported (Phillips-Campbell et al., 2012; Figure 1).
FIGURE 1.
Traditional targets of antimicrobial compounds at various stages of chlamydial developmental cycle. The traditional targets of current class of antibiotics (in black text) are DNA or RNA synthesis, protein synthesis and cell wall synthesis. Novel targets are chlamydial virulence factors, membrane structures and enzymes involved in metabolism with examples of these inhibitors indicated (in green text).
Chlamydiae are regularly reported in domesticated (Borel et al., 2018) and wild animals (Burnard and Polkinghorne, 2016). In livestock, chlamydial infections of pigs, cattle, sheep, goats, horses and poultry can cause major economic impacts and production losses, worldwide (Borel et al., 2018). C. suis, C. psittaci, C. abortus, and C. pecorum are the major livestock pathogens with clinical manifestations ranging from conjunctivitis, arthritis, reproductive disease, and pneumonia posing significant impacts on animal health and economic loss (Borel et al., 2018). Bacteria outside of the genus Chlamydia but in the broader phylum Chlamydiae have also been reported in animals (including fish notably) and humans with associations to adverse reproductive outcomes, respiratory infections, and potential zoonosis (Taylor-Brown et al., 2015; Taylor-Brown and Polkinghorne, 2017).
In the near-complete absence of viable chlamydial vaccines for any host, administration of antibiotics and, in particular, the use of tetracyclines, macrolides (inhibitors of protein synthesis), quinolones and rifampins (inhibitors of nucleic acid synthesis) is required for control (Kohlhoff and Hammerschlag, 2015; Figure 1). While the use of these antibiotics is widely accepted, there is growing concern over the emergence of phenotypic antibiotic resistance and treatment failure in the chlamydiae. While most of the attention has focused on treatment failure in humans (Somani et al., 2000; Kong and Hocking, 2015; Kong et al., 2015), the strongest evidence for this is actually in animals where genetically stable TETR and sulfadiazine resistance in C. suis strains infecting pigs has been well documented (Sandoz and Rockey, 2010; Borel et al., 2016). Studies of genetically acquired and phenotypic antibiotic resistance patterns in environmental chlamydiae have thus far revealed a similar trend to that of the traditional Chlamydiaceae (Baud and Greub, 2011), although there appear to be exceptions (Vouga et al., 2015).
General information on antimicrobial therapy and its associated complications with therapy failure, genotypic and phenotypic resistance in veterinary chlamydial infections is very limited. To expand on these issues, this review will summarize and discuss the evidence for the use of antimicrobials in the control of veterinary chlamydiae.
Antimicrobial Treatment of Veterinary Chlamydial Pathogens
Over the last six decades, control of the major veterinary chlamydial pathogens (C. abortus, C. psittaci, and C. pecorum) has centered on the use of tetracycline via TET-supplemented feed. Long acting oxytetracycline or its derivative doxycycline can also be administered orally with the duration and dose varying based on individual farm management practices and the form of tetracycline used for treatment (Ungemach et al., 2006). The mode of action of this antibiotic involves inhibition of chlamydial protein synthesis by binding of the antibiotic to the 30S ribosomal subunit (Figure 1). Additionally, doxycycline also has anti-inflammatory and immunomodulatory properties that result from inhibition of inducible nitric oxide synthase and proinflammatory cytokines (Sykes and Papich, 2014). While the tetracycline class of drugs have been the frontline antichlamydials in the treatment of uncomplicated chlamydial infections, macrolides, phenicols (protein synthesis inhibitors), quinolones, rifampins (nucleic acid synthesis inhibitors), and rarely β-lactams (bacterial cell wall synthesis inhibitors) antibiotic classes have also been successful in treating chlamydial infections (Borel et al., 2016; Figure 1). In the following sections, the use of antibiotics for treating the major veterinary chlamydial pathogens, C. suis, C. abortus, C. psittaci, and C. pecorum will be reviewed.
Treatment of C. abortus Infections
Chlamydia abortus is the causative agent of EAE and a zoonotic pathogen posing potential threat to pregnant women when in contact with infected ewes (Table 1). Globally, C. abortus is a serious cause of economic loss to the sheep production industry (Pospischil et al., 2002; Longbottom and Coulter, 2003). Treatment of early abortion and suspected EAE involves long-acting oxytetracycline (20 mg/kg) during the last month of pregnancy flock-wide (Supplementary Table 1). This administration has been shown to reduce the severity of C. abortus infections, pathological damage and eventually to increase the chances of live birth (Aitken et al., 1982; Greig et al., 1982). Usually a single dose is recommended to avoid emergence of TET resistance, however, fortnightly routine administration (oral tetracycline type product included in the feed at 400–500 mg/hd/day) until lambing seems to further suppress chlamydial shedding, which is crucial to prevent excretion of C. abortus at birth as well as on-farm spread of the infection (Rodolakis et al., 1980; Supplementary Table 1). Prophylactic use of tetracycline could potentially lead to emergence of acquired TET resistance, moreover, the use of therapy does not guarantee eradication of C. abortus infection with a small percentage of the pregnant flock still producing stillborn and weak born lambs whilst potentially carrying C. abortus post-treatment (Essig and Longbottom, 2015; Rodolakis and Laroucau, 2015; Table 1). To date tetracycline-resistant strains in C. abortus have not been isolated yet, however, relapse of infection or presence of C. abortus shedding post-treatment is suggestive of treatment failure due to the establishment of antibiotic protected reservoirs. Simultaneous detection of C. suis and C. abortus in semen of boars and conjunctiva of sows in pig production has been reported (Schautteet et al., 2010), further highlighting the potential risk of the spread of TETR resistance to other animal chlamydiae if significant selective pressure is maintained (Suchland et al., 2009). Despite being a major veterinary pathogen of zoonotic importance, there appears to be a lack of in vitro and in vivo models/studies investigating the role of antibiotics in the treatment of C. abortus infections in humans and animals.
Table 1.
Members of the order Chlamydiales and their animal host pathogenicity, antibiotic susceptibility, treatment and resistance.
| Species | Pathogenicity in animals | Antibiotic susceptibility | Treatment | Resistance/treatment failure/in vitro evidence of antibiotic-induced persistence | Reference |
|---|---|---|---|---|---|
| Chlamydia suis∗ | Respiratory disease, diarrhea, conjunctivitis, and reproductive disorders in pigs | Rifaximin, levofloxacin, and doxycycline | Aminoglycoside; β-lactams; fluoroquinolone; or tetracycline. Pro-/metaphylactic herd treatment: amoxicillin; chlortetracycline; MDT – chlortetracycline, sulfadimidine, tylosin; or MDT – trimethoprim, sulfadimidine, sulfathiazole. | Tetracyclineˆ and sulfadiazineˆ | Hoffmann et al., 2015 |
| Chlamydia abortus∗ | Ovine enzootic abortion | Tetracycline | Tetracycline, oxytetracycline, erythromycin, and clarithromycin. | – | Aitken et al., 1982 |
| Chlamydia psittaci∗ | Respiratory, joint, and reproductive disease in poultry, cattle, and horses | Doxycycline and enrofloxacin | Tetracycline, doxycycline, and rifampicin. | β-lactams $, tetracycline#, and rifampicin# | Butaye et al., 1998; Goellner et al., 2006 |
| Chlamydia pecorum | Ruminants: joint and ocular disease Koalas: ocular, urogenital, and reproductive disease | Tetracycline (ruminants), chloramphenicol, and florfenicol (koalas) | Tetracycline, chloramphenicol, and florfenicol. | β-lactams $ | Pudjiatmoko et al., 1998; Black et al., 2015; Leonard et al., 2017 |
| Parachlamydia acanthamoebae∗ | Miscarriage and pneumonia in bovines | Macrolides, tetracyclines, and rifampin | Azithromycin, clarithromycin, and/or doxycycline. | Quinolonesˆ, amoxicillinˆ, ceftriaxoneˆ, and imipenemˆ | Greub, 2009; Vouga et al., 2015 |
| Simkaniaceae∗ | Granulomatous lesions in reptiles | Macrolides, clindamycin, cyclines, rifampin, and quinolones | azithromycin, clarithromycin, and/or doxycycline. | β-lactams ˆ, fosfomycinˆ, and vancomycinˆ | Friedman et al., 2003; Vouga et al., 2017 |
| Waddlia chondrophila∗ | Miscarriage and pneumonia in cattle | Doxycycline and azithromycin | Azithromycin, clarithromycin, and/or doxycycline@. | β-lactams ˆ and fluoroquinolonesˆ | Goy and Greub, 2009 |
| Environmental CLOs | Epitheliocystis in fish | Tetracycline | Oxytetracycline. | Enrofloxacin# | Goodwin et al., 2005; Polkinghorne et al., 2010 |
∗Zoonotic and/or pathogenic in humans. ∧Antibiotic resistance. #Antibiotic treatment failure. $In vitro evidence of antibiotic-induced chlamydial stress response. @Empirical antimicrobial treatment in the absence of in vivo efficacy data.
Treatment of C. psittaci Infections
Chlamydia psittaci is an avian pathogen capable of causing systemic wasting disease in wild birds and production species such as chickens and ducks (Knittler and Sachse, 2015). Infection spill-over to other hosts is also a concern with C. psittaci recognized as a serious zoonotic agent of atypical pneumonia in humans (Stewardson and Grayson, 2010; Knittler and Sachse, 2015) with evidence growing for spill-over of infections and disease to other mammalian hosts as well (Van Loo et al., 2014; Jenkins et al., 2018). Human cases of psittacosis are effectively treated using orally administered doxycycline and tetracycline hydrochloride for a period of 10–14 days (Beeckman and Vanrompay, 2009; Senn et al., 2005). In patients for whom tetracycline is contra-indicated, i.e., in pregnant woman and children under the age of 8 years treatment with azithromycin and erythromycin at a dose of 250–500 mg PO qd for 7 days has proven to be the best alternative (Senn et al., 2005; Beeckman and Vanrompay, 2009). This is probably the main reason for the general decline in psittacosis cases worldwide, particularly those with fatal outcome, in the past decades. However, use of quinolones to treat chlamydia infections in humans has resulted in reports of treatment failure (Beeckman and Vanrompay, 2009).
The antibiotics of choice in veterinary medicine for the treatment of C. psittaci infections are doxycycline or other tetracyclines and the fluoroquinolone enrofloxacin administered orally (feed/drinking water) or parenterally (intramuscular or subcutaneous routes) (Flammer, 1989; Butaye et al., 1998; Supplementary Table 1). In terms of persistent infections, studies in bovine respiratory models have shown that treatment with tetracycline or rifampicin revealed evidence of clinical recovery of respiratory symptoms although re-isolation of the organism was still possible in some animals with no significant reduction in chlamydial shedding 14-days post-treatment of antibiotics (Prohl et al., 2015a,b; Table 1). In vitro studies also suggest that the development of drug-resistant C. psittaci strains is possible (Binet and Maurelli, 2005) and that C. psittaci is also capable of entering a persistent state upon treatment with penicillin G conceivably playing a role in the development of chronic infections, as well as in failure of antibiotic therapy and immunoprophylaxis (Goellner et al., 2006; Table 1). Although, TETR seems to be a problem only in C. suis, lack of antimicrobial resistance screening in routine diagnostic testing from C. psittaci field isolates in poultry and cattle impairs the assessment of the actual situation. While treatment with doxycycline and/or azithromycin seems to be efficacious for C. psittaci infections in birds, the widespread use of tetracycline in feed and/drinking water and long periods of treatment (21–25 days) in the poultry and bird industry (Guzman et al., 2010; Krautwald-Junghanns et al., 2013) can also lead to an accumulation of sub-therapeutic drug plasma concentrations (Tell et al., 2003), supporting the emergence of drug-resistant C. psittaci strains (Supplementary Table 1). Subclinical, persistent and chronic disease and infection relapse post-treatment is also plausible suggesting that there is need for pre- and post-antimicrobial treatment surveillance of C. psittaci infections in animals.
Treatment of C. suis Infections
Antibiotic therapy and the associated resistance reported for C. suis have been thoroughly reviewed recently (Borel et al., 2016). Briefly, C. suis is an endemic GIT pathogen of pigs. While a range of pathologies have been reported in association with C. suis infection (respiratory disease, diarrhea, conjunctivitis and reproductive disorders), the high rates of GIT positivity for this pathogen are commonly reported in the absence of disease (Schautteet and Vanrompay, 2011). Due to the endemic nature of C. suis in most pig production facilities, infections are rarely treated by antibiotics such as oxytetracycline. Quinolones (enrofloxacin) or macrolides (erythromycin) can be administered, in case of an infection with a TETR C. suis strain (Schautteet and Vanrompay, 2011; Table 1). However, due to emergence of TETR in C. suis, alternative treatment strategies such as the short-term treatment of C. suis infections with enrofloxacin and tiamulin was unsuccessful resulting in recurrence of C. suis infections in pigs (Reinhold et al., 2011a). The TETR feature of this bacterium, namely that it is the first and only species of intracellular bacteria known to have genetically acquired antibiotic resistance (Sandoz and Rockey, 2010) is of significant interest. The basis of this stable TETR phenotype is the presence of a Tet-island in the genome of C. suis, consisting of tetC gene encoding a TET efflux pump, TET repressor gene (tetR) (Dugan et al., 2004). These loci share high nucleotide sequence identity with several other Gram-negative bacterial-resistance plasmids, one of them being the fish bacteria Aeromonas salmonicida mobilizable plasmid pRAS3.2 (Dugan et al., 2004). Expanded studies of this Tet-island found that even in very distinct C. suis evolutionary lineages, this Tet island is present in the same genomic location adjacent to an rRNA operon (Seth-Smith et al., 2017). Based on studies at the herd-level, antibiotic treatment appears to promote the emergence of TETR and further spread of this resistance cassette among Tet-sensitive C. suis strains (Hoffmann et al., 2015; Wanninger et al., 2016).
It should be noted that acquisition of Tet Island is associated with mobile genetic elements, raising concerns over the potential spread and distribution of these elements across diverse set of bacteria, particularly into C. trachomatis, the most closely related currently described chlamydial species to C. suis. This potential risk has been confirmed experimentally with studies showing that C. suis can confer TETR to C. trachomatis in vitro (Suchland et al., 2009). Further highlighting this risk, C. suis has also been documented in ocular infections in humans with trachoma (Dean et al., 2013) and in workers in a pig production facility (De Puysseleyr et al., 2014). While most of the current risk of C. suis TETR resistance is confined to pigs, C. suis has also been detected in other animals including livestock, horses, cats, poultry (Teankum et al., 2006; Polkinghorne et al., 2009; Pantchev et al., 2010; Szymanska-Czerwinska et al., 2013; Guo et al., 2016) and wildlife (e.g., frogs) (Blumer et al., 2007).
Treatment of C. pecorum Infections
There is limited information on the efficacy of antibiotics against C. pecorum, with a single in vitro study suggesting that livestock isolates of this pathogen are susceptible to macrolides, tetracyclines and quinolones with potential recovery upon removal of the antibiotic not evaluated to further understand chlamydial latency (Pudjiatmoko et al., 1998; Table 1). In practice, treatment of C. pecorum-infected animals displaying evidence of arthritis, sporadic bovine encephalitis and conjunctivitis involves the use of intramuscular injections of long-acting oxytetracycline (300 mg/mL at a dose rate of 1 mL per 10 kg bodyweight) once a week, twice (Walker et al., 2016; Supplementary Table 1). Potential issues of chlamydial latency rather than infection clearance (Mårdh and Löwing, 1990; Smith, 2002) have been reported in association with this treatment with detectable chlamydial DNA loads as high as pre-treatment levels, three to 6 weeks post-treatment reported in some studies (Parkinson et al., 2010; Reinhold et al., 2011b; Walker, 2013; Table 1). In vitro data also suggests that penicillin G induces the chlamydial stress response (persistence) and is not bactericidal for this chlamydial species (Leonard et al., 2017; Table 1). This is of particular concern in livestock production industry where it is likely that some animals with endemic, asymptomatic C. pecorum infection are treated with both veterinary-approved and off-label antibiotics for other infections and purposes.
The treatment of C. pecorum infections is also of relevance to the veterinary treatment of the iconic Australian marsupial, the koala. Koalas infected by C. pecorum can develop ocular and urogenital tract disease that may lead to animals being admitted into wildlife hospitals for veterinary treatment (Polkinghorne et al., 2013). Treatment of clinical and subclinical koala chlamydiosis most commonly involves the administration of chloramphenicol due to its perceived safety and anecdotal effectiveness, despite a lack of information on therapeutic efficacy or pharmacokinetics in this marsupial host (Black et al., 2015; Table 1). Chloramphenicols are preferred over the efficacious first-line antichlamydials, azithromycin, or tetracyclines, as use of the latter antibiotics have been associated with gastrointestinal dysbiosis and emaciation in koalas (Osawa and Carrick, 1990). Summary of treatment regimens and associated complications of ocular, urogenital and reproductive tract disease in koalas have been reviewed in detail and can be found elsewhere (Vogelnest and Portas, 2018; Supplementary Table 1).
Treatment of Chlamydia-Related Bacteria (CRBs)
The discovery of new family level lineages in the order Chlamydiales such as the Parachlamydiaceae, Simkaniaceae, Criblamydiaceae, and Waddliaceae has prompted investigations into the pathogenic potential of this bacteria. Thus far, a range of studies have suggested that these bacteria may be linked to adverse pregnancy outcomes and respiratory disorders in humans and animals, with animal contact as a potential risk factor for higher prevalence (Taylor-Brown et al., 2015; Ammerdorffer et al., 2017; Borel et al., 2018; Table 1). While the pathogenic potential of these chlamydiae is yet to be fully defined, more recently described chlamydiae spread across several family-level taxonomic groups are well recognized causes of the gill disease of fish, epitheliocystis (Blandford et al., 2018).
There are very limited studies reported so far on antibiotic treatment regimens for CRBs in humans and animals with most of the knowledge of antibiotic efficacy and/or phenotypic resistance based on in vitro studies (Friedman et al., 2003; Goy and Greub, 2009; Greub, 2009; Vouga et al., 2015, 2017). These in vitro studies have revealed that most CRBs are resistant to quinolones and β-lactams with Parachlamydia and Neochlamydia spp. also demonstrating phenotypic resistance to amoxicillin, ceftriaxone and imipenem (MIC ≥32 μg/ml) (Vouga et al., 2015; Table 1). In the absence of data from animal models and from case reports, azithromycin, clarithromycin and/or doxycycline might be used therapeutically in case of Parachlamydia acanthamoebae infections (Greub, 2009). For Simkania, a single case study reported that simkania-associated pneumonia was successfully treated with a regimen of erythromycin (Lieberman et al., 1997). Oxytetracyclines have been found to be effective in treating epitheliocystis infections in several fish species, usually, mixed in the feed at a dose of 50 mg/kg/d for 3–5 consecutive days (Goodwin et al., 2005; Chang et al., 2016; Supplementary Table 1). Enrofloxacin failed to treat a leopard shark with epitheliocystis (Polkinghorne et al., 2010). Apart from the use of antibiotics in aquaculture, several alternative strategies have been used for treating epitheliocystis such as sterilization of rearing water using ultraviolet light (Miyaki et al., 1998), chemical treatments such as formalin, salt, benzalkonium chloride, potassium permanganate, and water exchange (Somridhivej et al., 2009; Blandford et al., 2018).
Future Directions and Concluding Remarks
While there is extensive clinical evidence supporting the use of antibiotics for the treatment of the most common chlamydial infections, this review has highlighted that, for most veterinary chlamydiae, comprehensive in vivo studies of the efficacy of these antibiotics have not been performed till date. This is obviously concerning given the growing body of evidence to suggest the potential for chlamydial antimicrobial resistance and treatment failure and the patterns and underlying causes of antibiotic resistance, treatment failure and relapse of infection post antibiotic treatment.
This issue becomes even more pressing when considering the continuing new information emerging about the host range of previously described chlamydiae as well as the range of novel chlamydiae being detected in animals (Taylor-Brown and Polkinghorne, 2017). In terms of the former, there is growing evidence that the ‘host barriers’ previously defined for veterinary chlamydiae are looser than first thought, with evidence that important chlamydial pathogens such as C. psittaci can infect a diverse range of animal hosts (Knittler et al., 2014) while others such as C. caviae are a more serious zoonotic risk than previously thought (Ramakers et al., 2017). Antimicrobial efficacy studies are lacking to inform treatment options in these new host species. In the absence of such information for novel chlamydiae, clinicians have no choice but to use treatment regimens used for existing chlamydiae. For example, the reported treatment for the newly emerging and apparently widespread chlamydial agent, C. gallinacea, involves tetracyclines or macrolides based on the treatment regimen for C. psittaci infections in poultry (Brown et al., 2016). Recent studies describing antibiotic sensitivity to tetracycline and moxifloxacin and phenotypic resistance to azithromycin in the newly described chlamydial species infecting snakes, C. serpentis and C. poikilothermis (Staub et al., 2018), demonstrate the potential for considerable variability in the antibiotic resistance profile of bacteria in the genus Chlamydia. As new chlamydial species continue to emerge in animals, the demonstration or prediction of antibiotic resistance to inform clinical treatment of these infections will become increasingly important.
In terms of the factors that may influence the success of antimicrobial control in animals, the clearance of GIT infections is still a major concern for existing antimicrobials and novel antichlamydials under development (Yeruva et al., 2013; Zhang et al., 2015). The intestinal site appears to be a natural habitat for infection of chlamydiae infecting mammalian and avian hosts, wherein studies have reported long-term GIT infections with continual shedding of the pathogen in the feces (Meyer and Eddie, 1933; York and Baker, 1951; Yang et al., 2014). This is particularly important because GIT infections associated with fecal shedding in flocks and herds appear to be the precursor to abortion, encephalitis, polyarthritis, conjunctivitis, and pneumonia in ruminants (Campos-Hernández et al., 2014; Hoffmann et al., 2015; Walker et al., 2016; Bommana et al., 2018). Ruminal and small animal models also suggest that neither the host immune system nor the use of antimicrobials is successful in clearing chlamydiae from the gut (Yeruva et al., 2013; Rank and Yeruva, 2014), due to the establishment of an antibiotic-protected reservoir in the GIT and down regulatory mechanisms further inhibiting the adaptive immune response from resolving GIT infections (Igietseme et al., 2001). Future studies to demonstrate the in vivo efficacy of existing and novel anti-chlamydial agents will need to account for GIT infection reservoirs if chlamydial “cure,” rather than clearance of symptoms, is the goal of such treatment.
In terms of acquired antibiotic resistance, co-infections of C. suis with C. trachomatis in humans and veterinary chlamydial species in animals poses the potential threat for horizontal transfer of TETR in these chlamydial species with tetracycline sub-therapeutic treatment/dosing potentially inducing selective pressure for emergence of TETR. The emergence of antibiotic resistance should be the driver for development and application of new antichlamydials in veterinary medicine. Efforts for these are already underway, including in animals (Lawrence et al., 2016), exploiting a range of strategies to target Chlamydia-specific cell structures and/or known virulence factors (Ur-Rehman et al., 2012; Marti et al., 2014; Koroleva et al., 2015; Rahn et al., 2016; Donati et al., 2017; Papa et al., 2017; Figure 1). Regardless of years of research into chlamydia control through immunoprophylaxis there are almost no viable chlamydial vaccines to date. In case of C. abortus, there is a live vaccine used in Europe and elsewhere that has been greatly beneficial in reducing the use of antimicrobials and emergence of resistance in sheep production, however, use of this live vaccine has been linked to the more recent OEA outbreaks and vaccine breakdown (Wheelhouse et al., 2010; Longbottom et al., 2018). Such efforts will be vital to meeting the demands to continue to control chlamydial infections in animals successfully in the 21st century.
Author Contributions
SB and AP conceptualized and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- EAE
enzootic abortion of ewes
- EB
elementary body
- CRBs
Chlamydia-related bacteria
- GIT
gastrointestinal tract
- MIC
minimum inhibitory concentration
- RB
reticulate body
- TETR
tetracycline resistance.
Footnotes
Funding. AP’s research was supported by an Australian Research Council Linkage Project Grant (LP160101599).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00113/full#supplementary-material
References
- Abdelrahman Y. M., Belland R. J. (2005). The chlamydial developmental cycle. FEMS Microbiol. Rev. 29 949–959. 10.1016/j.femsre.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Aitken I. D., Robinson G. W., Anderson I. E. (1982). Long-acting oxytetracycline in the treatment of enzootic abortion in ewes. Vet. Rec. 111 445–446. 10.1136/vr.111.19.445 [DOI] [PubMed] [Google Scholar]
- Ammerdorffer A., Stojanov M., Greub G., Baud D. (2017). Chlamydia trachomatis and chlamydia-like bacteria: new enemies of human pregnancies. Curr. Opin. Infect. Dis. 30 289–296. 10.1097/qco.0000000000000369 [DOI] [PubMed] [Google Scholar]
- Baud D., Greub G. (2011). Intracellular bacteria and adverse pregnancy outcomes. Clin. Microbiol. Infect. 17 1312–1322. 10.1111/j.1469-0691.2011.03604.x [DOI] [PubMed] [Google Scholar]
- Bavoil P. M. (2014). What’s in a word: the use, misuse, and abuse of the word “persistence” in Chlamydia biology. Front. Cell. Infect. Microbiol. 4:27 10.3389/fcimb.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman D. S. A., Vanrompay D. C. G. (2009). Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 15 11–17. 10.1111/j.1469-0691.2008.02669.x [DOI] [PubMed] [Google Scholar]
- Binet R., Maurelli A. T. (2005). Fitness cost due to mutations in the 16s rRNA associated with spectinomycin resistance in Chlamydia psittaci 6BC. Antimicrob. Agents Chemother. 49 4455–4464. 10.1128/AAC.49.11.4455-4464.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L. A., Higgins D. P., Govendir M. (2015). In vitro activity of chloramphenicol, florfenicol and enrofloxacin against Chlamydia pecorum isolated from koalas (Phascolarctos cinereus). Aust. Vet. J. 93 420–423. 10.1111/avj.12364 [DOI] [PubMed] [Google Scholar]
- Blandford M. I., Taylor-Brown A., Schlacher T. A., Nowak B., Polkinghorne A. (2018). Epitheliocystis in fish: an emerging aquaculture disease with a global impact. Transbound Emerg. Dis. 65 1436–1446. 10.1111/tbed.12908 [DOI] [PubMed] [Google Scholar]
- Blumer C., Zimmermann D. R., Weilenmann R., Vaughan L., Pospischil A. (2007). Chlamydiae in free-ranging and captive frogs in Switzerland. Vet. Pathol. 44 144–150. 10.1354/vp.44-2-144 [DOI] [PubMed] [Google Scholar]
- Bommana S., Walker E., Desclozeaux M., Jelocnik M., Timms P., Polkinghorne A., et al. (2018). Molecular and serological dynamics of Chlamydia pecorum infection in a longitudinal study of prime lamb production. PeerJ 6:e4296. 10.7717/peerj.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel N., Leonard C., Slade J., Schoborg R. V. (2016). Chlamydial antibiotic resistance and treatment failure in veterinary and human medicine. Curr. Clin. Microbiol. Rep. 3 10–18. 10.1007/s40588-016-0028-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel N., Polkinghorne A., Pospischil A. (2018). A review on chlamydial diseases in animals: still a challenge for pathologists? Vet. Pathol. 55 374–390. 10.1177/0300985817751218 [DOI] [PubMed] [Google Scholar]
- Brown M. A., Potroz M. G., Teh S.-W., Cho N.-J. (2016). Natural products for the treatment of Chlamydiaceae infections. Microorganisms 4:39. 10.3390/microorganisms4040039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnard D., Polkinghorne A. (2016). Chlamydial infections in wildlife–conservation threats and/or reservoirs of ‘spill-over’ infections? Vet. Microbiol. 196 78–84. 10.1016/j.vetmic.2016.10.018 [DOI] [PubMed] [Google Scholar]
- Butaye P., Ducatelle R., De Backer P., Vermeersch H., Remon J., Haesebrouck F. (1998). In vitro activities of doxycycline and enrofloxacin against European Chlamydia psittaci strains from turkeys. Antimicrob. Agents Chemother. 41 2800–2801. 10.1128/AAC.41.12.2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Hernández E., Vázquez-Chagoyán J. C., Salem A. Z. M., Saltijeral-Oaxaca J. A., Escalante-Ochoa C., López-Heydeck S. M., et al. (2014). Prevalence and molecular identification of Chlamydia abortus in commercial dairy goat farms in a hot region in Mexico. Trop. Anim. Health Production 46 919–924. 10.1007/s11250-014-0585-6 [DOI] [PubMed] [Google Scholar]
- Chang H. K., Li K. P., Tsai Y. L., Wu Y.-C., Shien J. H., Hsuan S.-L., et al. (2016). Pathobiological findings of epitheliocystis in giant grouper (Epinephelus lanceolatus) in Taiwan. Pak. Vet. J. 36 118–120. [Google Scholar]
- De Puysseleyr K., De Puysseleyr L., Dhondt H., Geens T., Braeckman L., Morré S. A., et al. (2014). Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect. Dis. 14:560. 10.1186/s12879-014-0560-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D., Rothschild J., Ruettger A., Kandel R. P., Sachse K. (2013). Zoonotic Chlamydiaceae species associated with trachoma. Nepal. Emerg. Infect. Dis. 19 1948–1955. 10.3201/eid1912.130656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati M., Cenacchi G., Biondi R., Papa V., Borel N., Vecchio Nepita E., et al. (2017). Activity of synthetic peptides against Chlamydia. Biopolymers 108:e23032. 10.1002/bip.23032 [DOI] [PubMed] [Google Scholar]
- Dugan J., Rockey D. D., Jones L., Andersen A. A. (2004). Tetracycline resistance in Chlamydia suis mediated by genomic islands inserted into the chlamydial inv-like gene. Antimicrob. Agents Chemother. 48 3989–3995. 10.1128/AAC.48.10.3989-3995.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig A., Longbottom D. (2015). Chlamydia abortus: new aspects of infectious abortion in sheep and potential risk for pregnant women. Curr. Clin. Microbiol. Rep. 2 22–34. 10.1007/s40588-015-0014-2 [DOI] [Google Scholar]
- Flammer K. (1989). Treatment of chlamydiosis in exotic birds in the United States. J. Am. Vet. Med. Assoc. 195 1537–1540. [PubMed] [Google Scholar]
- Friedman M. G., Dvoskin B., Kahane S. (2003). Infections with the chlamydia-like microorganism Simkania negevensis, a possible emerging pathogen. Microbes Infect. 5 1013–1021. 10.1016/S1286-4579(03)00188-6 [DOI] [PubMed] [Google Scholar]
- Gerlach H. (1999). “Chlamydia,” in Avian Medicine: Principles and Application, eds Ritchie B. W., Harrison G. J., Harrison L. R. (Delray Beach, FL: HBD International Inc.), 984–996. [Google Scholar]
- Goellner S., Schubert E., Liebler-Tenorio E., Hotzel H., Saluz H. P., Sachse K. (2006). Transcriptional response patterns of Chlamydophila psittaci in different in vitro models of persistent infection. Infect. Immunity 74 4801–4808. 10.1128/IAI.01487-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin A. E., Park E., Nowak B. F. (2005). Successful treatment of largemouth bass, Micropterus salmoides (L.), with epitheliocystis hyperinfection. J. Fish Dis. 28 623–625. 10.1111/j.1365-2761.2005.00662.x [DOI] [PubMed] [Google Scholar]
- Goy G., Greub G. (2009). Antibiotic susceptibility of Waddlia chondrophila in Acanthamoeba castellanii amoebae. Antimicrob. Agents Chemother. 53 2663–2666. 10.1128/aac.00046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig A., Linklater K. A., Dyson D. A. (1982). Long-acting oxytetracycline in the treatment of enzootic abortion in ewes. Vet. Rec. 111 445–446. 10.1136/vr.111.19.445 [DOI] [PubMed] [Google Scholar]
- Greub G. (2009). Parachlamydia acanthamoebae, an emerging agent of pneumonia. Clin. Microbiol. Infect. 15 18–28. 10.1111/j.1469-0691.2008.02633.x [DOI] [PubMed] [Google Scholar]
- Guo W., Li J., Kaltenboeck B., Gong J., Fan W., Wang C. (2016). Chlamydia gallinacea, not C. psittaci, is the endemic chlamydial species in chicken (Gallus gallus). Sci. Rep. 6:19638. 10.1038/srep19638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman D. S., Diaz-Figueroa O., Tully T., Jr., Ciembor P., Morgan T., Walden M., et al. (2010). Evaluating 21-day doxycycline and azithromycin treatments for experimental Chlamydophila psittaci infection in cockatiels (Nymphicus hollandicus). J. Avian Med. Surg. 24 35–45. 10.1647/2009-009r.1 [DOI] [PubMed] [Google Scholar]
- Hoffmann K., Schott F., Donati M., Di Francesco A., Hässig M., Wanninger S., et al. (2015). Prevalence of Chlamydial infections in fattening pigs and their influencing factors. PLoS One 10:e0143576. 10.1371/journal.pone.0143576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt H., Orbell G. M. B., Buckle K. N., Ha H. J., Lawrence K. E., Fairley R. A., et al. (2016). First report and histological features of Chlamydia pecorum encephalitis in calves in New Zealand. N. Z. Vet. J. 64 364–368. 10.1080/00480169.2016.1208781 [DOI] [PubMed] [Google Scholar]
- Igietseme J. U., Portis J. L., Perry L. L. (2001). Inflammation and clearance of Chlamydia trachomatis in enteric and nonenteric mucosae. Infect. Immunity 69 1832–1840. 10.1128/IAI.69.3.1832-1840.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C., Jelocnik M., Micallef M. L., Galea F., Taylor–Brown A., Bogema D. R., et al. (2018). An epizootic of Chlamydia psittaci equine reproductive loss associated with suspected spillover from native Australian parrots. Emerg. Microbes Infect. 7:88. 10.1038/s41426-018-0089-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittler M. R., Berndt A., Bocker S., Dutow P., Hanel F., Heuer D., et al. (2014). Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int. J. Med. Microbiol. 304 877–893. 10.1016/j.ijmm.2014.06.010 [DOI] [PubMed] [Google Scholar]
- Knittler M. R., Sachse K. (2015). Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog. Dis. 73 1–15. 10.1093/femspd/ftu007 [DOI] [PubMed] [Google Scholar]
- Kohlhoff S. A., Hammerschlag M. R. (2015). Treatment of chlamydial infections: 2014 update. Expert Opin. Pharmacother. 16 205–212. 10.1517/14656566.2015.999041 [DOI] [PubMed] [Google Scholar]
- Kong F. Y., Tabrizi S. N., Fairley C. K., Vodstrcil L. A., Huston W. M., Chen M., et al. (2015). The efficacy of azithromycin and doxycycline for the treatment of rectal Chlamydia infection: a systematic review and meta-analysis. J. Antimicrob. Chemother. 70 1290–1297. 10.1093/jac/dku574 [DOI] [PubMed] [Google Scholar]
- Kong F. Y. S., Hocking J. S. (2015). Treatment challenges for urogenital and anorectal Chlamydia trachomatis. BMC Infect. Dis. 15:293. 10.1186/s12879-015-1030-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroleva E. A., Kobets N. V., Zayakin E. S., Luyksaar S. I., Shabalina L. A., Zigangirova N. A. (2015). Small molecule inhibitor of type three secretion suppresses acute and chronic Chlamydia trachomatis infection in a novel urogenital Chlamydia model. Biomed. Res. Int. 2015:484853. 10.1155/2015/484853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautwald-Junghanns M. E., Stolze J., Schmidt V., Bohme J., Sachse K., Cramer K. (2013). Efficacy of doxycycline for treatment of chlamydiosis in flocks of racing and fancy pigeons. Tierarztl Prax Ausg K Kleintiere Heimtiere 41 392–398. [PubMed] [Google Scholar]
- Lawrence A., Fraser T., Gillett A., Tyndall J. D. A., Timms P., Polkinghorne A., et al. (2016). Chlamydia serine protease inhibitor, targeting HtrA, as a new treatment for koala Chlamydia infection. Sci. Rep. 6:31466. 10.1038/srep31466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C., Schoborg R., Borel N. (2017). Productive and penicillin-stressed Chlamydia pecorum infection induces nuclear factor kappa b activation and interleukin-6 secretion In Vitro. Front. Cell. Infect. Microbiol. 7:180. 10.3389/fcimb.2017.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D., Kahane S., Lieberman D., Friedman M. G. (1997). Pneumonia with serological evidence of acute infection with the Chlamydia-like microorganism “Z”. Am. J. Respir. Crit. Care Med. 156(2 Pt 1), 578–582. 10.1164/ajrccm.156.2.9608081 [DOI] [PubMed] [Google Scholar]
- Longbottom D., Coulter L. J. (2003). Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128 217–244. 10.1053/jcpa.2002.0629 [DOI] [PubMed] [Google Scholar]
- Longbottom D., Sait M., Livingstone M., Laroucau K., Sachse K., Harris S. R., et al. (2018). Genomic evidence that the live Chlamydia abortus vaccine strain 1B is not attenuated and has the potential to cause disease. Vaccine 36 3593–3598. 10.1016/j.vaccine.2018.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Löwing C. (1990). Treatment of chlamydial infections. Scand. J. Infect. Dis. Suppl. 68 23–30. [PubMed] [Google Scholar]
- Marti H., Koschwanez M., Pesch T., Blenn C., Borel N. (2014). Water-filtered infrared a irradiation in combination with visible light inhibits acute chlamydial infection. PLoS One 9:e102239. 10.1371/journal.pone.0102239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. F., Eddie B. (1933). Latent psittacosis infections in shell parrakeets. Proc. Soc. Exp. Biol. Med. 30 484–488. 10.3181/00379727-30-6539 [DOI] [Google Scholar]
- Miyaki K., Mizuta K., Yamamoto N., Yoshikoshi K., Kanai K., Tabeta O. (1998). Mass mortality of hatchery-reared juveniles of bartail flat-head, Platycephalus sp. due to epitheliocystis-like disease. Bull. Nagasaki Prefectural Inst. Fish. 24 7–10. [Google Scholar]
- Osawa R., Carrick F. N. (1990). Use of a dietary supplement in koalas during systemic antibiotic treatment of chlamydial infection. Aust. Vet. J. 67 305–307. 10.1111/j.1751-0813.1990.tb07805.x [DOI] [PubMed] [Google Scholar]
- Pantchev A., Sting R., Bauerfeind R., Tyczka J., Sachse K. (2010). Detection of all Chlamydophila and Chlamydia spp. of veterinary interest using species-specific real-time PCR assays. Comp. Immunol. Microbiol. Infect. Dis. 33 473–484. 10.1016/j.cimid.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Papa V., Ginocchietti L., Budriesi R., Micucci M., Costa R., Biondi R., et al. (2017). In vitro activity of a partially purified and characterized bark extract of Castanea sativa Mill. (ENC(R)) against Chlamydia spp. Ultrastruct. Pathol. 41 147–153. 10.1080/01913123.2016.1275909 [DOI] [PubMed] [Google Scholar]
- Parkinson T. J., Vermunt J. J., Malmo J. (2010). Sporadic Bovine Encephalomyelitis. Diseases of Cattle in Australasia, A Comprehensive Textbook. Wellington, NZ: New Zealand Veterinary Association Foundation for Continuing Education, 832–833. [Google Scholar]
- Phillips-Campbell R., Kintner J., Whittimore J., Schoborg R. V. (2012). Chlamydia muridarum enters a viable but non-infectious state in amoxicillin-treated balb/c mice. Microbes Infect. 14 1177–1185. 10.1016/j.micinf.2012.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkinghorne A., Borel N., Becker A., Lu Z. H., Zimmermann D. R., Brugnera E., et al. (2009). Molecular evidence for chlamydial infections in the eyes of sheep. Vet. Microbiol. 135 142–146. 10.1016/j.vetmic.2008.09.034 [DOI] [PubMed] [Google Scholar]
- Polkinghorne A., Hanger J., Timms P. (2013). Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet. Microbiol. 165 214–223. 10.1016/j.vetmic.2013.02.026 [DOI] [PubMed] [Google Scholar]
- Polkinghorne A., Schmidt-Posthaus H., Meijer A., Lehner A., Vaughan L. (2010). Novel Chlamydiales associated with epitheliocystis in a leopard shark Triakis semifasciata. Dis. Aquat. Organ. 91 75–81. 10.3354/dao02255 [DOI] [PubMed] [Google Scholar]
- Pospischil A., Thoma R., Hilbe M., Grest P., Zimmermann D., Gebbers J. O. (2002). Abortion in humans caused by Chlamydophila abortus (Chlamydia psittaci serovar 1). Schweiz Arch. Tierheilkd 144 463–466. 10.1024/0036-7281.144.9.463 [DOI] [PubMed] [Google Scholar]
- Prohl A., Lohr M., Ostermann C., Liebler-Tenorio E., Berndt A., Schroedl W., et al. (2015a). Enrofloxacin and macrolides alone or in combination with rifampicin as antimicrobial treatment in a bovine model of acute Chlamydia psittaci infection. PLoS One 10:e0119736. 10.1371/journal.pone.0119736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohl A., Lohr M., Ostermann C., Liebler-Tenorio E., Berndt A., Schroedl W., et al. (2015b). Evaluation of antimicrobial treatment in a bovine model of acute Chlamydia psittaci infection: tetracycline versus tetracycline plus rifampicin. Pathog. Dis. 73 1–12. 10.1111/2049-632x.12212 [DOI] [PubMed] [Google Scholar]
- Pudjiatmoko, Fukushi H., Ochiai Y., Yamaguchi T., Hirai K. (1998). In vitro susceptibility of Chlamydia pecorum to macrolides, tetracyclines, quinolones and beta-lactam. Microbiol. Immunol. 42 61–63. 10.1111/j.1348-0421.1998.tb01971.x [DOI] [PubMed] [Google Scholar]
- Rahn C., Marti H., Frohns A., Frohns F., Blenn C., Leonard C. A., et al. (2016). Water-filtered infrared A reduces chlamydial infectivity in vitro without causing ex vivo eye damage in pig and mouse models. J. Photochem. Photobiol. B 165 340–350. 10.1016/j.jphotobiol.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Ramakers B. P., Heijne M., Lie N., Le T. N., van Vliet M., Claessen V. P. J., et al. (2017). Zoonotic Chlamydia caviae presenting as community-acquired pneumonia. N. Engl. J. Med. 377 992–994. 10.1056/NEJMc1702983 [DOI] [PubMed] [Google Scholar]
- Rank R. G., Yeruva L. (2014). Hidden in plain sight: Chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect. Immunity 82 1362–1371. 10.1128/IAI.01244-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold P., Liebler-Tenorio E., Sattler S., Sachse K. (2011a). Recurrence of Chlamydia suis infection in pigs after short-term antimicrobial treatment. Vet. J. 187 405–407. 10.1016/j.tvjl.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Reinhold P., Sachse K., Kaltenboeck B. (2011b). Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet. J. 189 257–267. 10.1016/j.tvjl.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Rodolakis A., Laroucau K. (2015). Chlamydiaceae and chlamydial infections in sheep or goats. Vet. Microbiol. 181 107–118. 10.1016/j.vetmic.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Rodolakis A., Souriau A., Raynaud J. P., Brunault G. (1980). Efficacy of a long-acting oxytetracycline against chlamydial ovine abortion. Ann. Rech. Vet. 11 437–444. [PubMed] [Google Scholar]
- Sachse K., Laroucau K., Riege K., Wehner S., Dilcher M., Creasy H. H., et al. (2014). Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. nov. and Chlamydia gallinacea sp. nov. Syst. Appl. Microbiol. 37 79–88. 10.1016/j.syapm.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Sandoz K. M., Rockey D. D. (2010). Antibiotic resistance in Chlamydiae. Future Microbiol. 5 1427–1442. 10.2217/fmb.10.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schautteet K., Beeckman D. S. A., Delava P., Vanrompay D. (2010). Possible pathogenic interplay between Chlamydia suis, Chlamydophila abortus and PCV-2 on a pig production farm. Vet. Rec. 166 329–333. 10.1136/vr.b4714 [DOI] [PubMed] [Google Scholar]
- Schautteet K., Vanrompay D. (2011). Chlamydiaceae infections in pig. Vet. Res. 42:29. 10.1186/1297-9716-42-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn L., Hammerschlag M. R., Greub G. (2005). Therapeutic approaches to Chlamydia infections. Expert Opin. Pharmacother. 6 2281–2290. 10.1517/14656566.6.13.2281 [DOI] [PubMed] [Google Scholar]
- Seth-Smith H. M. B., Wanninger S., Bachmann N., Marti H., Qi W., Donati M., et al. (2017). The Chlamydia suis genome exhibits high levels of diversity, plasticity, and mobile antibiotic resistance: comparative genomics of a recent livestock cohort shows influence of treatment regimes. Genome Biol. Evol. 9 750–760. 10.1093/gbe/evx043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. P. (2002). Large Animal Internal Medicine. St. Louis, MO: Harcourt. [Google Scholar]
- Somani J., Bhullar V. B., Workowski K. A., Farshy C. E., Black C. M. (2000). Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J. Infect. Dis. 181 1421–1427. 10.1086/315372 [DOI] [PubMed] [Google Scholar]
- Somridhivej B., Taveekijakarn P., Me-anan C., Somsiri T. (2009). “Treatment of epitheliocystis in juvenile tilapia,” in Proceedings of the 47th Kasetsart University Annual Conference, (Kasetsart: Kasetsart University; ). [Google Scholar]
- Staub E., Marti H., Biondi R., Levi A., Donati M., Leonard C. A., et al. (2018). Novel Chlamydia species isolated from snakes are temperature-sensitive and exhibit decreased susceptibility to azithromycin. Sci. Rep. 8:5660. 10.1038/s41598-018-23897-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewardson A. J., Grayson M. L. (2010). Psittacosis. Infect. Dis. Clin. 24 7–25. 10.1016/j.idc.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Suchland R. J., Sandoz K. M., Jeffrey B. M., Stamm W. E., Rockey D. D. (2009). Horizontal transfer of tetracycline resistance among Chlamydia spp. In vitro. Antimicrob. Agents Chemother. 53 4604–4611. 10.1128/aac.00477-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. E., Papich M. G. (2014). “Chapter 8 – antibacterial drugs,” in Canine and Feline Infectious Diseases, ed. Sykes J. E. (Saint Louis, MO: W.B. Saunders; ), 66–86. [Google Scholar]
- Szymanska-Czerwinska M., Niemczuk K., Galinska E. M. (2013). Serological and nested PCR survey to determine the occurrence of Chlamydia infections in the Polish cattle population. Ann. Agric. Environ. Med. 20 682–686. [PubMed] [Google Scholar]
- Taylor-Brown A., Bachmann N. L., Borel N., Polkinghorne A. (2016). Culture-independent genomic characterisation of Candidatus Chlamydia sanzinia, a novel uncultivated bacterium infecting snakes. BMC Genomics 17:710. 10.1186/s12864-016-3055-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Brown A., Polkinghorne A. (2017). New and emerging chlamydial infections of creatures great and small. New Microbes New Infect. 18 28–33. 10.1016/j.nmni.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Brown A., Spang L., Borel N., Polkinghorne A. (2017). Culture-independent metagenomics supports discovery of uncultivable bacteria within the genus Chlamydia. Sci. Rep. 7:10661. 10.1038/s41598-017-10757-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Brown A., Vaughan L., Greub G., Timms P., Polkinghorne A. (2015). Twenty years of research into Chlamydia-like organisms: a revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog. Dis. 73 1–15. 10.1093/femspd/ftu009 [DOI] [PubMed] [Google Scholar]
- Teankum K., Pospischil A., Janett F., Burgi E., Brugnera E., Hoelzle K., et al. (2006). Detection of chlamydiae in boar semen and genital tracts. Vet. Microbiol. 116 149–157. 10.1016/j.vetmic.2006.03.021 [DOI] [PubMed] [Google Scholar]
- Tell L. A., Sun Y., Needham M., Johnson J. R., Shukla A. (2003). In vivo release of oxytetracycline from a biodegradable controlled-release gel injected subcutaneously in Japanese quail (Coturnix coturnix japonica). J. Vet. Pharmacol. Ther. 26 239–245. 10.1046/j.1365-2885.2003.00481.x [DOI] [PubMed] [Google Scholar]
- Ungemach F. R., Muller-Bahrdt D., Abraham G. (2006). Guidelines for prudent use of antimicrobials and their implications on antibiotic usage in veterinary medicine. Int. J. Med. Microbiol. 296(Suppl. 41), 33–38. 10.1016/j.ijmm.2006.01.059 [DOI] [PubMed] [Google Scholar]
- Ur-Rehman T., Slepenkin A., Chu H., Blomgren A., Dahlgren M. K., Zetterström C. E., et al. (2012). Pre-clinical pharmacokinetics and anti-chlamydial activity of salicylidene acylhydrazide inhibitors of bacterial type III secretion. J. Antibiot. 65 397–404. 10.1038/ja.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo H., Pardon B., De Schutter P., De Bleecker K., Vanrompay D., Deprez P., et al. (2014). Detection of Chlamydia psittaci in Belgian cattle with signs of respiratory disease and milk drop syndrome. Vet. Rec. 175:562. 10.1136/vr.102527 [DOI] [PubMed] [Google Scholar]
- Vogelnest L., Portas T. (2018). “Chlamydiosis in koalas,” in Medicine of Australian Mammals, eds Vogelnest L., Woods R. (Clayton city, MO: CSIRO Publishing; ). [Google Scholar]
- Vorimore F., Hsia R. C., Huot-Creasy H., Bastian S., Deruyter L., Passet A., et al. (2013). Isolation of a New Chlamydia species from the Feral Sacred Ibis (Threskiornis aethiopicus): Chlamydia ibidis. PLoS One 8:e74823. 10.1371/journal.pone.0074823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouga M., Baud D., Greub G. (2017). Simkania negevensis, an example of the diversity of the antimicrobial susceptibility pattern among Chlamydiales. Antimicrob. Agents Chemother. 61:e00638-17. 10.1128/AAC.00638-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouga M., Diabi H., Boulos A., Baud D., Raoult D., Greub G. (2015). Antibiotic susceptibility of Neochlamydia hartmanellae and Parachlamydia acanthamoebae in amoebae. Microbes Infect. 17 761–765. 10.1016/j.micinf.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Walker E. (2013). “Branhamella ovis and Chlamydia pecorum isolated from a case of conjunctivitis (with some polyarthritis) in lambs,” in Proceedings of the 2013 District Veterinarian Conference, Armidale. [Google Scholar]
- Walker E., Moore C., Shearer P., Jelocnik M., Bommana S., Timms P., et al. (2016). Clinical, diagnostic and pathologic features of presumptive cases of Chlamydia pecorum-associated arthritis in Australian sheep flocks. BMC Vet. Res. 12:193. 10.1186/s12917-016-0832-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanninger S., Donati M., Di Francesco A., Hässig M., Hoffmann K., Seth-Smith H. M. B., et al. (2016). Selective pressure promotes tetracycline resistance of Chlamydia suis in fattening pigs. PLoS One 11:e0166917. 10.1371/journal.pone.0166917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelhouse N., Aitchison K., Laroucau K., Thomson J., Longbottom D. (2010). Evidence of Chlamydophila abortus vaccine strain 1B as a possible cause of ovine enzootic abortion. Vaccine 28 5657–5663. 10.1016/j.vaccine.2010.04.114 [DOI] [PubMed] [Google Scholar]
- Yang R., Jacobson C., Gardner G., Carmichael I., Campbell A., Ryan U. (2014). Longitudinal prevalence and faecal shedding of Chlamydia pecorum in sheep. Vet. J. 201 322–326. 10.1016/j.tvjl.2014.05.037 [DOI] [PubMed] [Google Scholar]
- Yeruva L., Spencer N., Bowlin A. K., Wang Y., Rank R. G. (2013). Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog. Dis. 68 88–95. 10.1111/2049-632X.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York C. J., Baker J. A. (1951). A new member of the psittacosis-lymphogranuloma group of viruses that causes infection in calves. J. Exp. Med. 93 587–604. 10.1084/jem.93.6.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Huang Y., Gong S., Yang Z., Sun X., Schenken R., et al. (2015). In Vivo and Ex Vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect. Immunity 83 3568–3577. 10.1128/IAI.00673-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.